Acarbose is again on the stage
INTRODUCTION
Obesity is a key factor in the prevalence of type 2 diabetes mellitus (T2DM) worldwide. Therefore, in treating diabetes, researchers focus on the consequences of eliminating the negative effects of obesity, especially abdominal obesity, on reducing cardiovascular events and death. In a recently published study, Song
[1] aimed to examine the effect of acarbose on abdominal obesity, and its determining factors in comparison with metformin[1]. They evaluated Metformin and AcaRbose in Chinese as the initial Hypoglycemic treatment (MARCH) study data[2] using a new anthropometric measure: Waist-to-height ratio (WHtR). The MARCH study is a randomized, open-labeled, noninferiority trial on Type 2 diabetes patients that was published in 2014[2]. It has been showen in this study that acarbose treatment is as effective and safe as metformin at the 24
and 48
weeks. A group of 343 patients who were newly diagnosed with T2DM were treated with acarbose, and 333 other patients were treated with metformin. The new report by Song
[1] clarified that WHtR had significantly decreased in both groups in the 24
week after treatment, with women showing a more pronounced decrease. Between the beginning of the study and the 24
week of the treatment, the change in the waist-to-height ratio (ΔWHtR) was divided into two sets with large differences in one group and small differences in the other, thus, these data were subject to post-hoc analysis. In the acarbose group, women and those with a lower area under the glucagon-like peptide 1 (GLP-1) curve (AUCGLP-1) had a greater ΔWHtR. Among those using metformin, weight loss was greater in women as well as those with a high baseline AUCGLP-1. In conclusion, Song
[1] found a relationship between high WHtR in the treatment of acarbose with gender, GLP-1 level, fasting glucose, and lipid profile. In addition, Song
[1] emphasized the importance of WHtR for the measurement of abdominal obesity. They argued that, in both groups, a greater reduction in waist circumference in women was independent of the drug and was due to women’s excessive desire and attempts to lose weight. The study observed that the circulating GLP-1 level increased over time in acarbose users. Previous studies reported that alpha glucosidase enzyme inhibition increased circulating GLP-1 levels by stimulating GLP-1 secretion and inhibiting dipeptidyl peptidase 4 (DPP-4) enzymes in healthy and T2DM patients[3-7]. Moreover, a recently published study showed this effect to be inhibited by exendin, a GLP-1 receptor antagonist[8]. This study found that acarbose is more effective for abdominal obesity, especially in those with low GLP-1 levels. The effect of lifestyle change on the results was not evaluated in the article, which is an important limiting factor.
So the Princess got the golden apple, but when the girl went up to the Prince s apartment that night he was asleep,57 for the Princess had so contrived24 it
The work of Song
[1] throws up a question: “What role should acarbose play in the treatment of diabetes?” While acarbose continued to be part of diabetes guidelines and treatment algorithms, the appearance of new treatment agents in the last 10 to 15 years pushed acarbose to the background. In fact, there are large-scale studies that solidify the role of acarbose in treating impaired glucose tolerance (IGT) and T2DM. Over the past year, however, acarbose seems to have regained its importance. Prominent studies, such as the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) and the Acarbose Cardiovascular Evaluation (ACE) study, show that acarbose prevents the development of diabetes regardless of age, gender, and body mass index[9,10]. It has also been found that acarbose reduces cardiovascular events in patients with IGT and T2DM. In a recently published study, Zhang
[11] found a 50% relative risk (RR) reduction in myocardial infarction and a 52% RR reduction in all-cause deaths after a 10-year follow-up with regard to acarbose therapy in patients with T2DM[11]. This effect is due to the reduction of oxidative stress caused by the lowering of postprandial two-hour blood sugar. Some studies have claimed that it is effective in quickly providing joint target controls. However, the fact that the study was conducted only in Chinese patients is an important limiting factor. An increasing number of studies focus on the mechanisms with which acarbose acts in diabetes treatment and how it provides additional benefits[8]. The possible effect mechanisms of acarbose on diabetic patients are shown in Table 1.
