Management of diabetic foot ulcers and the challenging points: An endocrine view
INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disorder that has become a global health problem in the last decades[1]. DM has several complications that affect not only life expectancy but also the quality of life[2,3]. Diabetic foot ulcers (DFU) are one of the most challenging complications of DM. Up to one-third of diabetic patients may suffer from DFUs during their life[4,5]. The global prevalence of DFUs is reported at 6.3%, with DFUs being more common in men than women and in type 2 DM than type 1 DM[6]. The recurrence rate of DFUs is also high. The value reaches 40% within 1 year and 65% within 3 years[4]. Hence, studies should focus on establishing prevention strategies against DFU[4,5].
PATHOPHYSIOLOGY AND PREDISPOSING FACTORS
Peripheral artery disease (PAD) and diabetic neuropathy (DNP) are well-known chronic complications of diabetes[7]. Along with immune dysfunction, PAD and DNP are the main pathophysiological factors that predispose to DFUs[8]. DFUs are associated with DM duration, the presence of DNP, and PAD[9]. DNP is present in 80% of patients with DFUs, and it facilitates ulcer formation by causing decreased pain and pressure sensation. DNP also promotes the formation of anatomic deformities, such as prominent plantar metatarsal heads, hammertoes, Charcot foot,
[4,10]. Patients with diabetes should be assessed for DNP periodically after the diagnosis of type 2 DM and after the fifth year of type 1 DM. Pain, burning, and numbness should be questioned. Small fibers (by pinprick test and temperature sensation), large fibers (by vibration perception and 10 g monofilament test), and protective sensation (by 10 g monofilament test) should be tested. The tests predict the risk of complications besides screening the dysfunction[7,11,12].
Nearly half of the patients with DFUs have PAD, which is significantly associated with the increased risk of adverse limb events[13]. Vascular symptoms, including reduction in effort capacity, leg fatigue, and claudication, should be assessed. All peripheral pulses should be palpated together with an assessment of extremity appearance and warmth to evaluate perfusion[8,13]. Patients should also undergo the ankle-brachial index (ABI) testing as a part of the examination. The normal value of ABI is between 0.9 and 1.3, which is higher than 1.0[13,14]. A high ABI may be measured falsely in the presence of vascular calcifications[13]. Toe-brachial index (TBI) measurement is also recommended, especially in combination with ABI and arterial Doppler study. The diagnosis of PAD is unlikely in the presence of triphasic Doppler waveforms when the TBI is ≥ 0.75, and the ABI is between 0.9-1.3[13]. In addition, disrupted blood flow may be present at the microvascular level despite the intact or well-treated macrovascular component[15]. Dysfunctional signs of blood flow at the microvascular level can be detected by laser Doppler flowmetry[16]. Furthermore, DM causes immunological dysfunctions at the cellular level, leading to poor healing response and susceptibility to infections[8,17].
CLINICAL SIGNIFICANCE
DFUs are a serious healthcare problem globally. A potentially preventable event, such as a minor trauma, usually has dramatic results. DM remains the primary cause of nontraumatic lower-limb loss worldwide[18-20]. DFUs pose a serious financial burden worldwide, and nearly one-third of expenses for DM is estimated to be for DFUs[21-23]. The presence of DFU is associated with the increased risk of mortality in DM, and this association is stronger than the presence of any macrovascular disease alone[3,24]. The five-year survival rate in patients presenting with DFUs is poorer than that associated with the most common cancers[21]. Therefore, the best approach in the management of DFUs is the implementation of preventive measures based on the risk class[7,10,25].
Call it a glove-cleaner if you like, said the old man indifferently. Maybe it will clean gloves. I have never tried. One might call it a life-cleaner. Lives need cleaning sometimes.
IDENTIFICATION AND FOLLOW-UP OF PATIENTS AT RISK
DNP and PAD are the major predisposing factors of DFU development[28,29]. Thus, the neurosensory and vascular systems of the extremities must be protected to prevent or delay the development of DFUs. The onset and progression of diabetic microvascular complications (retinopathy, nephropathy, and neuropathy) can be delayed by intensive glycemic control. This finding has been shown in type 1 DM, but the current evidence in type 2 DM is weak[30-32]. However, no specific therapeutic agent or approach other than glycemic control can modify the progression of microvascular complications[7,10].
