Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Two Diphenyl Ether Tetracarboxylic Acid?Co Coordination Polymers
ZHAO Su?Qin GU Jin?Zhong
(1College of Physics and Electronic Information Engineering,Qinghai University for Nationalities,Xining 810007,China)
(2College of Chemistry and Chemical Engineering,Lanzhou University,Lanzhou 730000,China)
Abstract:Two cobalt coordination polymers,namely[Co2(μ3?deta)(H2biim)3(H2O)2]n(1)and{[Co2((μ6?deta)(phen)2]·H2O}n(2),have been constructed hydrothermally using H4deta(2,3,3',4'?diphenyl ether tetracarboxylic acid),H2biim(2,2'?biimidazole),phen(1,10?phenanthroline),and cobalt chloride at 160 ℃.The products were isolated as stable crystalline solids and were characterized by IR spectra,elemental analyses,thermogravimetric analyses,and single?crystal X?ray diffraction analyses.Single?crystal X?ray diffraction analyses revealed that compounds 1 and 2 crystallize in the triclinic and monoclinic systems,space groups and P21/c,respectively.Compound 1 discloses a 1D chain structure,and compound 2 features a 2D network.The catalytic activities in Knoevenagel condensation reaction of the compounds were investigated.Compound 1 exhibited excellent catalytic activity in Knoevenagel con?densation reaction at room temperature.CCDC:2069400,1;2069401,2.
Keywords:coordination polymer;tetracarboxylic acid;catalytic property;Knoevenagel condensation reaction
0 Introduction
The field of coordination polymers has attracted tremendous attention due to their structural and topo?logical diversity as well as their potential applications as functional materials[1?11].In the last ten years,organic carboxylate ligands have been widely used in synthesiz?ing coordination polymers due to the strong coordina?tion ability of the carboxyl group and the rich coordina?tion modes[5?6,12?15].Among them,ether?bridged carboxyl?icacids have been extensively applied as versatile building blocks toward the assembly of metal?organic architectures[16?17].
The 2,3,3',4'?diphenyl ether tetracarboxylic acid(H4deta)is a good bridging ligand for constructing coor?dination polymers[18],under considering structural semi?rigidity,which has multiple coordinate sites involving four carboxylate oxygen atoms and one O?ether donor.Knoevenagel condensation is one of the imperative and essential condensation processes in synthetic organic chemistry,in whichα,β?unsaturated products formed via carbon?carbon double bond involve a nucleophilic addition reaction between active methylene and carbon?yl compounds followed by a dehydration reaction[19?23].Products obtained are extensively used as specialty chemicals and intermediates in the synthesis of fine chemicals such as carbocyclic,substituted alkenes,biologically active compounds,therapeutic drugs,calci?um antagonists,natural products,functional polymers,coumarin derivatives,flavors,and perfumes.Transition metal?catalyzed Knoevenagel condensation reactions have recently received much attention[24?26],mainly due to the low price and moderate toxicity of the catalysts in combination with their high activity.
Herein,we report the synthesis,crystal structures,and catalytic activity of two Cocoordination poly?mers with H4deta,2,2'?biimidazole(H2biim)and 1,10?phenanthroline(phen)ligands.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spec?trometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10 ℃·min-1.Powder X?ray dif?fraction patterns(PXRD)were measured on a Rigaku?Dmax 2400 diffractometer using CuKαradiation(λ=0.154 06 nm);the X?ray tube was operated at 40 kV and 40 mA;the data collection range was between 5°and 45°.Solution1H NMR spectra were recorded on a JNM ECS 400M spectrometer.
1.2 Synthesis of[Co2(μ3?deta)(H2biim)3(H2O)2]n(1)
A mixture of CoCl2·6H2O(0.048 g,0.2 mmol),H4deta(0.035 g,0.1 mmol),H2biim(0.027 g,0.2 mmol),NaOH(0.016 g,0.4 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon?lined stainless?steel vessel,and heated at 120℃for 3 d,followed by cooling to room temperature at a rate of 10 ℃·h-1.Orange block?shaped crystals were isolated manually,and washed with distilled water.Yield:35%(based on H4deta).Anal.Calcd.for C34H28Co2N12O11(%):C 45.45,H 3.14,N 18.71;Found(%):C 45.62,H 3.12,N 18.59.IR(KBr,cm-1):3 384m,2 972w,1 628w,1 549s,1 483m,1430m,1398s,1377s,1341m,1271w,1231w,1182w,1 120w,1 084w,1 049w,991w,907w,867w,828w,756w,694m,624w.
1.3 Synthesis of{[Co2(μ6?deta)(phen)2]·H2O}n(2)
Synthesis of 2 was similar to 1 except using phen(0.040 g,0.2 mmol)instead of H2biim.Purple block?shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:60%(based on H4deta).Anal.Calcd.for C40H24Co2N4O10(%):C 57.30,H 2.88,N 6.68;Found(%):C 57.12,H 2.86,N 6.70.IR(KBr,cm-1):3 660w,3 428w,3 066w,1 626s,1 581s,1514m,1492w,1425m,1391s,1377s,1302w,1262w,1 235w,1 151w,1 089w,969w,924w,894w,840m,818w,770m,725m,685w,641w.
The compounds are insoluble in water and com?mon organic solvents,such as methanol,ethanol,ace?tone,and DMF.