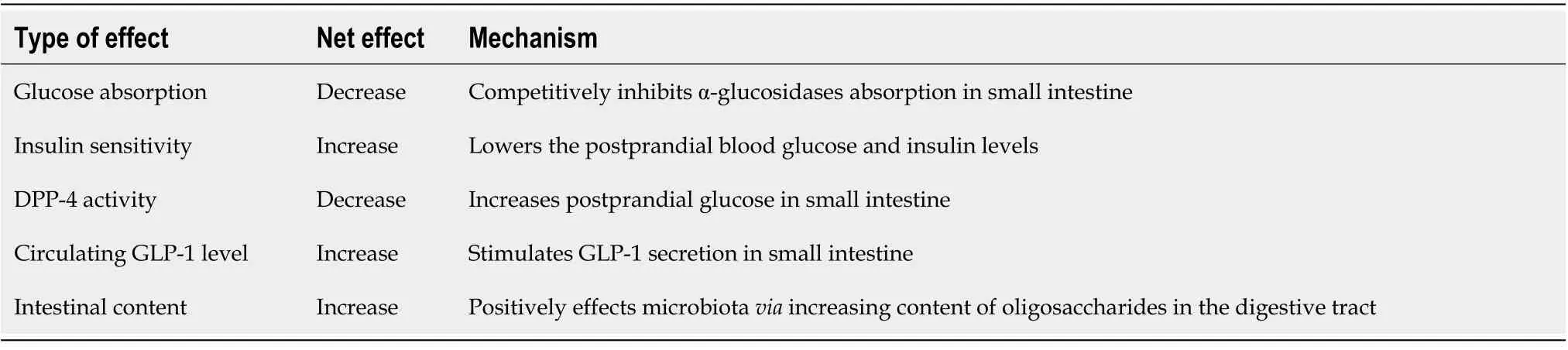
Acarbose inhibits carbohydrate digestion by competitively inhibiting the alpha glucosidase enzyme in the small intestine lumen. Consequently, it reduces glucose absorption, prevents postprandial hyperglycemia and hyperinsulinemia, and increases insulin sensitivity[12]. For this reason, it has been used in clinical practice since the 1990s, whether in monotherapy for mild cases of type 2 diabetes or as a combination agent with insulin and other antidiabetics in severe and advanced cases. Some studies have shown that acarbose has positive effects on intestinal flora[13]. In order to reduce gastrointestinal intolerance, a daily dose of 50 mg is offered just before meals, and a dose of 100 mg is offered three times a day after four to six weeks, when weekly titrations are reached. Acarbose can decrease hemoglobin A1c (HBA1c) by 0.5% to 1.5% and is especially effective on postprandial hyperglycemia[12].
The following are the advantages of acarbose: It is one of the rare agents that has been shown to prevent diabetes in the pre-diabetic period; the rate of hypoglycemia is low; its annual cost is lower than that of new antidiabetic drugs; it has weight-loss properties, or at least is weight neutral; it has a positive effect on the lipid profile by lowering the triglyceride level; and there is increasing evidence to show that it reduces the risk factors of cardiovascular disease. However, it shouldn’t be forgotten that this hasn’t yet been proven in Cardio Vascular Outcome Trials (CVOTs).
I didn t taste a bit of breakfast that morning. Dad seemed in a jovial5() mood as he described an exceptional6 Yankee game seen through the eyes of Mel Allen on the radio last night.
The disadvantages of acarbose are that it has to be used three times a day, and gastrointestinal side effects, such as gas, bloating, and diarrhea are relatively frequent.
CONCLUSION
In my opinion, we should remember that acarbose is an effective alternative to controlling postprandial hypoglycemia in countries that predominantly consume carbohydrates, like China or Turkey. The increasing evidence on its effects on GLP-1 and cardiovascular protection may lead to an extension of its use. It seems that acarbose, which has a high efficacy and is safe in terms of its side-effect profile, will be at the forefront of diabetes guidelines in the near future.
1 Song LL, Wang X, Yang ZJ, Kong XM, Chen XP, Zhang B, Yang WY. Factors associated with improvement in waist-to-height ratio among newly diagnosed type 2 diabetes patients treated with acarbose or metformin: A randomized clinical trial study.
2020; 11: 514-526 [PMID: 33269063 DOI: 10.4239/wjd.v11.i11.514]
2 Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, Weng J, Jia W, Lu J, Xu Y, Yang Z, Chen W. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial.
