Small bowel adenocarcinoma:An overview
INTRODUCTION
The small intestine is the longest part of the entire digestive tract, which constitutes about 75% of the total length (about 6 m in length and 4 times the length of the large intestine). It constitutes 90% of the absorptive surface area of the gastro-intestinal tract.It has three parts:duodenum, jejunum, and ileum. It is the primary site of absorption of proteins, lipids, and carbohydrates as well as the synthesis of vitamin B12.Malignant neoplasms arising from the small intestine are very rare worldwide, but their incidence has been on the rise in the past few decades, with an estimated growth of around 100%[1]. Malignancies of the small intestine are primarily small bowel adenocarcinoma (SBA) (40%) and neuroendocrine tumors (40%), with others including gastrointestinal stromal tumor, lymphoma, sarcoma
Since SBA is a rare cancer,there is no consensus regarding its diagnostic approach and management strategies. In general, resectable and advanced stages of SBA are treated as an extension of colorectal cancer (CRC) despite poor patient outcomes. However, recent data on molecular profiling have highlighted the settings where it may not be possible to treat SBA as an extension of CRC. This review article will focus only on SBA and discuss in brief its etiopathogenesis, presentation, and management.
EPIDEMIOLOGY
According to the United States (US) National Cancer Database, there has been a rapid rise in the incidence of small bowel tumors, from 11.8 cases/million in 1973 to 22.7 cases/million in 2004[2]. The lifetime risk of developing SBA in the US was only about 0.3% in 2015, and this was 2-5 times less than the risk of developing CRC[3]. African Americans show disproportionate gender affliction, with incidences of 4.2 and 3.5 among men and women, respectively, while Native Americans and Asians are the least likely to be diagnosed with SBA. Duodenal primaries constitute about 50% of SBA, while jejunal and ileal primaries contribute to 30% and 20% of SBAs, respectively[4]. The median age at diagnosis is around 60 years, of which over 85% present above 50 years of age and with a relatively higher incidence among males (relative risk:2.6 for males, 2 for female)[5,6]. SBA is mostly diagnosed in the late stage. This highlights that SBA is difficult to diagnose and enlightens the lack of adequate screening programs, even for the high-risk individuals.
About 60% of patients are symptomatic at presentation, and the most common symptom is related to stenosis[7]. Symptomatic presentation was more common for jejuno-ileal primaries (84%) as compared to duodenum (54%). For duodenal primaries,patients present with complaints suggestive of both stenosis or bleeding.
ETIOLOGY AND PATHOPHYSIOLOGY
In contrast to CRC, studies evaluating the pathogenesis of SBA are lacking due to the rarity of the disease. As seen with most alimentary tract cancers, smoking and alcohol consumption are associated with a higher risk of SBA. Some studies have reported high consumption of refined carbohydrates, red meat, or smoked food to be related to higher risk, but higher intake of coffee and vegetables appear to be protective[8].
Since SBA is a rare disease, it has been difficult to construct a hypothesis regarding its pathogenesis. However, the probable hypotheses include rapid turnover of small intestinal epithelium due to accumulated genetic damage and increased lymphoid tissue allowing increased immune surveillance. On the other hand, the inherent nature of the small intestine allows less exposure to carcinogenic agents due to rapid transit,dilute alkaline milieu, and lack of bacterial degradation[9]. The intestinal epithelium also has a wide array of microsomal enzymes, including various hydroxylases, that protect them against possible carcinogens[10].
Small bowel cancers are staged according to the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) system, eighth edition.
Surgery remains the treatment of choice for localized SBA. Complete resection with negative margins with adequate lymph node dissection is the mainstay of treatment.The principle of surgical resection is to remove the tumor with at least 5 cm proximal and distal margin, with resection of the adjoining mesentery and adequate lymphnode dissection. The technique and type of resection depend on the segment of small bowel involved. Segmental resection with lymph node dissection is the treatment of choice for tumors located in jejunum and ileum. Lymphatic drainage of ileum and jejunum is to mesenteric nodes, which include superior mesenteric nodes. Tumors of the distal ileum or ileocaecal valve require ileo-caecal resection or right hemicolectomy with resection of ileocolic artery and associated lymph nodes[40]. The lymphatic drainage of the ileocecum is to appendicular, ileocolic, and superior mesenteric lymph nodes. Segmental resection with lymph node dissection can be performed for tumors of first and third part of duodenum. For adenocarcinoma arising from the second part of duodenum or invading into the ampulla or pancreas, pancreaticoduodenectomy should be considered[41]. Lymphatic drainage of duodenum is to pancreaticoduodenal, pyloric, hepatic (pericholedochal, cystic, hilar), and superior mesenteric nodes.
PROGNOSTIC FACTORS
Small bowel video capsule endoscopy (VCE) is a non-invasive diagnostic modality used for visualization of small bowel mucosa. In a pooled analysis of 530 patients, out of 106 diagnosed neoplasms, 20 were missed at capsule endoscopy (miss rate 18.9%),while 67 were missed by the comparison modality (miss rate 63.2%)[35]. VCE is limited by its inability to perform tissue sampling and it cannot be performed in small bowel obstruction or stricture due to risk of capsule retention. Enteroscopy allows visualization and biopsy of small bowel lesions. For complete evaluation, enteroscopy should be performed both
oral and anal approach, as the diagnostic accuracy for any suspected small intestinal pathology is almost 80%. The limitations of enteroscopy are its invasive nature, and the need of expertise and equipment for performing it.