The importance and role of adequate glycemic control for delaying or preventing chronic complications of DM are discussed above. Although RCTs have shown an association between intensive glycemic control before DFU formation and the low risk of LEAs, to our knowledge, the role of glycemia in the management of active DFU has never been studied in RCTs[42,43]. Considering the known negative effect of hyperglycemia on wound healing and immune defense, hyperglycemia may be associated with negative consequences in patients with DFUs[8,17,44]. Several meta-analyses of observational studies addressed this point[43,45,46]. Margolis
[46] published a meta-analysis of five observational studies including DFUs. Glycemic control was not associated with wound healing according to this study. The other two meta-analyses reported that the high fasting plasma glucose and Hba1c levels were associated with a high rate of amputations[43,45].


CURRENT EVIDENCE FOR PREVENTION
Several randomized clinical trials (RCT) evaluated the primary prevention strategies of DFUs, but none of them were high-quality research[27]. Conducting RCT to determine the primary prevention strategies and evaluate their efficacy is a considerable challenge, given that numerous patients and a long follow-up period will be required[21]. On the other hand, conducting RCT on the prevention of ulcer recurrence is technically easier because the recurrence rate is high[4,21]. Suitable therapeutic footwear with appropriate pressure distribution prevents recurrence or worsening of plantar foot ulcers, with high-quality evidence[7].
DNP, PAD, foot deformity, and medical history of DFU are the most important risk factors for new DFU formation. These factors are the shadows of the coming event, which is DFU if the preventive measures are not applied in time[4,10,26]. Poor glycemic control, chronic kidney disease (especially dialysis), and smoking are also among the risk factors[7,8]. Diabetic patients should be categorized based on the risk of developing DFU. Thus, the risk factors for DFUs must be screened at least annually[7,12]. The risk classification system developed by the International Working Group on the Diabetic Foot (IWGDF) is useful in daily clinical practice (Table 1)[13].
PAD is one of the macrovascular complications of diabetes. The benefit of intensive glycemic control on macrovascular complications in diabetics has not been shown in RCTs, but several epidemiological analyses reported a correlation between an increased rate of cardiovascular disease (CVD) and chronic hyperglycemia[33-35]. The benefit of intensive therapy could not be shown in three large RCTs comparing intensive and conventional therapies in terms of cardiovascular benefits in patients with longstanding DM[36,37]. Unlike these studies, in a research investigating the effect of glycemic control on complications in newly diagnosed DM, the benefit of intensive glycemic control on CVD was shown after a 10-year follow-up on the postinterventional period[38]. The management of other CVD risk factors is particularly important in the prevention or delay of PAD and other macrovascular complications in patients with DM. Smoking cessation, effective treatment of hyperlipidemia and hypertension, weight loss, appropriate nutrition, and exercise habits are important points that should be emphasized in every patient with DM. Exercise should be considered with caution if the patient is in the risk group for DFU. Patients in the low- or moderate-risk group should be advised exercises that increase the motion of foot and ankle, relieve pressure, and decrease neuropathic symptoms. Patients in the risk group should avoid long walks, exercises that increase the pressure on the soles of feet, activities with a risk of trauma, and wearing inappropriate shoes[13].
CURRENT TECHNOLOGICAL OPPORTUNITIES FOR MONITORING
The recurrence rate of DFUs is also extremely high in patients who are under followup in specialized centers. Thus, systems that facilitate recognition of the early signs of DFU formation must be developed. Patients can refer to health care providers early, and preventive and/or therapeutic appropriate strategies can be executed on time[42]. Risky conditions for DFU formation, such as early signs of inflammation and pressureinduced plantar tissue stress by current technological opportunities, can be screened and followed-up[29]. The available technological devices had been invented for this purpose; these devices include instruments for daily monitoring plantar temperature, socks that enable temperature monitoring continuously, socks that monitor plantar pressure, smart insoles to screen sustained plantar pressure, alarm systems that warn patients to wear offloading devices, activity monitoring devices,
[29].
According to Marina Warner, Perrault had many animals to choose from for her version of the story, but purposely chose a donkey. Perrault picked the ass for effect; he was well acquainted with the vast Aesopian folklore54 about the jackass as fall guy. She speculates that Perrault wanted to mock the atmosphere of enchantment55 in the story with the donkey. She also notes that A. A. Milne s Eeyore stands in direct line of descent from this classically pathetic figure of fun (Warner 1994).Return to place in story.
