Crystal Structures and Magnetic Properties of LnⅢ2Complexes Based on a Polydentate Schiff Base Ligand
GAO Xiao?Li LEI Wen?Ting ZHANG Qing?Fang ZHOU Yu KANG Xiao?Min
(1Department of Chemistry,Taiyuan Normal University,Jinzhong,Shanxi 030619,China)
(2College of Chemistry and Chemical Engineering,Inner Mongolia University,Hohhot 010021,China)
Abstract:Three new LnⅢ2complexes based on a polydentate Schiff base ligand,namely[Ln2(L)2(acac)2(CH3OH)2]·2CH3OH(Ln=Sm(1),Gd(2),Ho(3),H2L=pyridine?2?carboxylic acid(1?methyl?3?oxo?butylidene)?hydrazide,Hacac=acetylacetone),have been synthesized by using solvothermal method.The crystal structures and magnetic proper?ties of these complexes have been systematically studied.The crystal structures measurement results reveal that complexes 1?3 are isostructural and each eight?coordinated Ln ion possesses a square antiprism geometry;the adjacent central rare earth Ln ions are connected by two μ2?O to form a parallelogram[Ln2O2]core.The magnetic study showed that complex 2 displayed significant magnetic refrigeration property with a larger magnetic entropy(-ΔSmax)of 31.9 J·K-1·kg-1at ΔH=70 kOe and T=2.0 K;while complex 3 shows slow magnetic relaxation behavior.CCDC:2052182,1;2052183,2;2052184,3.
Keywords:LnⅢ2complexes;crystal structures;magnetic properties;magnetic refrigeration property;slow magnetic relaxation behavior
0 Introduction
In recent years,the study of lanthanide?based complexes has attracted increasing attention of chem?ists and material scientists not only due to their beauti?ful and fascinating crystal structures but also because of the potential applications in functional materials,including interesting magnetic properties,lumines?cence,gas adsorption,and catalysis[1?4].Among these potential applications of lanthanide?based complexes,the molecular?based magnetic material is one of the research hotspots for inorganic chemistry and material chemists[5],and magnetic refrigeration and single?molecule magnets(SMMs)are particularly attractive[6?9].Key to the potential magnetic refrigeration application of molecular?based magnetic materials is its large mag?netocaloric effect(MCE)[10],and an excellent magnetic refrigeration material featuring large MCE should possess negligible magnetic anisotropy and a large magnetic density[11].Hence,the isotropic Gdion with a high spin state(S=7/2)is the best candidate for designing and constructing Ln?based complexes,which would be a promising magnetic refrigerant mate?rial to perform significant MCE[12].Based on this,lots of polynuclear or high?nuclear Gd?based clusters with fascinating structures and larger MCE have been reported over the past decade[13?16].It is worth mention?ing that Zheng and Long's group has conducted out?standing work on the magnetic refrigeration materials of Gd?based clusters[17?18].These studies promote the design and synthesis of lanthanide?based complexes with outstanding and excellent magnetic properties.On the other hand,lanthanide?based SMMs have caused public attention in recent years[19],and designing lanthanide?based SMMs with large energy barriers(Ueff)and high blocking temperatures(TB)is a great chal?lenge[20].Given this,lots of Ln?based SMMs exhibit?ing significant magnetic behavior have appeared since the first Ln?based SMM(Bu4N)[Tb(Pc)2]reported by Ishikawa et al.in 2003[21].
To seek and study the magnetic behaviors of lan?thanide?based complexes,an attractive polydentate ligand H2L(Scheme 1)has been selected to construct lanthanide complexes,and three LnⅢ2complexes[Ln2(L)2(acac)2(CH3OH)2]·2CH3OH(Ln=Sm(1),Gd(2),and Ho(3),H2L=pyridine?2?carboxylic acid(1?methyl?3?oxo?butylidene)?hydrazide,Hacac=acetylace?tone)have been synthesized.The magnetic refrigera?tion and slow magnetic relaxation behavior of 1?3 have been studied.