The prognosis of SBA remains poor even after surgical resection. The 5-year survival ranges from 20%-50%[2,24,43-45]. The pattern of relapse is predominantly systemic. In a study by Dabaja
[24], out of 146 patients who underwent curative resection, 58 patients had disease recurrence. The patterns of recurrence were:distant, carcinomatosis, abdominal wall, and local recurrence in 33, 11, 4, and 10 patients, respectively.Since SBA is a rare disease, randomized controlled trials (RCTs) on the use of adjuvant therapy are largely lacking. The role of adjuvant treatment in SBA is still not clear. The use of chemotherapy in the adjuvant setting has been extrapolated from colon cancers because of clinicopathological and molecular similarities. The rationale and regimen of chemotherapy is the same as colon cancer. A prospective phase 3 randomized trial,BALLAD, is recruiting patients to investigate the benefit of adjuvant chemotherapy in SBA.
He missed his family. His mom raised four kids by herself on a forty?acre farm in Missouri but no matter how scarce money was, she d always made sure they had a good Christmas. He thought about his box of gifts in the truck.
And now her two sisters found her to be that fine, beautiful lady whom they had seen at the ball. They threw themselves at her feet to beg pardon60 for all the ill- treatment they had made her undergo. Cinderella took them up, and, as she embraced them, cried that she forgave them61 with all her heart, and desired them always to love her.
CLINICAL PRESENTATION
The diagnosis of SBA is often delayed due to vague and non-specific symptoms. Also,the small bowel has been a challenging anatomical site for evaluation by endoscopic and radiological techniques. The disease is often advanced by the time the patient presents to the health care facilities. Abdominal pain, which is intermittent and crampy, is the most common presenting symptom followed by nausea and vomiting,anemia, gastrointestinal bleeding, weight loss, and jaundice[6,7]. Though initial symptoms are nonspecific abdominal discomfort, they are often missed and SBA usually presents as an emergency due to an occlusion or bleeding. Obstruction may occur due to narrowing of the lumen by large intraluminal mass or due to an apple core lesion. However, it is difficult to differentiate between benign and malignant lesions based on clinical presentation. The physical findings depend on the stage and degree of involvement. The differential diagnoses include adhesions, IBD, irritable bowel syndrome, diverticulitis, adenomas, polyposis syndromes, benign neoplasms of small bowel, and peptic ulcer disease.
And when she had done this, she went up to the feast, and everyone stepped out of her way, for nobody knew her, and they thought she must be a King s daughter
DIAGNOSIS AND STAGING
Multiple imaging techniques are available to evaluate small bowel neoplasms but the best imaging strategy is still not clear. Plain abdominal X-rays may show partial or complete obstruction; however, they are of limited value. Upper gastrointestinal series with small bowel follow-through involves administration of barium to delineate the small bowel and to pick up mucosal abnormalities; however, it does not contribute to staging and may miss smaller lesions. Enteroclysis provides improved and detailed evaluation of small bowel segments and can be performed by three methods :single contrast, air contrast, and methylcellulose enteroclysis. A thin nasogastric tube is passed beyond the stomach into the small bowel. However, enteroclysis is timeconsuming, technically complex, and causes discomfort to the patient, and the procedure may miss flat infiltrating lesions and extramural disease.
Then he called loudly Itchi, Itchi! Rabbit, my friend, be quick, be quick! Don t you hear how my skin is crackling ? And the rabbit came in a great hurry and pulled him out
Harvesting eight lymph nodes are considered adequate for lymph node evaluation[42]. Extended lymph node dissection does not appear to be beneficial in small bowel cancers[40].
CT enterography and magnetic resonance (MR) enterography are the preferred diagnostic and staging modality for small bowel neoplasms. The technique requires careful patient preparation, which includes administration of neutral or low-density oral contrast media (1.5-2 litres over 45-60 minutes) with enteric phase CT (45 seconds after infusion of contrast) to optimize contrast resolution between mucosa and lumen,thus enabling evaluation of mucosal abnormalities arising from the small bowel wall[33]. Positron emission tomography-CT (PET-CT) helps in the initial diagnosis, disease staging, and assessment of response to treatment and restaging or ruling out recurrence of disease but is not routinely recommended[34].
The disease staging of SBA is similar to CRC and the prognosis is directly related to the stage of the disease. Approximately 10% of the patients present with stage I disease, 30% with stage II, 25% with stage III, and 35% with stage IV disease, which reflects the delay in diagnosis[23]. The 5-year overall survival (OS) for SBA is around 20%-30%[13]. The stage-wise 5-year OS is 55%, 50%, 30%, and 5% for Stages I, II, III,and IV, respectively[24]. Moreover, the 5-year disease-free survival (DFS) in stage III patients is related to the number of lymph nodes involved. The DFS is 58% with two or less positive lymph nodes
35% with three or more[25]. In general, the prognosis of SBA is worse than CRC but better than gastric cancer. Other factors associated with poor prognosis are advanced age, poor performance status, duodenal primary, low serum albumin, high carcinoembryonic antigen (CEA) or cancer antigen 19-9 (CA 19.9), poorly differentiated tumor, and positive surgical margins[26,27]. Those with duodenal primary have the worst prognosis, probably related to under-staging and incomplete lymph node dissection[25].