POINTS TO BE CONSIDERED IN DFU MANAGEMENT
10.Cut off her hands:Hands connect a person to god (though the act of prayer) and mean loyalty57 and (Biedermann 163). It should also be noted that the father was the head and controlling figure of the family.
Glycemic control
A diabetic patient with very low risk (IWGDF group 0) must be examined annually for DNP and PAD. The patients who have a higher risk (IWGDF group 1-3) should be examined more frequently (Table 1), and preventive measures should be executed (Table 2)[7,13]. Patients who have moderate-to-high risk should wear therapeutic shoes to reduce plantar pressure and the risk of ulceration. Pre-ulcerative lesions, abundant callus, stinging toenails, and fungal infections (tinea pedis, onychomycosis,
) should be treated properly. Surgical interventions should be performed to fix deformities, if necessary[7,10,13]. The patient’s feet with DNP should be inspected every visit, and the patients at risk should be encouraged and educated about self-care and preventive measures[7].
In the summer time we didn t get much to eat for Sunday supper, except watermelon and then we had to eat it outside behind the dining room so we would not make a mess on the tables inside. About the only time that I would see him was through the high chain-link fence that surrounded the orphanage when we ate our watermelon outside.
DFUs are predisposed to infection. The exact diagnosis of infection should be performed correctly the first time to manage the infection in DFUs. The classical manifestations of inflammation (warmth, erythema, tenderness, and swelling), extent of infection, involvement of deep tissues and/or bones, and presence of an abscess and/or fistula tract should be evaluated. The clinician should be acquainted with the clinical findings of necrotizing infections. Systemic manifestations of infection (including findings of systemic inflammatory response syndrome and sepsis) and hemodynamic status should also be assessed carefully along with the wound characteristics[58,59]. The presence of severe infection, extensive gangrene, necrotizing infection, deep abscess, compartment syndrome, and/or limb-threatening ischemia needs immediate consultation with a surgeon[59].
An intercurrent illness (trauma, infection, surgery,
) causes impaired glycemic control in diabetics and necessitates adjustment of the therapy. Here, DM patients are predisposed to severe hyperglycemia, diabetic ketoacidosis, and nonketotic hyperosmolar state. Patients treated with noninsulin antidiabetics require insulin. ADA and AACE recommend insulin regimens for critically ill and noncritically ill hospitalized patients[48].
DFU is the major cause of nontraumatic lower extremity amputations (LEA), worldwide[20,39]. Once DFU occurs, the management strategies should be implemented without delay. Numerous studies emphasized the importance of a multidisciplinary team approach in the management of these patients[20,40,41]. The multidisciplinary team should focus on four major points; glycemic control, diagnosis and treatment of vascular disease, evaluation and local management of wound, diagnosis, and treatment of infection[41].
Several oral antidiabetics have other properties besides the glucose-lowering effect. For instance, canagliflozin, a sodium-glucose cotransporter-2 (SGLT-2) inhibitor, is associated with an approximately two-fold increased risk of LEA (primarily at the level of toe or metatarsal) in patients with type-2 DM and established CVD (or at risk for CVD)
placebo[49]. On the other hand, in RCTs of empagliflozin and dapagliflozin, the risk of amputation was similar between the treatment and placebo arms[50]. An increased risk of LEAs was reported with canagliflozin, empagliflozin, and dapagliflozin (for toe amputations) in a pharmacovigilance study. This study relied on several LEA cases[51]. Recent meta-analyses found no associations between SGLT-2 inhibitors and increased LEA risk; however, Chang
[53] compared the use of SGLT-2 inhibitors with other oral antidiabetics and reported that SGLT-2 inhibitors may contribute to the increased risk of LEA[49,52]. A study examining systematic reviews, which evaluated the adverse effects of SGLT-2 inhibitors, summarized the scarcity of high-quality systematic reviews on this topic[54]. To our opinion, SGLT-2 inhibitors may increase the risk of LEA in patients with DFU as a group effect. Conflicting data are available regarding this traffic; thus, exercising cautiousness is reasonable.
In addition to the effect of hyperglycemia on the wound healing process, hyperglycemia causes impaired immune functions and decreased response to infections[20,47]. American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) recommend targeting glucose levels between 140-180 mg/dL without causing hypoglycemia in the majority of inpatients[48]. These levels should be aimed at patients with DFUs treated in inpatient setting.