1.4 Structure determination
Two single crystals with dimensions of 0.25 mm×0.20 mm×0.18 mm(1)and 0.23 mm×0.22 mm×0.20 mm(2)were collected at 293(2)K on a Bruker SMART APEX Ⅱ CCD diffractometer with MoKα(λ=0.071 073 nm).The structures were solved by direct methods and refined by full?matrix least?squares onF2using the SHELXTL?2014 program[27].All non?hydro?gen atoms were refined anisotropically.All the hydro?gen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compounds 1 and 2 are given in Table 3 and 4.
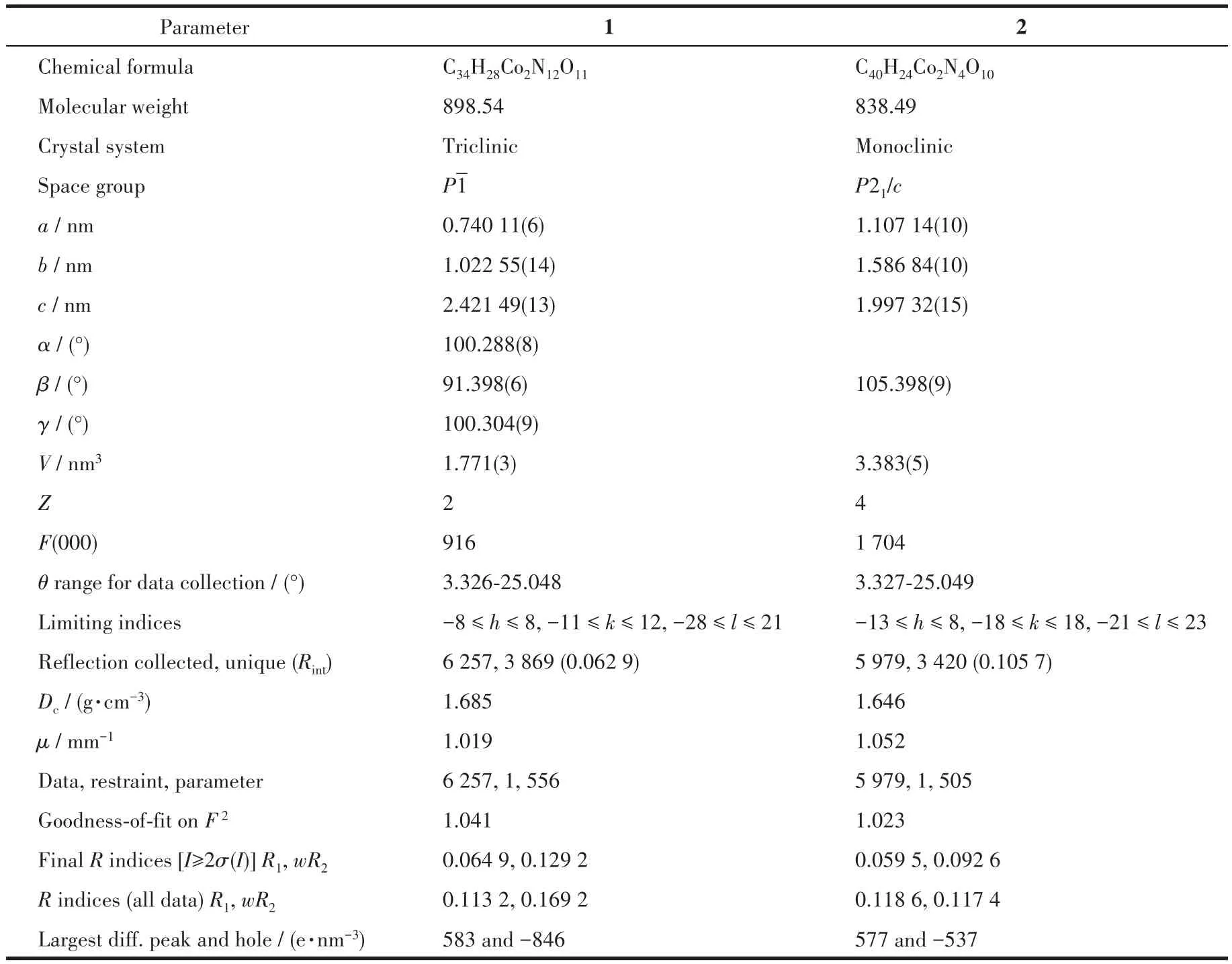
Table 1 Crystal data for compounds 1 and 2
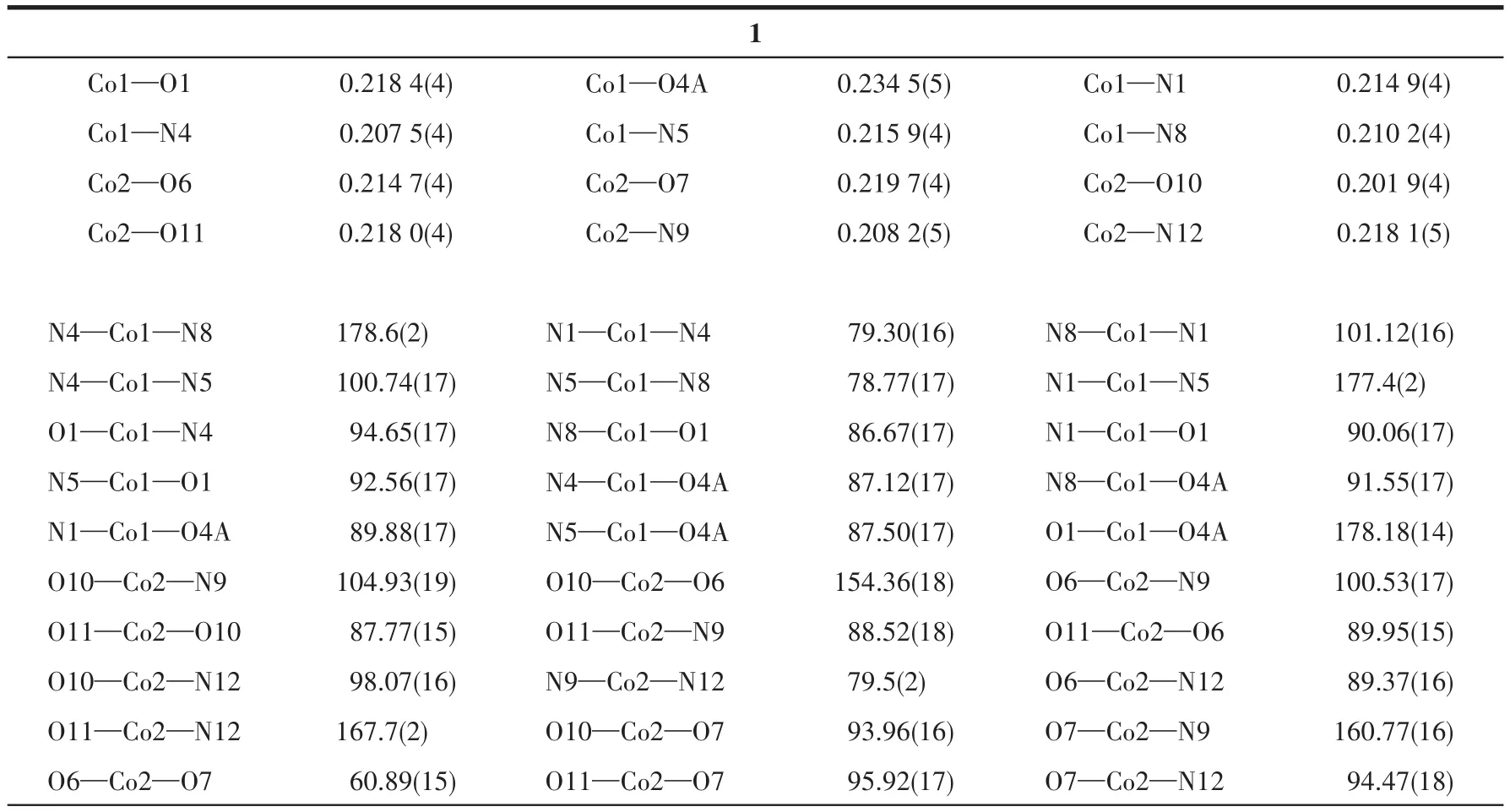