2014; 2: 46-55 [PMID: 24622668 DOI: 10.1016/S2213-8587(13)70021-4]
3 Moritoh Y, Takeuchi K, Hazama M. Chronic administration of voglibose, an alpha-glucosidase inhibitor, increases active glucagon-like peptide-1 levels by increasing its secretion and decreasing dipeptidyl peptidase-4 activity in ob/ob mice.
2009; 329: 669-676 [PMID: 19208898 DOI: 10.1124/jpet.108.148056]
4 Ueno H, Tsuchimochi W, Wang HW, Yamashita E, Tsubouchi C, Nagamine K, Sakoda H, Nakazato M. Effects of Miglitol, Acarbose, and Sitagliptin on Plasma Insulin and Gut Peptides in Type 2 Diabetes Mellitus: A Crossover Study.
2015; 6: 187-196 [PMID: 26055217 DOI: 10.1007/s13300-015-0113-3]
5 Lee A, Patrick P, Wishart J, Horowitz M, Morley JE. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics.
2002; 4: 329-335 [PMID: 12190996 DOI: 10.1046/j.1463-1326.2002.00219.x]
6 Borg MJ, Jones KL, Sun Z, Horowitz M, Rayner CK, Wu T. Metformin attenuates the postprandial fall in blood pressure in type 2 diabetes.
2019; 21: 1251-1254 [PMID: 30615231 DOI: 10.1111/dom.13632]
7 Br?nden A, Albér A, Rohde U, Rehfeld JF, Holst JJ, Vilsb?ll T, Knop FK. Single-Dose Metformin Enhances Bile Acid-Induced Glucagon-Like Peptide-1 Secretion in Patients With Type 2 Diabetes.
2017; 102: 4153-4162 [PMID: 28938439 DOI: 10.1210/jc.2017-01091]
8 Dalsgaard NB, Gasbjerg LS, Hansen LS, Hansen NL, Stensen S, Hartmann B, Rehfeld JF, Holst JJ, Vilsb?ll T, Knop FK. The role of GLP-1 in the postprandial effects of acarbose in type 2 diabetes.
2021; 184: 383-394 [PMID: 33449919 DOI: 10.1530/EJE-20-1121]
9 Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial.
2002; 359: 2072-2077 [PMID: 12086760 DOI: 10.1016/S0140-6736(02)08905-5]
10 Gerstein HC, Coleman RL, Scott CAB, Xu S, Tuomilehto J, Rydén L, Holman RR; ACE Study Group. Impact of Acarbose on Incident Diabetes and Regression to Normoglycemia in People With Coronary Heart Disease and Impaired Glucose Tolerance: Insights From the ACE Trial.
2020; 43: 2242-2247 [PMID: 32641379 DOI: 10.2337/dc19-2046]
11 Zhang XL, Yuan SY, Wan G, Yuan MX, Yang GR, Fu HJ, Zhu LX, Zhang JD, Li YL, Gao DY, Cui XL, Wang ZM, Xie RR, Chen YJ. The effects of acarbose therapy on reductions of myocardial infarction and all-cause death in T2DM during 10-year multifactorial interventions (The Beijing Community Diabetes Study 24).
2021; 11: 4839 [PMID: 33649485 DOI: 10.1038/s41598-021-84015-0]
12 Alssema M, Ruijgrok C, Blaak EE, Egli L, Dussort P, Vinoy S, Dekker JM, Denise Robertson M. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: a systematic review and meta-analysis.
2021; 11: 11 [PMID: 33658478 DOI: 10.1038/s41387-021-00152-5]
13 Wang Z, Wang J, Hu J, Chen Y, Dong B, Wang Y. A comparative study of acarbose, vildagliptin and saxagliptin intended for better efficacy and safety on type 2 diabetes mellitus treatment.
2021; 274: 119069 [PMID: 33460667 DOI: 10.1016/j.lfs.2021.119069]
 World Journal of Diabetes2022年1期
World Journal of Diabetes2022年1期
- World Journal of Diabetes的其它文章
- Gut microbiota-derived metabolites are novel targets for improving insulin resistance
- Role of nutritional ketosis in the improvement of metabolic parameters following bariatric surgery
- High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition
- Management of diabetic foot ulcers and the challenging points: An endocrine view
- Polycystic ovary syndrome and type 2 diabetes mellitus: A state-oftheart review