Vascular disease
The prevalence of PAD among DFU patients reaches 50%. The presence of PAD is significantly related to adverse limb events. All patients with DFU should be examined clinically for PAD. Doppler sonographic study should be performed with a combination ABI and/or TBI test. No single modality has been defined as optimal. Vascular imaging (and revascularization if PAD is present) should always be considered when the ulcer remains unhealed in 4-6 wk despite the appropriate treatment and normal condition (ABI and TBI)[7,13]. The threshold for performing vascular studies should be very low in DFU patients, especially for those who are unresponsive to treatment[55]. Based on the vascular structure and clinical conditions, surgical bypass or endovascular treatment can be applied as a revascularization therapy[15,55].
Local wound management
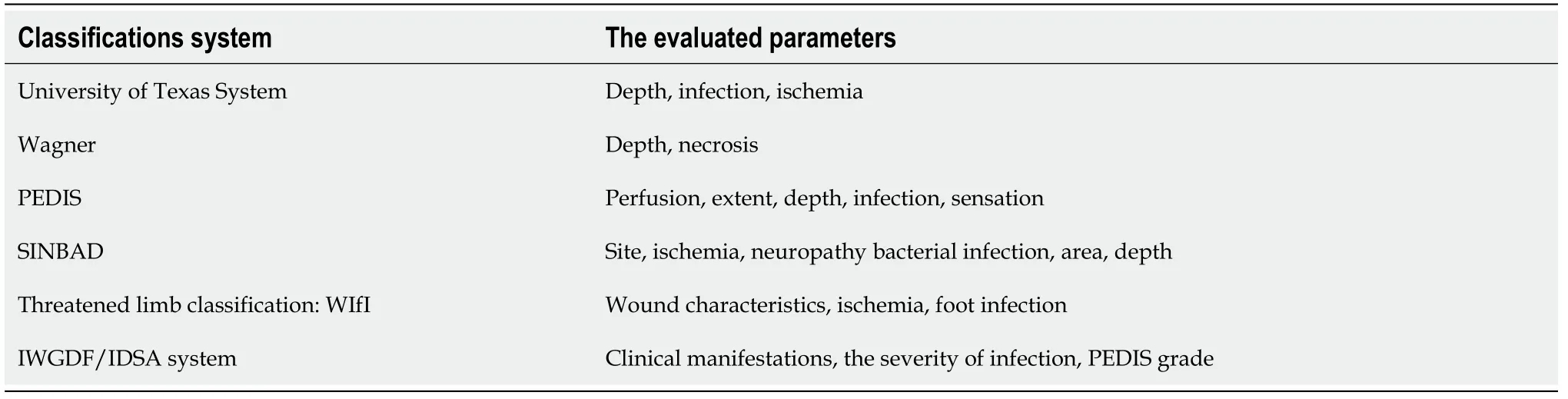
The first step in the treatment of DFUs is to classify the wound and assess the patient’s medical condition. The depth and width of the ulcer, the presence of ischemia, and infection should be evaluated. Classification systems have been developed for DFUs (Table 3). Wound classification helps in the prediction of prognosis, along with determining the type and intensity of treatment[20,56,57]. All infected and nonvitalized tissues should be removed by surgical debridement, and the abscess should be drained, if present[58]. Other debridement methods, such as mechanical, enzymatic, and biological debridement, are available other than surgical procedures[20]. Surgical debridement is the most effective and preferred method[20,58].
Post-debridement wound care is vital. Further tissue injury should be avoided. Proper wound coverage and dressing are necessary. Negative pressure therapy can be used if the wound is clean. Wound characteristics are determinative of the dressing procedure. Pressure reduction is another important point for wound healing. Several available methods of mechanical offloading (cast walkers, wedge shoes, bed rest,
) are also applied. Surgical pressure reduction may be needed occasionally[20,56,57].
Management of infection
As for Tsarevitch Ivan, dead and cut into pieces, he lay on the green plain for thirty days. And on the thirty- first day it chanced that the Gray Wolf passed that way. He knew at once by his keen scent45 that the body was that of Tsarevitch Ivan. While he sat grieving for his friend, there came flying an iron-beaked she-crow with two fledglings, who alighted on the ground and would have eaten of the flesh, but the Wolf leaped up and seized one of the young birds.