Table 2 Selected bond distances(nm)and bond angles(°)for compounds 1 and 2
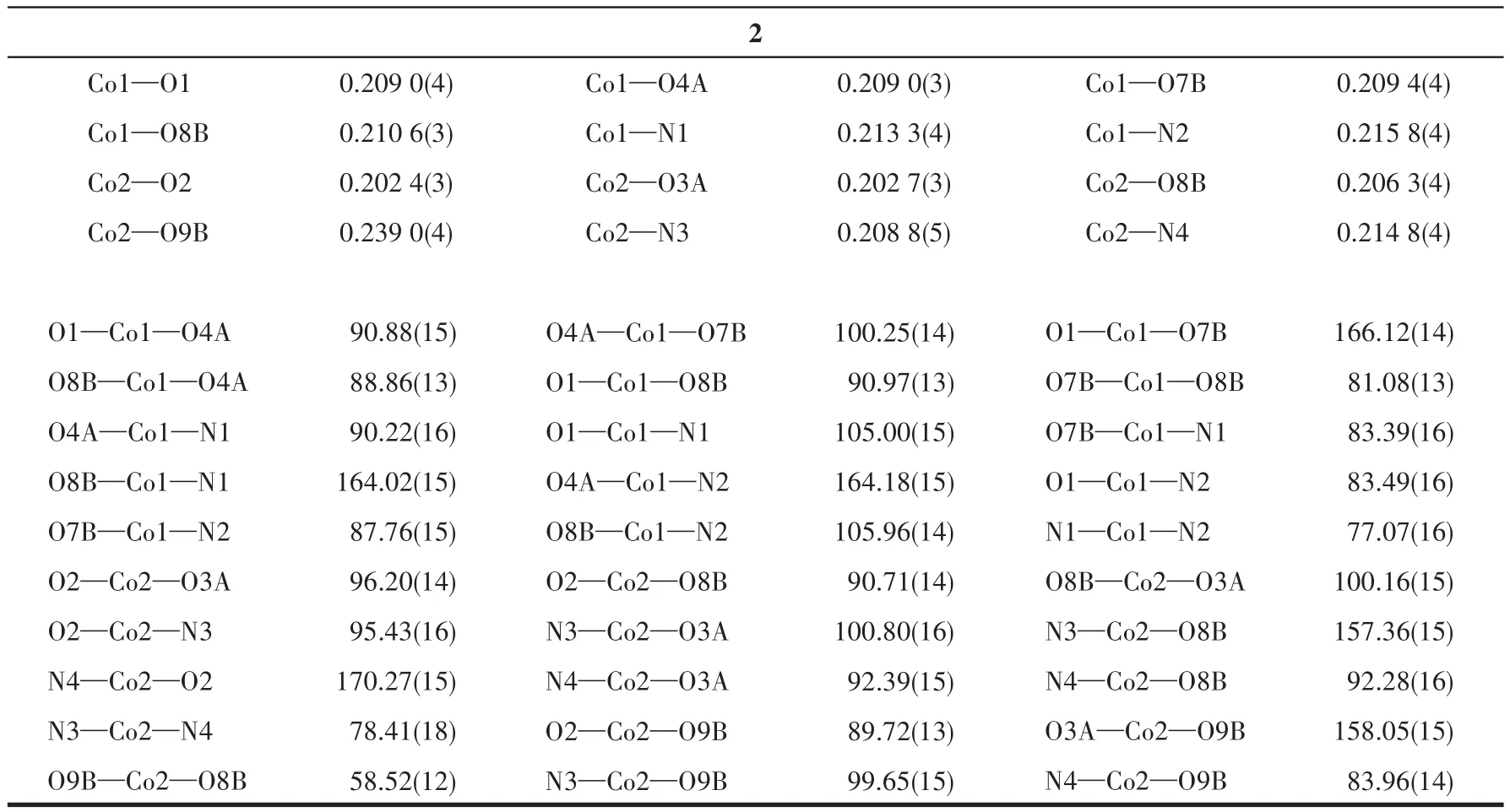
Continued Table 2
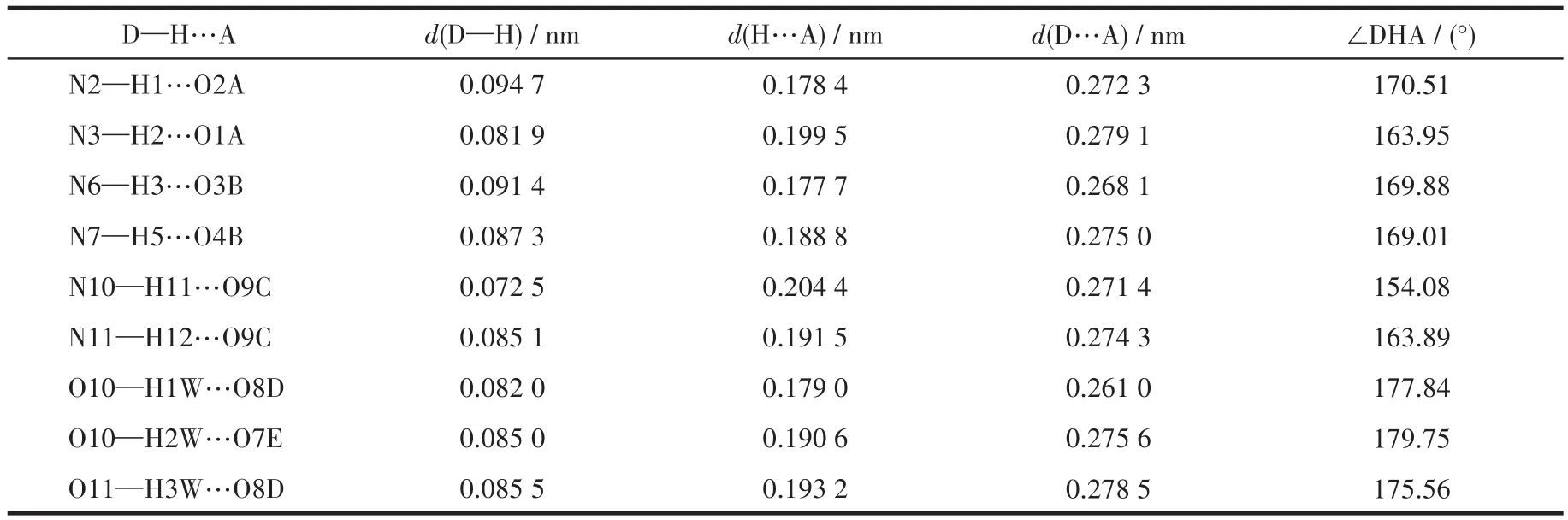
Table 3 Hydrogen bond parameters of compound 1
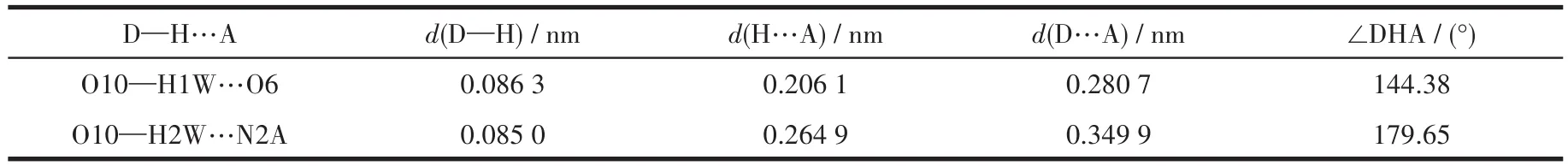
Table 4 Hydrogen bond parameters of compound 2
CCDC:2069400,1;2069401,2.
1.5 Catalytic activity for Knoevenagel condensation reaction of aldehydes
Prior to the catalytic activity study,compound 1 was activated in a vacuum oven at 210℃for 10 h.In a typical test,a suspension of an aromatic aldehyde(0.50 mmol,benzaldehyde as a model substrate),malononi?trile(1.0 mmol),and catalyst(Molar fraction:2%)in methanol(1.0 mL)was stirred at room temperature.After the desired reaction time,the catalyst was removed by centrifugation,followed by evaporation of the solvent from the filtrate under reduced pressure to give a crude solid.This solid was dissolved in CDCl3and analyzed by1H NMR spectroscopy for quantifica?tion of products(Fig.S1,Supporting information).To perform the recycling experiment,the catalyst was iso?lated by centrifugation,washed with dichloromethane,dried at room temperature,and reused.The subsequent steps were performed as described above.