Scheme 1 Structure of ligand H2L
1 Experimental
1.1 Material and measurement
Solvents(methanol,dichloromethane)and starting chemical reagent(Ln(NO3)3·6H2O,Ln=Sm,Gd,and Ho)were purchased commercially and used without further purification.Acetylacetone and 2?pyridine carboxylic acid hydrazide were purchased from Aladdin Reagent(Shanghai)Co.,Ltd.Ln(acac)3·2H2O(Ln=Sm,Gd,and Ho)was prepared using a reported method[22].The ele?mental analyses(C,H,and N)of complexes 1?3 were measured on a PerkinElmer 240 CHN elemental ana?lyzer.Powder X?ray diffraction(PXRD)of complexes 1?3 were performed using an UltimaⅣ(Rigaku)with CuKαradiation in a 2θrange from 5°to 50°.The oper?ating voltage and current were 40 kV and 25 mA,respectively.Thermogravimetric analyses(TGA)of complexes 1?3 were performed on a TG 209 apparatus(Netzsch)under an air atmosphere.Magnetic properties for complexes 1?3 were measured using a Quantum Design MPMS?XL7 and a PPMS?9 ACMS magnetome?ter.Diamagnetic corrections were estimated with Pascal's constants for all atoms[23].
1.2 Preparation of complexes 1?3
2?Pyridine carboxylic acid hydrazide(0.04 mmol),Ln(acac)3·2H2O(0.04 mmol,Ln=Sm(1),Gd(2),and Ho(3)),methanol(8 mL),and dichloromethane(2.0 mL)were enclosed in a 15 mL glass vial,and then the mixture was stirred at room temperature for about 2.0 h.Whereafter,the mixture was heated to 70℃and kept for 48 h,and then the temperature was decreased to room temperature slowly.Yellow block crystals suit?able for X?ray diffraction were obtained.
[Sm2(L)2(acac)2(CH3OH)2]·2CH3OH(1):Yieldbased on Sm(acac)3·2H2O:36%.Elemental analysis Calcd.for C36H52N6O12Sm2(%):C 40.66,H 4.89,N 7.91;Found(%):C 40.64,H 5.03,N 8.00.
[Gd2(L)2(acac)2(CH3OH)2]·2CH3OH(2):Yield based on Gd(acac)3·2H2O:32%.Elemental analysis Calcd.for C36H52N6O12Gd2(%):C 40.10,H 5.01,N 7.80;Found(%):C 40.21,H 4.97,N 7.75.
[Ho2(L)2(acac)2(CH3OH)2]·2CH3OH(3):Yield based on Ho(acac)3·2H2O:41%.Elemental analysis Calcd.for C36H52N6O12Ho2(%):C 39.57,H 4.76,N 7.69;Found(%):C 39.62,H 4.89,N 7.72.
1.3 X?ray crystallography
The X?ray diffraction measurements for complexes 1?3 were performed on a Bruker SMART APEX Ⅱ CCD diffractometer equipped with a graphite mono?chromatized MoKαradiation(λ=0.071 073 nm)by usingφ?ωscan mode.Multi?scan absorption correction was applied to the intensity data using the SADABS program.The structures were solved by direct methods and refined by full?matrix least?squares onF2using the SHELXTL?2018 program.All non?hydrogen atoms were refined anisotropically.All the other H atoms were positioned geometrically and refined using a riding model.Details of the crystal data and structure refinement parameters for 1?3 are summarized in Table 1,and selected bond lengths and angles of 1?3 are listed in Table S1?S3(Supporting information).