So that evening, when the Princess came once more with her sleeping-drink, he pretended to drink, but threw it away behind him, for he suspected that it was a sleeping-drink
Other than imaging, faecal occult blood test should be done in addition to complete hemogram and kidney and liver function tests. The role of tumor markers is limited,and serum CEA may be elevated but cannot be used for diagnostic and prognostic purposes.
On histopathology, SBA may present as polypoidal, infiltrative, or constrictive lesions. The duodenal primaries are mostly exophytic. Jejunal and ileal adenocarcinomas are usually constricting apple-core lesions, which are large annular and have circumferential bowel involvement. They are histologically very similar to colorectal adenocarcinoma and are identified by their complex glandular architecture, degree of cellular and nuclear pleomorphism, loss of epithelial polarity, desmoplastic reaction,luminal necrosis, and invasion[36].
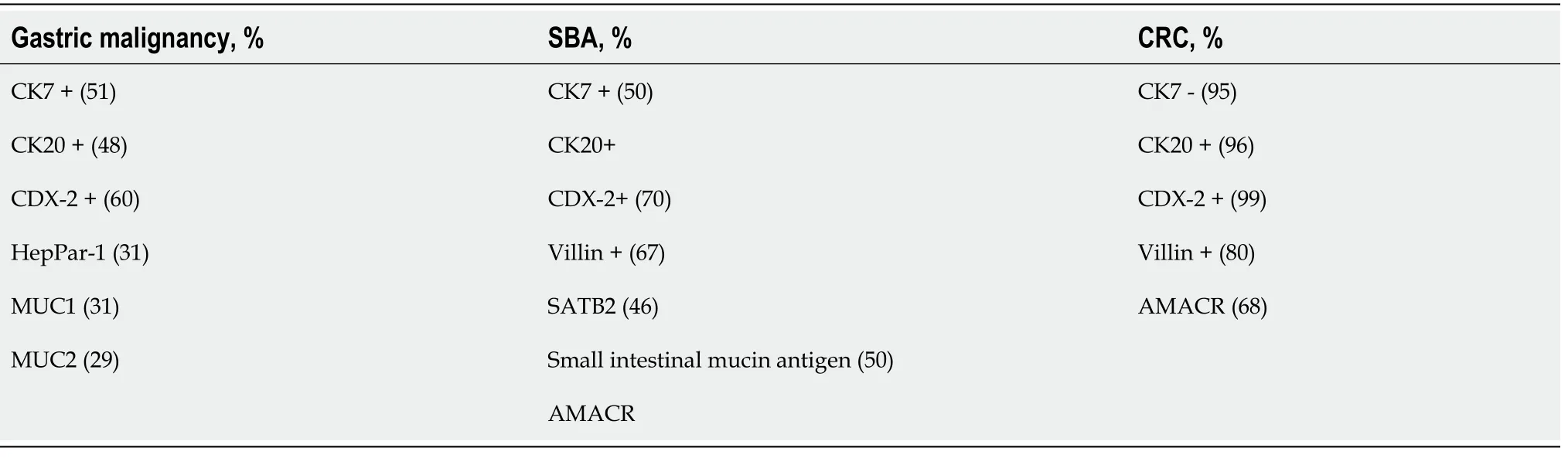
On immunohistochemistry (IHC), SBA are cytokeratin (CK)7 positive in more than 50% of cases. SBA may also be CK20, caudal-type homeobox 2 (CDX2), SMAD4, or villin positive. They may be easily distinguished from CRC which are CK7-/CK20+ on IHC[37]. There is also a significant difference in the expression of
-methylacyl coenzyme (a racemase overexpressed in CRC but very rare in SBA). IHC of the pathways of tumorigenesis reveals that the pathogenesis of SBA and CRC are significantly different. Complete loss of APC reactivity, nuclear expression of ?-catenin was more frequently associated with SBA, thus showing that Wnt signaling defects and MSI pathways are only responsible for 40% of SBA[38]. Rates of mutation in p53 and Rb gene were similar in both groups. However, an Italian study found that a subgroup of SBA with CK7?/CK20- were associated with mismatch repair (MMR)-deficiency, and those with CK7?/CK20? or CK7?/CK20+ SBAs had significantly better survival compared to those with CK7+/CK20? or CK7+/CK20+ cancers[39].Table 1 summarizes the IHC test results that differentiate between adenocarcinoma of stomach and small and large intestine.
Most of the cases of SBA are sporadic, but an increased risk is seen in inherited cancer syndromes, like Lynch syndrome, familial adenomatous polyposis, and Peutz-Jeghers syndrome[11]. The majority of sporadic cases are related to inflammatory bowel diseases (IBD) like the Crohn’s disease and celiac disease. A meta-analysis showed that the relative risk of developing SBA in patients with Crohn’s disease was 33.2 times greater than that of the general population[12]. The increased SBA risk in Crohn’s disease could be due to the location (with about 70% of cancers arising in ileum) and duration of disease (with approximated SBA risk of only 2% after 25 years)[13-15]. Some other risk factors for the development of SBA in Crohn’s disease are debateable, including male gender, old age, proximal small bowel disease, use of 6-mercaptopurine, corticosteroids, azathioprine or TNF-alpha antibodies, and younger age at the time of diagnosis. All these risk factors need to be validated in a large cohort study.