2 Results and discussion
2.1 Description of the structure
2.1.1 Structure of compound 1
X?ray crystallography analysis reveals that com?pound 1 crystallizes in the triclinic system space group.As shown in Fig.1,the asymmetric unit of 1 bears two crystallographically unique Coions(Co1 and Co2),oneμ3?deta4-block,three H2biim moieties,and two H2O ligands.The six?coordinated Co1 ion exhibits a distorted octahedral{CoN4O2}environment,which is occupied by two carboxylate O donors from two differ?entμ3?deta4-blocks and four N atoms from two H2biim moieties.The Co2 center is also six?coordinated and forms a distorted octahedral{CoN2O4}geometry.It is completed by two carboxylate O atoms from oneμ3?deta4-block,two O donors from two H2O ligands,and two N atoms from the H2biim moiety.The Co—O and Co—N bond distances are 0.218 4(4)?0.234 5(5)nm and 0.207 5(4)?0.218 1(5)nm,respectively;these are within the normal ranges observed in related Cocom?pounds[6,28?29].In compound 1,the ligand deta4-adopts a coordination mode Ⅰ(Scheme 1)with four COO-groups being uncoordinated,monodentate or bidentate.In deta4-ligand,a dihedral angle(between two aromat?ic rings)and a C—Oether—C angle are 78.88°and 118.26°,respectively.Theμ3?deta4-blocks connect Co ions to give a 1D chain(Fig.2).Adjacent chains are assembled into a 2D supramolecular sheet through O—H…O and N—H…O hydrogen bonds(Table 3 and Fig.3).
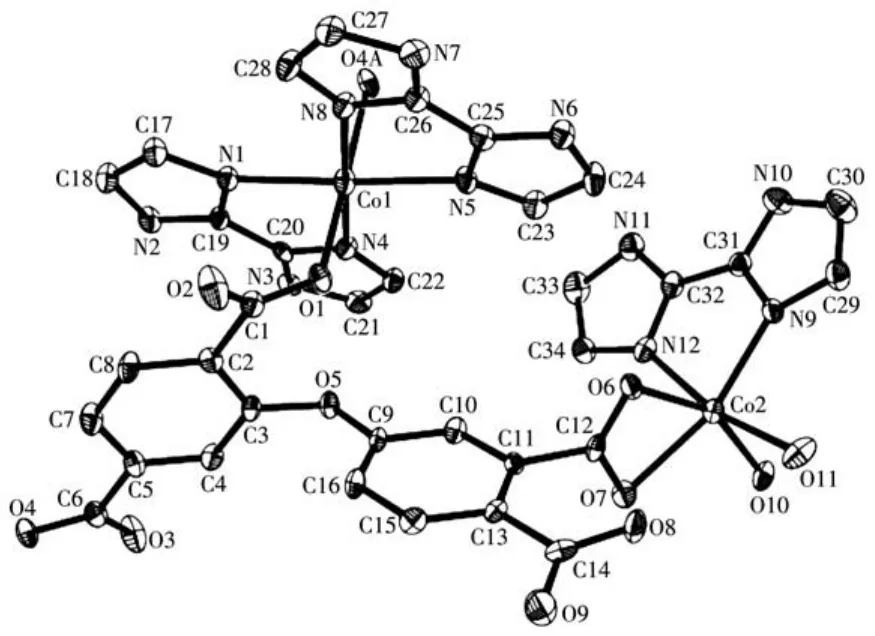
Fig.1 Drawing of asymmetric unit of compound 1 with 30% probability thermal ellipsoids
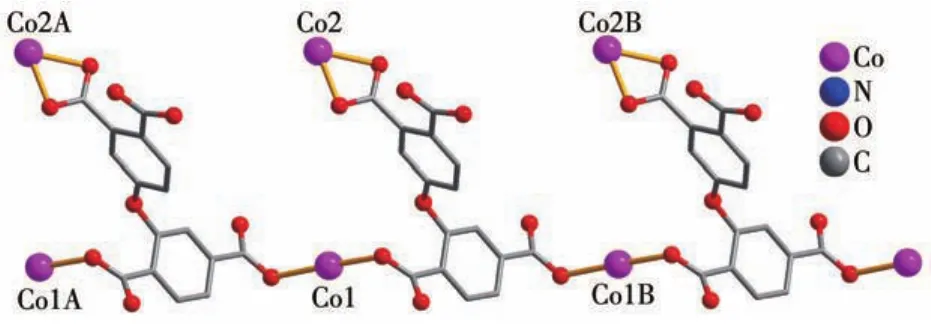
Fig.2 Perspective of 1D metal?organic chain along a axis
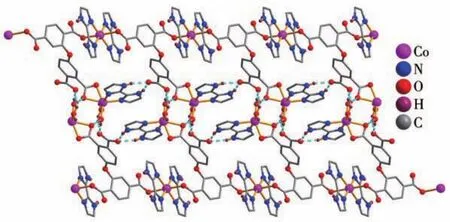
Fig.3 Perspective of 2D H?bonded network along a axis
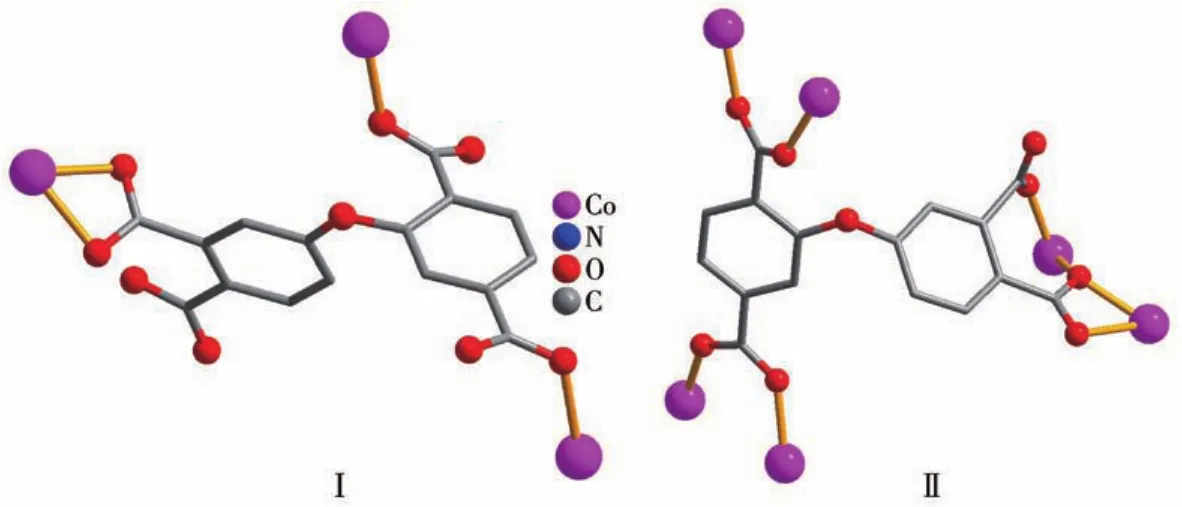
Scheme 1 Coordination modes of deta4-ligand in compounds 1 and 2
2.1.2 Structure of compound 2