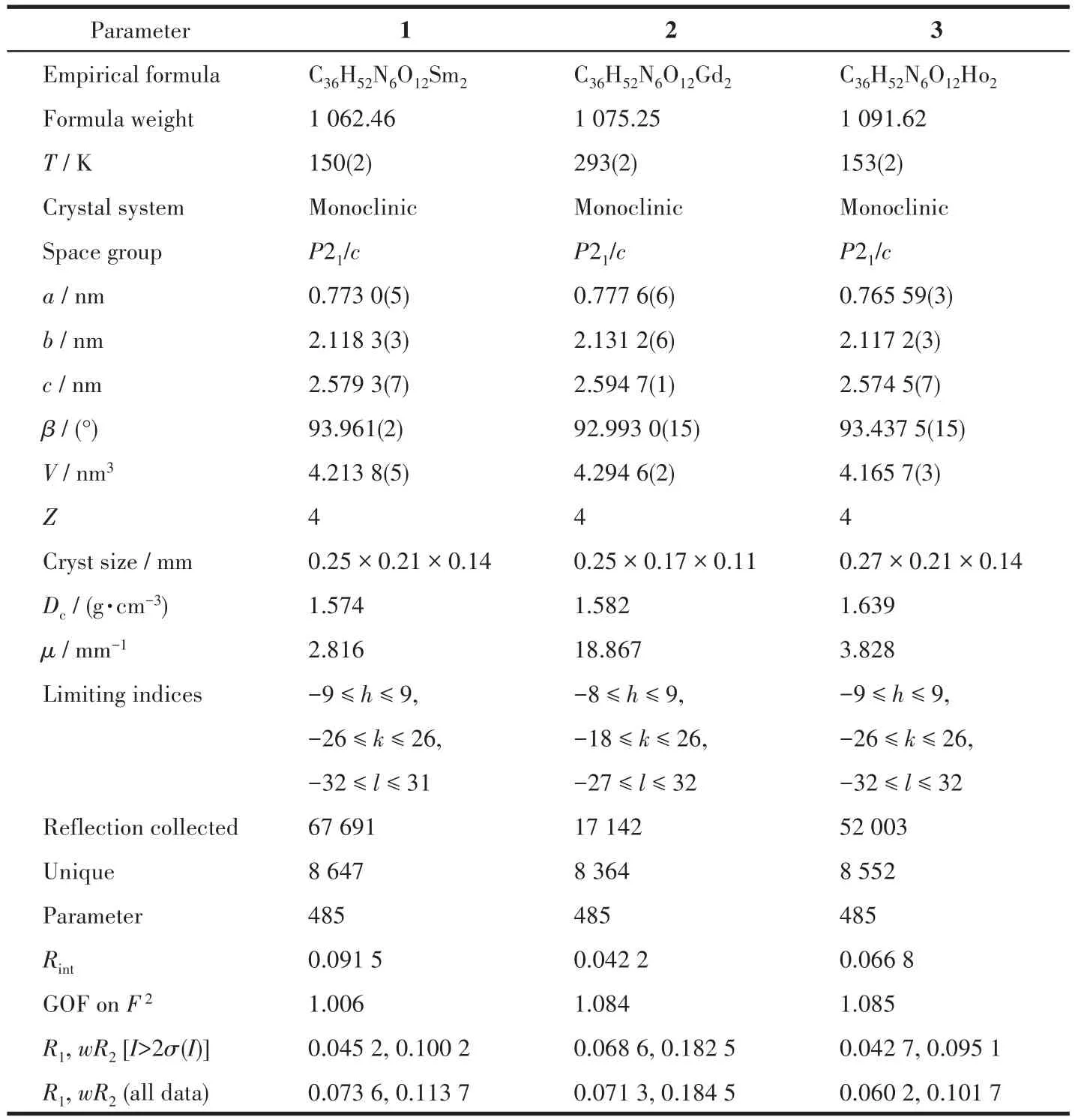
Table 1 Crystal data and structure refinement parameters for complexes 1?3
CCDC:2052182,1;2052183,2;2052184,3.