Most diabetic foot infections are polymicrobial. A wound specimen must be obtained for culture if no clinical sign of infection is observed[56]. However, the specimens for culture should always be collected in the presence of infection (especially in moderate-to-severe infection) before antibiotic administration[56,59]. Specimens for culture can be collected by aspiration of the abscess, curettage from the ulcer (after debridement), or biopsy during the surgical procedure (from deep tissue or bone) but not by superficial swab[58,59].
Empiric antimicrobial therapy should be considered in the presence of infection, and the selection of antibiotic should be based on clinical findings and the severity of infection[56,58,59]. An antibiotic regimen that covers gram-positive organisms only is preferable in antibiotic-naive patients with mild infections. In the case of antibiotic treatment in the last several weeks of, severely ischemic limb, or moderate-to-severe infections, the coverage of antibiotic therapy should include commonly isolated gramnegative organisms and anaerobes (in certain conditions) besides gram-positive organisms[59]. The clinical course and culture results should drive antibiotic therapy during follow-up[56,59].
Following round the wall he reached a lower part; he remembered the Divine Names and flung himself over, saying, Whatever happens is by the will of God
POTENTIAL ADJUNCTIVE THERAPIES
In addition to all these interventions, several adjunctive therapies may help the healing of DFUs [negative pressure wound therapy (vacuum-assisted closure), skin grafts and substitutes, hyperbaric oxygen therapy, shock wave therapy, growth factors, autologous combined leucocyte, platelet, fibrin, and placental derived products][56,60]. No high-quality evidence supports the recommendation of these interventions without concern, and none of these treatments is an alternative to the best standard therapy[60].
It took place at the Biltmore Hotel, which, to my eight-year-old mind, was just about the fancies place to eat in all of Providence6. My grandmother, my mother, and I were having lunch after a morning spent shopping. I grandly ordered a salisbury steak, confident in the knowledge that beneath that fancy name was a good old hamburger with gravy7. When brought to the table, it was accompanied by a plate of peas. I do not like peas now. I did not like peas then. I have always hated peas. It is a complete mystery to me why anyone would voluntarily eat peas. I did not eat them at home. I did not eat them at restaurants. And I certainly was not about to eat them now. Eat your peas, my grandmother said.
CONCLUSION
A DFU is a challenging complication of diabetes that has become a global health problem. The treatment process is troublesome for the patient and healthcare team, and the treatment results are often unsatisfactory, especially in advanced cases. Moreover, the recurrence rate is high despite the healing of ulcer. DFUs are one of the leading causes of morbidity in diabetic patients.
DFUs are potentially preventable. Hence, strict implementation of primary and secondary prevention strategies should be implemented. However, the scarcity of high-quality evidence especially in establishing preventive measures for primary prevention is a challenge.
The multidisciplinary team approach is the cornerstone of the management of DFU. All the team members should be experienced in their field. The evidence-based standard follow-up and treatment algorithms should be applied without delay once an ulcer develops.
Geographic heterogeneity in terms of access to adequate healthcare equipment and experienced healthcare team, poor adherence of the patients, late reference to health care providers, difficulties in achieving adequate perfusion of ulcer, the presence of DNP, the impossibility of restoring sensation, and high recurrence rates are the featured challenging points in the management of DFU.
ACKNOWLEDGEMENTS
Preparation for publication of this article is supported by the Society of Endocrinology and Metabolism of Turkey.
1 Blaslov K, Naran?a FS, Kruljac I, Renar IP. Treatment approach to type 2 diabetes: Past, present and future.
2018; 9: 209-219 [PMID: 30588282 DOI: 10.4239/wjd.v9.i12.209]
2 Pedras S, Carvalho R, Pereira MG. Quality of Life in Portuguese Patients with Diabetic Foot Ulcer Before and After an Amputation Surgery.
2016; 23: 714-721 [PMID: 27495905 DOI: 10.1007/s12529-016-9567-6]
3 Saluja S, Anderson SG, Hambleton I, Shoo H, Livingston M, Jude EB, Lunt M, Dunn G, Heald AH. Foot ulceration and its association with mortality in diabetes mellitus: a meta-analysis.
2020; 37: 211-218 [PMID: 31613404 DOI: 10.1111/dme.14151]
4 Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence.