The birth of Tommy, a healthy, beautiful son, was an event for celebration, and as time went by, it seemed as though every day brought another reason to celebrate the gift of Tommy’s life. He was sweet, thoughtful, fun-loving and a joy to be around.
TREATMENT
A study found that DNA copy number aberrations seen in SBA are more similar to CRCs than to gastric cancer[16]. The rates of mismatch repair deficiency, characterized by microsatellite instability (MSI), are similar (5%-35%) in both CRC and SBA[17,18].The common carcinogenesis pathway involved in both SBA and CRC include
mutations, loss of 18q and p53[19,20],and relatively lower rates of
mutations (0%-27%)[21]. This lack of
mutations could be responsible for the difference in incidence between SBA and CRC. Recently, a comprehensive genomic analysis of SBA demonstrated that it is a molecularly unique cancer[22]. The frequency of genetic mutations in SBA is significantly different from that in CRC (
:27%
76%,
:14.5%
2.6%) or gastric carcinoma (
:53.6%
14.2%,
:17.4%
5.2%). Though rates of
mutations were similar in CRC and SBA (7.6% and 9.1%),
mutations were much less frequent in SBA (only 10.3% of
-mutated patients). SBAs showed high
2 (
) mutations(8.2%), MSI (7.6%), and high tumor mutational burden (TMB) (9.5%). There were also distinct differences between the molecular profile of unspecified SBA compared to IBD-associated SBA. Targetable mutations were also identified in a significant group of patients, including
and
(
), and receptor tyrosine kinase fusions.
Computed tomography (CT) scans are routinely done to detect abnormalities in small bowel, extramural spread of disease, and to rule out lymphatic and distant metastasis. The oral contrast agent is selected based on the anatomical area of interest in the small bowel and on the clinically suspected diagnosis for a particular patient.The radiographic findings differ with the location of the SBA and therefore aid in diagnosis. Duodenal carcinomas are seen as polypoidal, well-delineated lesions[30,31], whereas jejunal and ileal carcinomas are seen as annular narrowing with abrupt concentric or irregular overhanging edge stenosis that could lead to partial or complete obstruction[32]. Moderate heterogeneous enhancement is usually seen after intravenous contrast administration. The absence of comb sign and the presence of a single focal lesion rather than multiple skip areas of bowel wall thickening differentiate adenocarcinoma of the ileum from Crohn’s disease[30].
ADJUVANT TREATMENT
An analysis from the SEER database showed that removal of 10 or more lymph nodes was associated with significantly improved survival in jejuno-ileal adenocarcinoma, but only for stage II patients[28]. Similar relation of improved survival with adequate lymph node dissection was also described for stage II duodenal adenocarcinoma[29]. But it is still debateable whether the improvement in survival was due to stage migration after ample lymph node dissection. Even adjuvant chemotherapy can never compensate for inadequate lymph node dissection in duodenal primaries.
So the young man came out from his hiding-place and begged the sun to tell him if in the course of his travels he had not seen somewhere a palace that had not its like in the whole world, for its laths were of gold and its tiles of diamond, and all the furniture in silver and gold
A meta-analysis of 26 observational studies showed that the 5-year OS was 46%after curative surgical resection. Lymph node involvement was associated with poor OS with 5-year survival rate of 21% for nodal metastases as compared to 65% for patients without lymph nodal involvement. Adjuvant treatment after curative resection did not result in any survival benefit even in patients with nodal metastasis[46]. The efficacy of adjuvant chemotherapy was investigated in a large database of 4746 patients of SBA who underwent curative resection[26]. A propensity score matched analysis was used to account for the effects of confounding by indication in different treatment groups. Patients who received adjuvant chemotherapy had a significant survival advantage as compared to patients with surgery alone (median OS, 63.2
44.5 mo;
< 0.001). There was trend towards improved OS with adjuvant chemotherapy in AJCC stage I patients (158.8
110.7 mo;
= 0.226) and AJCC stage II patients (104.0
79.6 mo;
= 0.185), patients with T4 tumor (64.0
47.4 mo;
=0.130) or a positive resection margin (44.4
31.0 mo;
= 0.333), but it did not reach level of statistical significance in either of them. Stage III patients who received adjuvant chemotherapy showed significantly superior survival as compared to surgery alone (42.4
26.1 mo;
< 0.001).
Completely resected stage I tumors are kept on observation. Adjuvant chemotherapy for 6 mo is recommended for patients with node-positive, completely resected disease. The regimens of choice are CAPOX (capecitabine and oxaliplatin) and FOLFOX (folinic acid, fluorouracil, and oxaliplatin). Observation or 6 mo of adjuvant chemotherapy are acceptable options for Stage II patients (T3, T4 node-negative), and treatment decision making is based on clinicopathological features, MMR status, and patient preference. Observation or 6 mo of adjuvant chemotherapy (5-fluro-uracil[FU]/leucovorin [LCV] or capecitabine) is preferred for stage IIA (T3N0M0) patients that are microsatellite stable (MSS) or proficient MMR (pMMR) with no high-risk features. High risk features include T4 disease, inadequate lymph node dissection,close or positive surgical margins, tumor perforation, lymphovascular invasion,perineural invasion, and poorly differentiated histology. Patients with deficient mismatch repair (dMMR) have better outcome and can be kept on observation; if adjuvant chemotherapy is to be given in this setting, then oxaliplatin containing regimen should be chosen over fluoropyrimidines alone. The choice of regimen for stage II patients with high risk features and MSS or pMMR is CAPOX, FOLFOX,FU/LCV or Capecitabine.