The asymmetric unit of compound 2 contains two crystallographically unique Coions(Co1 and Co2),oneμ6?deta4-block,two phen moieties,and one lattice water molecule.As depicted in Fig.4,both Co centers are six?coordinated and display a distorted octahedral{CoN2O4}geometry.It is taken by four carboxylate O atoms from three individualμ6?deta4-blocks and two N donors from the ligand phen.The bond lengths of Co—O are in a range of 0.202 4(3)?0.239 0(4)nm,while the Co—N bonds are 0.208 8(5)?0.215 8(4)nm,being comparable to those found in some reported Cocompounds[28?30].In 2,the deta4-block acts as aμ6?linker(mode Ⅱ,Scheme 1),in which four carboxylate groups adopt monodentate,μ?bridging bidentate or tridentate modes.Besides,theμ6?deta4-ligand is considerably bent showing a dihedral angle of 67.68°(between two aromatic rings)and the angle of C—Oether—C being 121.04°.Two adjacent Co1 and Co2 ions are joined via three carboxylate groups from three independentμ6?deta4-ligands to form a Co2subunit with a Co1…Co2 distance of 0.352 7(2)nm(Fig.4).These di?cobaltsubunits are further linked together through the remain?ing carboxylate groups ofμ6?deta4-blocks to form a 2D metal?organic network(Fig.5).Compounds 1 and 2 were assembled under similar conditions except for the type of auxiliary ligand used(H2biim for 1 and phen for 2),but they show different structures.
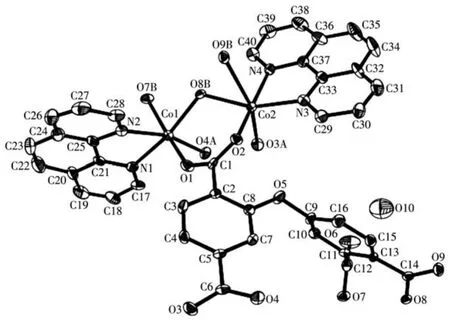
Fig.4 Drawing of asymmetric unit of compound 2 with 30% probability thermal ellipsoids
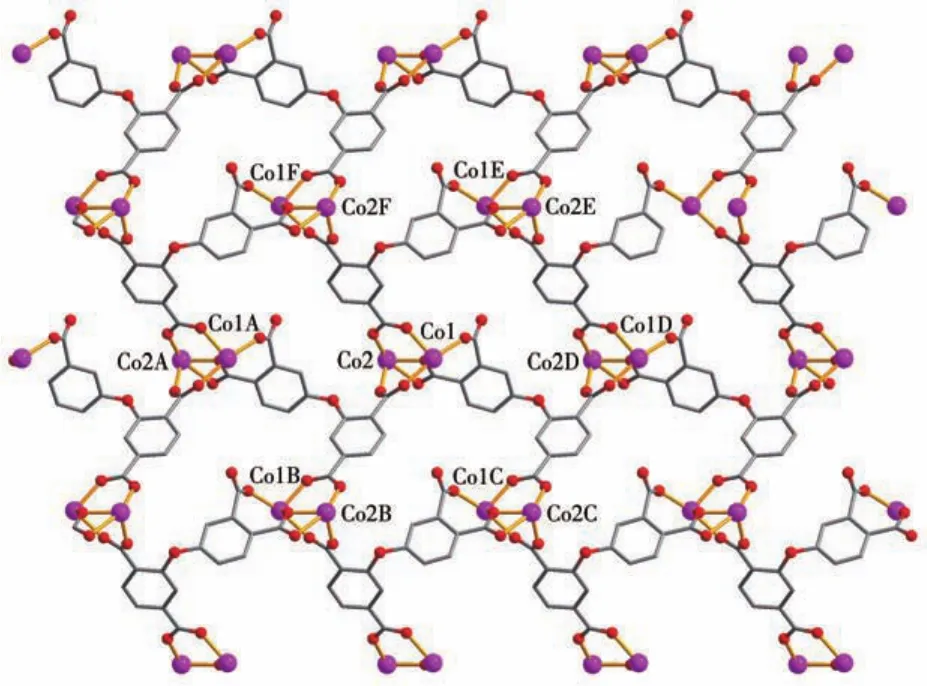
Fig.5 View of 2D metal?organic network parallel to ab plane
2.2 TGA for compounds 1 and 2
To determine the thermal stability of compounds 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by TGA.As shown in Fig.6,compound 1 lost its two coordinated water molecules in a range of 96?203℃ (Obsd.4.2%,Calcd.4.0%).Decomposition of the sample occurred above 305℃,corresponding to the removal of deta4-and H2biim ligands attached to the Co ions.For 2,one weight loss(Obsd.2.2%,Calcd.2.1%)in a range of 84?243 ℃corresponds to the removal of one lattice water mole?cule;the remaining sample started to decompose above 342℃,corresponding to the removal of deta4-and phen ligands attached to the Co ions.