2017; 376: 2367-2375 [PMID: 28614678 DOI: 10.1056/NEJMra1615439]
5 Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers.
2017; 110: 104-109 [PMID: 28116957 DOI: 10.1177/0141076816688346]
6 Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis
.
2017; 49: 106-116 [PMID: 27585063 DOI: 10.1080/07853890.2016.1231932]
7 American Diabetes Association. 11. Microvascular Complications and Foot Care:
.
2021; 44: S151-S167 [PMID: 33298422 DOI: 10.2337/dc21-S011]
8 Aumiller WD, Dollahite HA. Pathogenesis and management of diabetic foot ulcers.
2015; 28: 28-34 [PMID: 25853673 DOI: 10.1097/01.JAA.0000464276.44117.b1]
9 Margolis SA. Diabetic foot - A global health challenge.
2020; 49: 237 [PMID: 32416648 DOI: 10.31128/AJGP-05-20-1234e]
10 Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes.
2005; 293: 217-228 [PMID: 15644549 DOI: 10.1001/jama.293.2.217]
11 Aldana PC, Khachemoune A. Diabetic Foot Ulcers: Appraising Standard of Care and Reviewing New Trends in Management.
2020; 21: 255-264 [PMID: 31848923 DOI: 10.1007/s40257-019-00495-x]
12 Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, van Netten JJ; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update).
2020; 36 Suppl 1: e3269 [PMID: 32176451 DOI: 10.1002/dmrr.3269]
13 Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot (IWGDF). Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update).
2020; 36 Suppl 1: e3276 [PMID: 31958217 DOI: 10.1002/dmrr.3276]
14 Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes.
2011; 41: 110-116 [PMID: 21095144 DOI: 10.1016/j.ejvs.2010.09.020]
15 Cychosz CC, Phisitkul P, Belatti DA, Wukich DK. Preventive and Therapeutic Strategies for Diabetic Foot Ulcers.
2016; 37: 334-343 [PMID: 26475457 DOI: 10.1177/1071100715611951]
16 Jan YK, Shen S, Foreman RD, Ennis WJ. Skin blood flow response to locally applied mechanical and thermal stresses in the diabetic foot.
2013; 89: 40-46 [PMID: 23727385 DOI: 10.1016/j.mvr.2013.05.004]
17 Reardon R, Simring D, Kim B, Mortensen J, Williams D, Leslie A. The diabetic foot ulcer.
2020; 49: 250-255 [PMID: 32416652 DOI: 10.31128/AJGP-11-19-5161]
18 Hurley L, Kelly L, Garrow AP, Glynn LG, McIntosh C, Alvarez-Iglesias A, Avalos G, Dinneen SF. A prospective study of risk factors for foot ulceration: the West of Ireland Diabetes Foot Study.
2013; 106: 1103-1110 [PMID: 24072752 DOI: 10.1093/qjmed/hct182]
19 Lavery LA, Peters EJ, Armstrong DG. What are the most effective interventions in preventing diabetic foot ulcers?
2008; 5: 425-433 [PMID: 18593392 DOI: 10.1111/j.1742-481X.2007.00378.x]
20 Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer.
2015; 6: 37-53 [PMID: 25685277 DOI: 10.4239/wjd.v6.i1.37]
21 Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers.
2018; 41: 645-652 [PMID: 29559450 DOI: 10.2337/dc17-1836]
22 Kerr M, Barron E, Chadwick P, Evans T, Kong WM, Rayman G, Sutton-Smith M, Todd G, Young B, Jeffcoate WJ. The cost of diabetic foot ulcers and amputations to the National Health Service in England.
2019; 36: 995-1002 [PMID: 31004370 DOI: 10.1111/dme.13973]
23 Raghav A, Khan ZA, Labala RK, Ahmad J, Noor S, Mishra BK. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always.
2018; 9: 29-31 [PMID: 29344337 DOI: 10.1177/2042018817744513]
24 Brennan MB, Hess TM, Bartle B, Cooper JM, Kang J, Huang ES, Smith M, Sohn MW, Crnich C. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes.
2017; 31: 556-561 [PMID: 27993523 DOI: 10.1016/j.jdiacomp.2016.11.020]
25 Lin C, Liu J, Sun H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A meta-analysis.
2020; 15: e0239236 [PMID: 32936828 DOI: 10.1371/journal.pone.0239236]
26 Crawford F, Cezard G, Chappell FM, Murray GD, Price JF, Sheikh A, Simpson CR, Stansby GP, Young MJ. A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS).