The role of chemoradiation is limited to duodenal adenocarcinoma, as duodenal primaries have a high rate of local relapse. In a study by Kelsey
[47], the most common site of recurrence was the operative bed followed by retroperitoneal lymph nodes. None of the studies have demonstrated improvement in survival with the addition of adjuvant chemoradiation[46-48]. Chemoradiation in duodenal cancer has been used mainly for high-risk cases (node-positive, advanced T stage, resection margin positive, inadequate lymph node dissection, or poorly differentiated histology)[47,48]. In a propensity score-matched analysis of 1028 patients from 1998-2012 who received adjuvant therapy (chemotherapy in 478, chemoradiation in 550), there was no survival advantage observed in patients who received adjuvant chemoradiation compared to those who received adjuvant chemotherapy. Even in high-risk cases,additional use of radiotherapy did not result in improved survival[26].
METASTATIC DISEASE
Approximately one-third of the patients with SBA present with metastatic disease and common sites of metastasis are the liver and peritoneum[7,23]. Evidence supporting the role of metastasectomy in SBA is limited. Only patients with limited visceral metastasis may be considered as candidates for metastasectomy, but after discussing in a multidisciplinary tumor board. Peritoneal metastases are seen in 25%-50% of the patients with metastatic SBA and are more common in jejunal and ileal carcinomas as compared to duodenal cancers[24,49,50]. Peritoneal metastasis generally carries a poor prognosis. The treatment of choice in these patients remains systemic chemotherapy.Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy(HIPEC) may be considered in very selected patients (patients suitable for complete cytoreduction, absence of unresectable systemic disease, and good general condition of patient allowing a major surgical procedure), but there is limited evidence due to rarity of disease. Grade III-IV morbidity ranged from 12%-35%[51]. Recurrences are common and careful patient selection is of utmost importance[24].
There is a dearth of prospective data on chemotherapy regimens used in SBA. The majority of retrospective studies and phase II prospective studies support the use of fluoropyrimidine and oxaliplatin based chemotherapy[52-54]. The most commonly used regimens are FOLFOX and CAPOX. The addition of bevacizumab has been considered safe. In a recently published systematic review and meta-analysis[55], the use of bevacizumab in addition to chemotherapy improved the OS and DFS, but the results should be interpreted with caution, as there were no RCTs included in the analysis. The role of anti- epidermal growth factor receptor (EGFR) therapies(cetuximab and panitumumab) is unclear.
The young man was puzzled, and did not know what to reply, for, though he would gladly have married the princess without a sixpence, he had spent all his money in building the ship, and knew he could not give her all she wanted
Patients with progressive disease on first-line chemotherapy whose tumors are dMMR or MSI-high (H) or with high TMB (> 10 mutations per megabase) can be considered for immune check-point inhibitor therapy with anti- programmed cell death protein (PD)1 inhibitors. The percentage of SBA with dMMR or MSI-H range from 1%-16%[22,56,57]. Pembrolizumab is approved for patients with solid MSIH/dMMR malignancies which have progressed following prior treatment and for which no satisfactory alternative treatment options are available[58]. Efficacy of pembrolizumab in patients with MSI-H/dMMR non-colorectal malignancies has been established in phase II Keynote-158 study. Out of 19 patients of SBA, 3 had complete response and 5 had partial response. Overall response rate was 42.1% and median progression-free survival was 9.2 mo[59]. FOLFIRI (leucovorin calcium [calcium folinate], 5-fluorouracil, and irinotecan) and taxane-based chemotherapy are secondline treatment options for patients with pMMR/MSS and patients who are refractory to immunotherapy.
CONCLUSION
SBA are rare malignancies with a poor prognosis. These are often diagnosed in an advanced stage owing to the non-specific nature of symptoms. The clinical presentation is varied and vague and a high index of suspicion is required for prompt diagnosis and treatment. The most common site of presentation is the duodenum.Surgical resection with negative margins and adequate lymph node dissection remains the mainstay of treatment. Because of the rarity of ther disease, there is a paucity of prospective data. The treatment is generally extrapolated from the evidence available from colonic cancers.
1 Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database:Incidence—SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973-2013 Varying)—Linked To County Attributes—Total U.S., 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. [cited 13 February 2021]. Available from:https://seer.cancer.gov/statfacts/html/smint.html
2 Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States:changes in epidemiology, treatment, and survival over the last 20 years.
2009; 249:63-71 [PMID:19106677 DOI:10.1097/SLA.0b013e31818e4641]
3 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018; 68:394-424 [PMID:30207593 DOI:10.3322/caac.21492]
4 Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors:epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry.
2007; 142:229-235 [PMID:17372046 DOI:10.1001/archsurg.142.3.229]
5 Taghipour Zahir S, Heidarymeybodi Z, AleSaeidi S. Prognostic Factors and Survival Time in Patients with Small Bowel Tumors:A Retrospective Observational Study.
2019;2019:2912361 [PMID:31186956 DOI:10.1155/2019/2912361]
6 Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. A single-institution experience with 491 cases of small bowel adenocarcinoma.