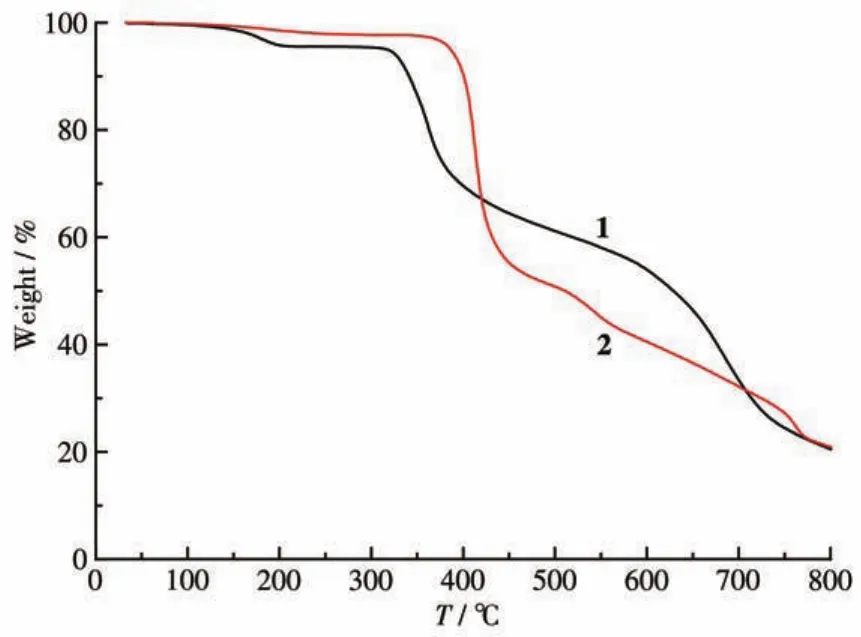
Fig.6 TGA curves of compounds 1 and 2
2.3 Catalytic activity for Knoevenagel condensation reaction
Given the potential of cobaltcoordination com?pounds to catalyze organic reactions[6,30?31],we explored the application of 1 and 2 as heterogeneous catalysts in the Knoevenagel condensation reaction of benzalde?hyde as a model substrate to give 2?(phenylmethylene)?propanedinitrile.Typical tests were carried out by reacting a mixture of benzaldehyde,malononitrile,and a Cocomplex catalyst in methanol at room tempera?ture(Scheme 2,Table 5).Such effects as reaction time,catalyst loading,solvent composition,catalyst recy?cling,and finally substrate scope were investigated.
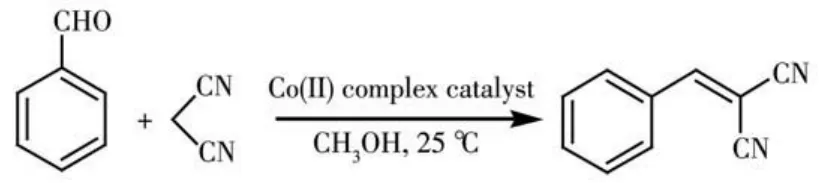
Scheme 2 Knoevenagel condensation reaction of benzaldehyde(model substrate)catalyzed by Cocoordination compounds
Table 5 Knoevenagel condensation reaction of benzaldehyde with malononitrile catalyzed by Cocoordination compounds

Table 5 Knoevenagel condensation reaction of benzaldehyde with malononitrile catalyzed by Cocoordination compounds
aMolar fraction;bCalculated by1H NMR spectroscopy:nproduct/naldehyde×100%.
Entry Catalyst 1 2 3 4 5 6 7 8 9 1 0 11 12 13 14 15 16 17 1 1 1 1 1 1 1 1 1 1 1 1 1 2 Blank CoCl2·6H2O H4deta Time/min 10 20 30 40 50 60 60 60 60 60 60 60 55 60 60 60 60 Catalyst loadinga/%2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 1.0 1.5 2.5 2.0—2.0 2.0 Solvent CH3OH CH3OH CH3OH CH3OH CH3OH CH3OH H2O C2H5OH CH3CN CHCl3 CH3OH CH3OH CH3OH CH3OH CH3OH CH3OH CH3OH Yieldb/%41 56 64 80 93 100 90 98 85 67 92 98 100 89 23 33 30
Upon using compound 1 as the catalyst(Molar fraction:2%),a high conversion rate of 100% of benzal?dehyde into 2?(phenyl methylene)?propanedinitrile was reached after 60 min in methanol at room temperature(Table 5,Entry 6).The product was accumulated with a yield increase from 41% to 100% on prolonging the reaction from 10 to 60 min(Table 5,Entry 1?6).The influence of catalyst amount was also investigated,revealing a product yield growth from 92% to 100% on increasing the loading of catalyst from 1% to 2.5%(Table 5,Entry 6 and 11?13).In addition to water,other solvents were tested,in particular,the reaction showed a comparable efficiency in H2O and ethanol(90% and 98% product yields,respectively).Acetoni?trile and chloroform were less suitable(85% and 67% product yields,respectively).It should be highlighted that under similar reaction conditions,the Knoevenagel condensation of benzaldehyde was significantly less ef?ficient in the absence of catalyst(only 23% product yield)or when using H4deta(30% yield)or CoCl2·6H2O(33% yield)as catalysts(Table 5,Entry 15?17).