2015; 19: 1-210 [PMID: 26211920 DOI: 10.3310/hta19570]
27 Hoogeveen RC, Dorresteijn JA, Kriegsman DM, Valk GD. Complex interventions for preventing diabetic foot ulceration.
2015; CD007610 [PMID: 26299991 DOI: 10.1002/14651858.CD007610.pub3]
28 Liao F, An R, Pu F, Burns S, Shen S, Jan YK. Effect of Exercise on Risk Factors of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis.
2019; 98: 103-116 [PMID: 30020090 DOI: 10.1097/PHM.0000000000001002]
29 Lung CW, Wu FL, Liao F, Pu F, Fan Y, Jan YK. Emerging technologies for the prevention and management of diabetic foot ulcers.
2020; 29: 61-68 [PMID: 32197948 DOI: 10.1016/j.jtv.2020.03.003]
30 Crasto W, Patel V, Davies MJ, Khunti K. Prevention of Microvascular Complications of Diabetes.
2021; 50: 431-455 [PMID: 34399955 DOI: 10.1016/j.ecl.2021.05.005]
31 King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes.
1999; 48: 643-648 [PMID: 10594464 DOI: 10.1046/j.1365-2125.1999.00092.x]
32 Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy.
2019; 5: 41 [PMID: 31197153 DOI: 10.1038/s41572-019-0092-1]
33 Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk.
2004; 141: 413-420 [PMID: 15381514 DOI: 10.7326/0003-4819-141-6-200409210-00006]
34 Ahmad OS, Morris JA, Mujammami M, Forgetta V, Leong A, Li R, Turgeon M, Greenwood CM, Thanassoulis G, Meigs JB, Sladek R, Richards JB. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease.
2015; 6: 7060 [PMID: 26017687 DOI: 10.1038/ncomms8060]
35 Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus.
2004; 141: 421-431 [PMID: 15381515 DOI: 10.7326/0003-4819-141-6-200409210-00007]
36 Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Zu?iga-Hernandez JA, McCoy RG. Benefits and harms of intensive glycemic control in patients with type 2 diabetes.
2019; 367: l5887 [PMID: 31690574 DOI: 10.1136/bmj.l5887]
37 Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research, and the American Diabetes Association. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association.
2015; 132: 691-718 [PMID: 26246173 DOI: 10.1161/CIR.0000000000000230]
38 Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes.
2008; 359: 1577-1589 [PMID: 18784090 DOI: 10.1056/NEJMoa0806470]
39 Rathnayake A, Saboo A, Malabu UH, Falhammar H. Lower extremity amputations and long-term outcomes in diabetic foot ulcers: A systematic review.
2020; 11: 391-399 [PMID: 32994867 DOI: 10.4239/wjd.v11.i9.391]
40 Buggy A, Moore Z. The impact of the multidisciplinary team in the management of individuals with diabetic foot ulcers: a systematic review.
2017; 26: 324-339 [PMID: 28598756 DOI: 10.12968/jowc.2017.26.6.324]
41 Musuuza J, Sutherland BL, Kurter S, Balasubramanian P, Bartels CM, Brennan MB. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers.
2020; 71: 1433-1446.e3 [PMID: 31676181 DOI: 10.1016/j.jvs.2019.08.244]
42 Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, Prokop LJ, Henke P, Tsapas A, Montori VM, Murad MH. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome.
2016; 63: 22S-28S.e1 [PMID: 26804364 DOI: 10.1016/j.jvs.2015.10.005]
43 Lane KL, Abusamaan MS, Voss BF, Thurber EG, Al-Hajri N, Gopakumar S, Le JT, Gill S, Blanck J, Prichett L, Hicks CW, Sherman RL, Abularrage CJ, Mathioudakis NN. Glycemic control and diabetic foot ulcer outcomes: A systematic review and meta-analysis of observational studies.
2020; 34: 107638 [PMID: 32527671 DOI: 10.1016/j.jdiacomp.2020.107638]
44 Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights.