2010; 199:797-803 [PMID:20609724 DOI:10.1016/j.amjsurg.2009.05.037]
7 Sakae H, Kanzaki H, Nasu J, Akimoto Y, Matsueda K, Yoshioka M, Nakagawa M, Hori S, Inoue M,Inaba T, Imagawa A, Takatani M, Takenaka R, Suzuki S, Fujiwara T, Okada H. The characteristics and outcomes of small bowel adenocarcinoma:a multicentre retrospective observational study.
2017; 117:1607-1613 [PMID:28982111 DOI:10.1038/bjc.2017.338]
8 Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, Locher C, Afchain P.Small bowel adenocarcinoma:epidemiology, risk factors, diagnosis and treatment.
2014; 46:97-104 [PMID:23796552 DOI:10.1016/j.dld.2013.04.013]
9 Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine.
2009; 19:58-69 [PMID:19064190 DOI:10.1016/j.annepidem.2008.10.004]
10 Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C. Pathogenesis and risk factors of small bowel adenocarcinoma:a colorectal cancer sibling?
2005; 100:703-710 [PMID:15743371 DOI:10.1111/j.1572-0241.2005.40605.x]
11 Duerr D, Ellard S, Zhai Y, Taylor M, Rao S. A Retrospective Review of Chemotherapy for Patients with Small Bowel Adenocarcinoma in British Columbia.
2016; 7:2290-2295 [PMID:27994666 DOI:10.7150/jca.16606]
12 Canavan C, Abrams KR, Mayberry J. Meta-analysis:colorectal and small bowel cancer risk in patients with Crohn's disease.
2006; 23:1097-1104 [PMID:16611269 DOI:10.1111/j.1365-2036.2006.02854.x]
13 Overman MJ. Rare but real:management of small bowel adenocarcinoma.
2013; 189-193 [PMID:23714497 DOI:10.14694/EdBook_AM.2013.33.189]
14 Barral M, Dohan A, Allez M, Boudiaf M, Camus M, Laurent V, Hoeffel C, Soyer P. Gastrointestinal cancers in inflammatory bowel disease:An update with emphasis on imaging findings.
2016; 97:30-46 [PMID:26315381 DOI:10.1016/j.critrevonc.2015.08.005]
15 Palascak-Juif V, Bouvier AM, Cosnes J, Flourié B, Bouché O, Cadiot G, Lémann M, Bonaz B,Denet C, Marteau P, Gambiez L, Beaugerie L, Faivre J, Carbonnel F. Small bowel adenocarcinoma in patients with Crohn's disease compared with small bowel adenocarcinoma de novo.
2005; 11:828-832 [PMID:16116317 DOI:10.1097/01.mib.0000179211.03650.b6]
16 Haan JC, Buffart TE, Eijk PP, van de Wiel MA, van Wieringen WN, Howdle PD, Mulder CJ, van de Velde CJ, Quirke P, Nagtegaal ID, van Grieken NC, Grabsch H, Meijer GA, Ylstra B. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers.
2012; 23:367-374 [PMID:21586687 DOI:10.1093/annonc/mdr122]
17 Overman MJ, Pozadzides J, Kopetz S, Wen S, Abbruzzese JL, Wolff RA, Wang H.Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine.
2010; 102:144-150 [PMID:19935793 DOI:10.1038/sj.bjc.6605449]
18 Suerink M, Kilin? G, Terlouw D, Hristova H, Sensuk L, van Egmond D, Farina Sarasqueta A,Langers AMJ, van Wezel T, Morreau H, Nielsen M; PALGA-group collaborators. Prevalence of mismatch repair deficiency and Lynch syndrome in a cohort of unselected small bowel adenocarcinomas.
2020 [PMID:33046565 DOI:10.1136/jclinpath-2020-207040]
19 Aparicio T, Svrcek M, Zaanan A, Beohou E, Laforest A, Afchain P, Mitry E, Taieb J, Di Fiore F,Gornet JM, Thirot-Bidault A, Sobhani I, Malka D, Lecomte T, Locher C, Bonnetain F, Laurent-Puig P. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study.
2013;109:3057-3066 [PMID:24196786 DOI:10.1038/bjc.2013.677]
20 Zaaimi Y, Aparicio T, Laurent-Puig P, Taieb J, Zaanan A. Advanced small bowel adenocarcinoma:Molecular characteristics and therapeutic perspectives.
2016; 40:154-160 [PMID:26547136 DOI:10.1016/j.clinre.2015.09.008]
21 Diosdado B, Buffart TE, Watkins R, Carvalho B, Ylstra B, Tijssen M, Bolijn AS, Lewis F, Maude K,Verbeke C, Nagtegaal ID, Grabsch H, Mulder CJ, Quirke P, Howdle P, Meijer GA. High-resolution array comparative genomic hybridization in sporadic and celiac disease-related small bowel adenocarcinomas.
2010; 16:1391-1401 [PMID:20179237 DOI:10.1158/1078-0432.CCR-09-1773]
22 Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma.
2017;3:1546-1553 [PMID:28617917 DOI:10.1001/jamaoncol.2017.1051]
23 Overman MJ, Hu CY, Kopetz S, Abbruzzese JL, Wolff RA, Chang GJ. A population-based comparison of adenocarcinoma of the large and small intestine:insights into a rare disease.