The results show that compound 1 is more active than compound 2.Although a relationship between structure and catalytic activity in the present study can not be clearly established,the highest conversation shown by compound 1 may eventually be associated with its 1D structure for easily accessible metal centers,together with the presence of the open metal sites[30,32].
We also compared the activities of catalyst 1 in the reactions of other substituted aromatic aldehydes with malononitrile,and the corresponding yields were in a range of 54% to 100%(Table 6).Aryl aldehydes bearing strong electron?withdrawing substituents(e.g.,nitro and chloro)exhibited high activities(Table 6,Entry 2?5),which may be related to an increase in the electrophilicity of the substrate.Aldehydes containing electron?donating groups(e.g.,methyl)showed lower reaction yields(Table 6,Entry 6?8),as expected.

Table 6 Knoevenagel condensation reaction of various aldehydes with malononitrile catalyzed by compound 1a
To examine the stability of 1 in the Knoevenagel condensation reaction,we tested the recyclability of this heterogeneous catalyst.For this purpose,upon completion of a reaction cycle,we separated the cata?lyst by centrifugation,washed it with CH2Cl2,and dried it at room temperature before its further use.We found that for catalyst 1,the catalytic system maintained the high activity over at least five consecutive cycles with the yields being 100%,100%,99%,and 98% for the second to the fifth run,respectively.According to the PXRD data(Fig.S2),the structure of 1 was essentially preserved after five catalytic cycles.
The achieved catalytic performance of compound 1 in the Knoevenagel condensation reaction of benzal?dehyde with malononitrile is superior to those exhibit?ed by the heterogeneous catalysts based on other metal?carboxylate coordination compounds(Table 7)[33?37].
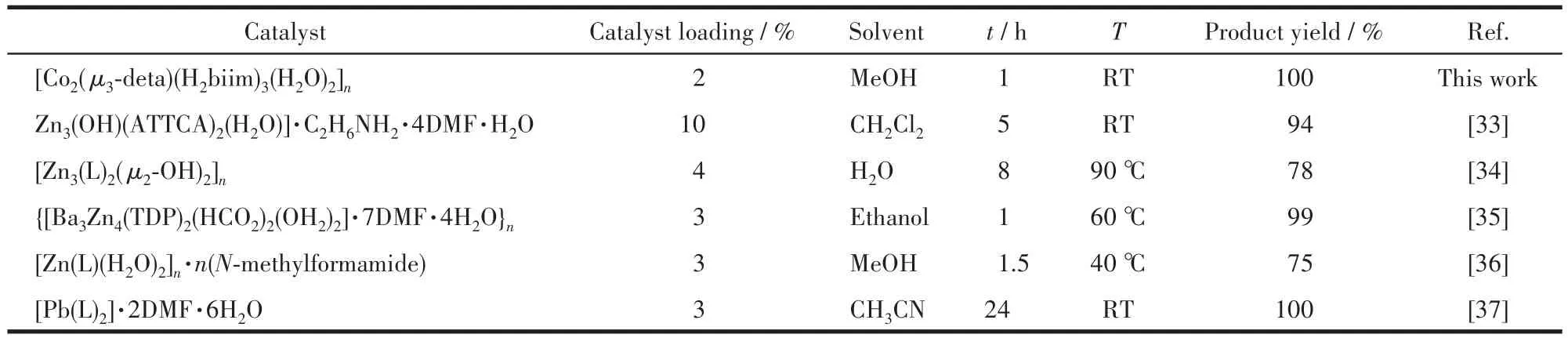
Table 7 Comparison among various catalysts for Knoevenagel condensation reaction between benzaldehyde and malononitrile
3 Conclusions
In summary,we have synthesized two Cocoor?dination polymers based on a tetracarboxylate ligand.Compound 1 discloses a 1D chain structure.Com?pound 2 features a 2D network.The catalytic proper?ties of both compounds were investigated.Compound 1 revealed an excellent catalytic activity in the Knoeve?nagel condensation reaction of benzaldehyde at room temperature.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- 《無機化學學報》投稿須知(NOTICE TO AUTHORS)
- Replacement of Carboxylate Ligand Substituent on Modulation of Structures and Magnetic Properties in Salen?Type Dinuclear Dy Complexes
- Mn?MOF Derived Mn2O3 Micromotors Applied to Removal of Methyl Blue in Water
- Crystal Structures and Magnetic Properties of LnⅢ2Complexes Based on a Polydentate Schiff Base Ligand
- Synthesis of a Water?Stable Zn?Based Metal?Organic Framework for Luminescence Detecting Fe3+and 2,6?Dichloro?4?nitroaniline
- Constructing and Photocatalytic Performance of Flower?like CeO2/TiO2 Heterostructures