2014; 31: 817-836 [PMID: 25069580 DOI: 10.1007/s12325-014-0140-x]
45 Kim JL, Shin JY, Roh SG, Chang SC, Lee NH. Predictive Laboratory Findings of Lower Extremity Amputation in Diabetic Patients: Meta-analysis.
2017; 16: 260-268 [PMID: 29141468 DOI: 10.1177/1534734617737660]
46 Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis.
2000; 136: 1531-1535 [PMID: 11115166 DOI: 10.1001/archderm.136.12.1531]
47 Llorente L, De La Fuente H, Richaud-Patin Y, Alvarado-De La Barrera C, Diaz-Borjón A, López-Ponce A, Lerman-Garber I, Jakez-Ocampo J. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology.
2000; 74: 239-244 [PMID: 11064109 DOI: 10.1016/s0165-2478(00)00255-8]
48 Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital.
2021; 9: 174-188 [PMID: 33515493 DOI: 10.1016/S2213-8587(20)30381-8]
49 Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and metaanalysis.
2020; 15: e0234065 [PMID: 32502190 DOI: 10.1371/journal.pone.0234065]
50 Cahn A, Raz I, Bonaca M, Mosenzon O, Murphy SA, Yanuv I, Rozenberg A, Wilding JPH, Bhatt DL, McGuire DK, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Jermendy G, Hadjadj S, Langkilde AM, Sabatine MS, Wiviott SD, Leiter LA. Safety of dapagliflozin in a broad population of patients with type 2 diabetes: Analyses from the DECLARE-TIMI 58 study.
2020; 22: 1357-1368 [PMID: 32239659 DOI: 10.1111/dom.14041]
51 Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect?
2018; 20: 1531-1534 [PMID: 29430814 DOI: 10.1111/dom.13255]
52 Huang CY, Lee JK. Sodium-glucose co-transporter-2 inhibitors and major adverse limb events: A trial-level meta-analysis including 51 713 individuals.
2020; 22: 2348-2355 [PMID: 32744411 DOI: 10.1111/dom.14159]
53 Chang HY, Singh S, Mansour O, Baksh S, Alexander GC. Association Between Sodium-Glucose Cotransporter 2 Inhibitors and Lower Extremity Amputation Among Patients With Type 2 Diabetes.
2018; 178: 1190-1198 [PMID: 30105373 DOI: 10.1001/jamainternmed.2018.3034]
54 Pelletier R, Ng K, Alkabbani W, Labib Y, Mourad N, Gamble JM. Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews.
2021; 12: 2042098621989134 [PMID: 33552467 DOI: 10.1177/2042098621989134]
55 Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL Sr, Murad MH. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine.
2016; 63: 3S-21S [PMID: 26804367 DOI: 10.1016/j.jvs.2015.10.003]
56 Diagnosis and Management of Diabetic Foot Complications. Arlington (VA): American Diabetes Association; 2018 Oct- [PMID: 30958663]
57 Everett E, Mathioudakis N. Update on management of diabetic foot ulcers.
2018; 1411: 153-165 [PMID: 29377202 DOI: 10.1111/nyas.13569]
58 Wukich DK, Armstrong DG, Attinger CE, Boulton AJ, Burns PR, Frykberg RG, Hellman R, Kim PJ, Lipsky BA, Pile JC, Pinzur MS, Siminerio L. Inpatient management of diabetic foot disorders: a clinical guide.
2013; 36: 2862-2871 [PMID: 23970716 DOI: 10.2337/dc12-2712]
59 Matheson EM, Bragg SW, Blackwelder RS. Diabetes-Related Foot Infections: Diagnosis and Treatment.
2021; 104: 386-394 [PMID: 34652105]
60 Rayman G, Vas P, Dhatariya K, Driver V, Hartemann A, Londahl M, Piaggesi A, Apelqvist J, Attinger C, Game F; International Working Group on the Diabetic Foot (IWGDF). Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update).
2020; 36 Suppl 1: e3283 [PMID: 32176450 DOI: 10.1002/dmrr.3283]
 World Journal of Diabetes2022年1期
World Journal of Diabetes2022年1期
- World Journal of Diabetes的其它文章
- Gut microbiota-derived metabolites are novel targets for improving insulin resistance
- Role of nutritional ketosis in the improvement of metabolic parameters following bariatric surgery
- High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition
- Polycystic ovary syndrome and type 2 diabetes mellitus: A state-oftheart review
- Acarbose is again on the stage