2012; 19:1439-1445 [PMID:22187121 DOI:10.1245/s10434-011-2173-6]
24 Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel:presentation,prognostic factors, and outcome of 217 patients.
2004; 101:518-526 [PMID:15274064 DOI:10.1002/cncr.20404]
25 Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma:analysis of the surveillance, epidemiology, and end results database.
2010; 116:5374-5382 [PMID:20715162 DOI:10.1002/cncr.25324]
26 Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, Fraker DL, Drebin JA,Karakousis GC, Roses RE. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma:A propensity score-matched analysis.
2016; 122:693-701 [PMID:26717303 DOI:10.1002/cncr.29840]
27 Zaanan A, Costes L, Gauthier M, Malka D, Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM,Sobhani I, Moulin V, Afchain P, Ta?eb J, Bonnetain F, Aparicio T. Chemotherapy of advanced smallbowel adenocarcinoma:a multicenter AGEO study.
2010; 21:1786-1793 [PMID:20223786 DOI:10.1093/annonc/mdq038]
28 Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma:understaged and undertreated?
2010; 17:2728-2732 [PMID:20458546 DOI:10.1245/s10434-010-1109-x]
29 Ecker BL, McMillan MT, Datta J, Dempsey DT, Karakousis GC, Fraker DL, Drebin JA, Mamtani R,Giantonio BJ, Roses RE. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma:A matched cohort study.
2016; 69:135-141 [PMID:27821316 DOI:10.1016/j.ejca.2016.09.027]
30 Anzidei M, Napoli A, Zini C, Kirchin MA, Catalano C, Passariello R. Malignant tumours of the small intestine:a review of histopathology, multidetector CT and MRI aspects.
2011; 84:677-690 [PMID:21586504 DOI:10.1259/bjr/20673379]
31 Sata N, Endo K, Shimura K, Koizumi M, Nagai H. A new 3-D diagnosis strategy for duodenal malignant lesions using multidetector row CT, CT virtual duodenoscopy, duodenography, and 3-D multicholangiography.
2007; 32:66-72 [PMID:16802199 DOI:10.1007/s00261-006-9008-0]
32 Sailer J, Zacherl J, Schima W. MDCT of small bowel tumours.
2007; 7:224-233[PMID:18083648 DOI:10.1102/1470-7330.2007.0032]
33 Ilangovan R, Burling D, George A, Gupta A, Marshall M, Taylor SA. CT enterography:review of technique and practical tips.
2012; 85:876-886 [PMID:22553291 DOI:10.1259/bjr/27973476]
34 Cronin CG, Scott J, Kambadakone A, Catalano OA, Sahani D, Blake MA, McDermott S. Utility of positron emission tomography/CT in the evaluation of small bowel pathology.
2012; 85:1211-1221 [PMID:22919004 DOI:10.1259/bjr/64534573]
35 Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials.
2005; 37(10):960-965 [PMID:16189768 DOI:10.1055/s-2005-870353]
36 Fatehullah A, Appleton PL, N?thke IS. Cell and tissue polarity in the intestinal tract during tumourigenesis:cells still know the right way up, but tissue organization is lost.
2013; 368(1629):20130014 [PMID:24062584 DOI:10.1098/rstb.2013.0014]
37 Chen ZM, Ritter JH, Wang HL. Differential expression of alpha-methylacyl coenzyme A racemase in adenocarcinomas of the small and large intestines.
2005; 29:890-896 [PMID:15958853 DOI:10.1097/01.pas.0000167364.90899.59]
38 Zhang MQ, Chen ZM, Wang HL. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma:a comparison with colorectal adenocarcinoma.
2006;19:573-580 [PMID:16501564 DOI:10.1038/modpathol.3800566]
39 Neri G, Arpa G, Guerini C, Grillo F, Lenti MV, Giuffrida P, Furlan D, Sessa F, Quaquarini E, Viglio A, Ubezio C, Pasini A, Ferrero S, Sampietro G, Ardizzone S, Latella G, Mescoli C, Rugge M,Zingone F, Barresi V, Ciccocioppo R, Pedrazzoli P, Corazza GR, Luinetti O, Solcia E, Paulli M, Di Sabatino A, Vanoli A. Small Bowel Adenocarcinomas Featuring Special AT-Rich Sequence-Binding Protein 2 (SATB2) Expression and a Colorectal Cancer-Like Immunophenotype:A Potential Diagnostic Pitfall.
2020; 12 [PMID:33228145 DOI:10.3390/cancers12113441]
40 Carrère N, Samalin E, Cellier C, Aparicio T, Becouarn Y, Bedenne L, Michel P, Parc Y, Pocard M,Chibaudel B, Bouché O; Thésaurus National de Cancérologie Digestive (TNCD). Small bowel adenocarcinoma:French intergroup clinical practice guidelines for diagnosis, treatments and followup (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO).
2018; 50:15-19[PMID:29174568 DOI:10.1016/j.dld.2017.09.123]
41 Cloyd JM, George E, Visser BC. Duodenal adenocarcinoma:Advances in diagnosis and surgical management.
2016; 8:212-221 [PMID:27022448 DOI:10.4240/wjgs.v8.i3.212]
42 National Comprehensive Cancer Networlk (NCCN) guidelines. [cited 24 February 2021].Available from:https://www.nccn.org/professionals/physician_gls/
43 Ojha A, Zacherl J, Scheuba C, Jakesz R, Wenzl E. Primary small bowel malignancies:single-center results of three decades.
2000; 30:289-293 [PMID:10777190 DOI:10.1097/00004836-200004000-00017]
44 Chaiyasate K, Jain AK, Cheung LY, Jacobs MJ, Mittal VK. Prognostic factors in primary adenocarcinoma of the small intestine:13-year single institution experience.
2008; 6:12 [PMID:18237404 DOI:10.1186/1477-7819-6-12]
45 Khan K, Peckitt C, Sclafani F, Watkins D, Rao S, Starling N, Jain V, Trivedi S, Stanway S,Cunningham D, Chau I. Prognostic factors and treatment outcomes in patients with Small Bowel Adenocarcinoma (SBA):the Royal Marsden Hospital (RMH) experience.
2015; 15:15[PMID:25603878 DOI:10.1186/s12885-015-1014-6]
46 Meijer LL, Alberga AJ, de Bakker JK, van der Vliet HJ, Le Large TYS, van Grieken NCT, de Vries R, Daams F, Zonderhuis BM, Kazemier G. Outcomes and Treatment Options for Duodenal Adenocarcinoma:A Systematic Review and Meta-Analysis.
2018; 25:2681-2692[PMID:29946997 DOI:10.1245/s10434-018-6567-6]
47 Kelsey CR, Nelson JW, Willett CG, Chino JP, Clough RW, Bendell JC, Tyler DS, Hurwitz HI,Morse MA, Clary BM, Pappas TN, Czito BG. Duodenal adenocarcinoma:patterns of failure after resection and the role of chemoradiotherapy.
2007; 69:1436-1441[PMID:17689032 DOI:10.1016/j.ijrobp.2007.05.006]
48 Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, Pawlik TM, Herman JM, Edil BH, Ahuja N, Choti MA, Wolfgang CL, Schulick RD. Duodenal adenocarcinoma:clinicopathologic analysis and implications for treatment.
2012; 19:1928-1935 [PMID:22167476 DOI:10.1245/s10434-011-2168-3]
49 Legué LM, Bernards N, Gerritse SL, van Oudheusden TR, de Hingh IH, Creemers GM, Ten Tije AJ,Lemmens VE. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013:a population-based study in The Netherlands.
2016; 55:1183-1189[PMID:27170100 DOI:10.1080/0284186X.2016.1182211]
50 Rovers KP, de Bree E, Yonemura Y, de Hingh IH. Treatment of peritoneal metastases from small bowel adenocarcinoma.
2017; 33:571-578 [PMID:27919181 DOI:10.1080/02656736.2016.1266700]
51 Saxena A, Valle SJ, Liauw W, Morris DL. Recurrence and Survival Outcomes After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Small Bowel Adenocarcinoma.
2017; 37:5737-5742 [PMID:28982894 DOI:10.21873/anticanres.12012]
52 Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL,Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater.
2009; 27:2598-2603 [PMID:19164203 DOI:10.1200/JCO.2008.19.7145]
53 Zhang L, Wang LY, Deng YM, Wang FH, Feng F, Chen YC, An X, Chen C, Xu RH, Li YH.Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma:a three-center study from China.
2011; 16:689-696 [PMID:22331723]
54 Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F, Zhan ZY, Feng M, Yan J, Zhao JG, Xiong JP. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma.
2012; 23:561-566 [PMID:22481063 DOI:10.1097/CAD.0b013e328350dd0d]
55 Vergara JP, Sacdalan DB, Amurao-Amante M, Sacdalan DL. Bevacizumab in metastatic smallbowel adenocarcinoma:a systematic review and meta-analysis.
2019; 11:2036361318825413 [DOI:10.1177/2036361318825413]
56 Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C,Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M,Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR,Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade.
2017; 357:409-413 [PMID:28596308 DOI:10.1126/science.aan6733]
57 Middha S, Zhang L, Nafa K, Jayakumaran G, Wong D, Kim HR, Sadowska J, Berger MF, Delair DF,Shia J, Stadler Z, Klimstra DS, Ladanyi M, Zehir A, Hechtman JF. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data.
2017; 2017 [PMID:30211344 DOI:10.1200/PO.17.00084]
58 Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS,Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM,Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N,Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.
2015; 372:2509-2520 [PMID:26028255 DOI:10.1056/NEJMoa1500596]
59 Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R,Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer:Results From the Phase II KEYNOTE-158 Study.
2020; 38:1-10 [PMID:31682550 DOI:10.1200/JCO.19.02105]
 World Journal of Gastrointestinal Oncology2022年2期
World Journal of Gastrointestinal Oncology2022年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Endoscopic ultrasound-guided ablation of solid pancreatic lesions:A systematic review of early outcomes with pooled analysis
- Prevention of late complications of endoscopic resection of colorectal lesions with a coverage agent:Current status of gastrointestinal endoscopy
- Predictive value of serum alpha-fetoprotein for tumor regression after preoperative chemotherapy for rectal cancer
- Chemotherapy predictors and a time-dependent chemotherapy effect in metastatic esophageal cancer
- Association and prognostic significance of alpha-L-fucosidase-1 and matrix metalloproteinase 9 expression in esophageal squamous cell carcinoma
- Comprehensive molecular characterization and identification of prognostic signature in stomach adenocarcinoma on the basis of energy-metabolism-related genes