2 Results and discussion
2.1 Crystal structures of complexes 1?3
Single?crystal X?ray diffraction analyses reveal that 1?3 are isostructural and crystallize in the mono?clinic space groupP21/c(Table 1).Hereon,we selected the structure of 2 as a representative for describing.As shown in Fig.1,the structure of 2 comprises two Gdions,two L2-,two acac-,and two coordinated CH3OH.Each central Gdion is coordinated by six oxygen atoms and two nitrogen atoms formed a[N2O6]coordina?tion environment.As shown in Fig.S1,the Gd1 ion is coordinated by two nitrogen atoms(N3 and N4)and six oxygen atoms(O2,O3,O4,O7,O8,and O9)of two L2-,one CH3OH and one acac-.The eight?coordinated Gd1 ion possesses a square antiprism geometry which is confirmed by using SHAPE 2.0 software(Table 2).The coordination mode of L2-and acac-are shown in Fig.2.The two central Gdions are connected by twoμ2?O atoms forming a parallelogram[Gd2O2]core.In the[Gd2O2]core,the Gd1…Gd2 distance is 0.392 8(8)nm,O2—Gd1—O4 bond angle is 62.201 1(9)°,and the Gd1—O2—Gd2 bond angle is 113.809 2(3)°.Further?more,the bond distances of Gd—O in complex 2 are in a range of 0.225 0(6)?0.244 5(6)nm,and the Gd1—N3,Gd1—N4,Gd2—N1,and Gd2—N6 bond lengths are 0.255 5(8),0.243 8(7),0.242 6(7),and 0.254 1(8)nm,respectively.The O—Gd—O bond angles are in a range of 66.18(19)°?147.9(2)°.These bond lengths and angles of 2 are compared to those of reported Gd2com?plexes[24?27].

Fig.1 Molecular structure of complex 2 shown with 30% probability displacement ellipsoids
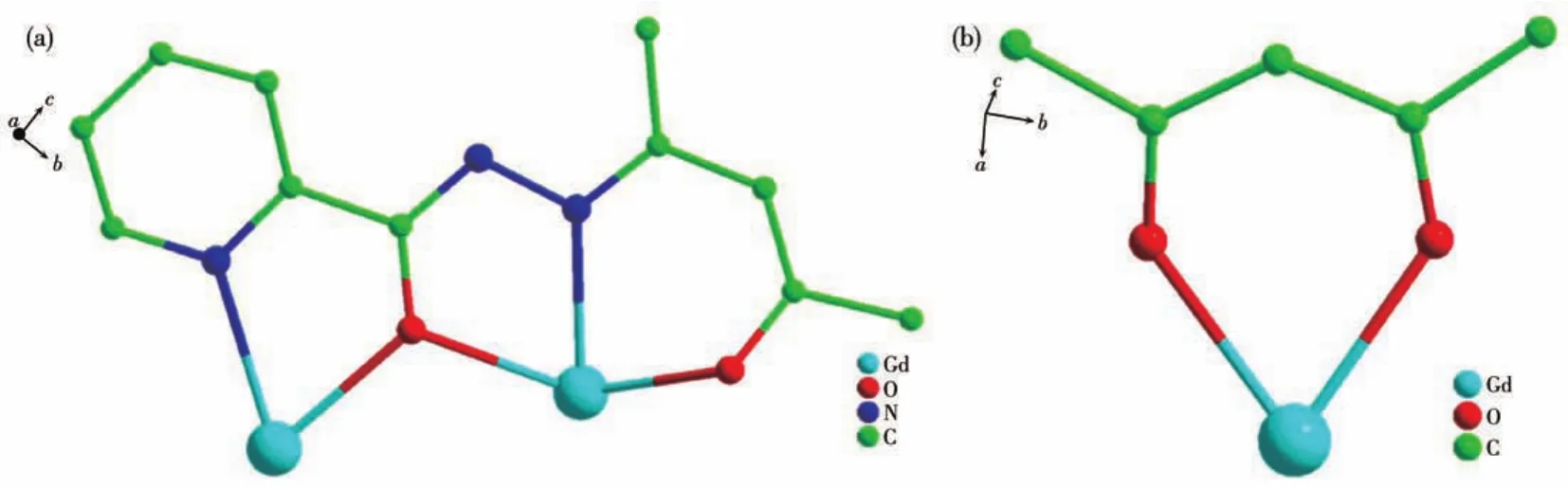
Fig.2 (a)Coordination mode of L2-in 2;(b)Coordination mode of acac-in 2
Table 2 Gdgeometry analysis by SHAPE 2.0 for complex 2
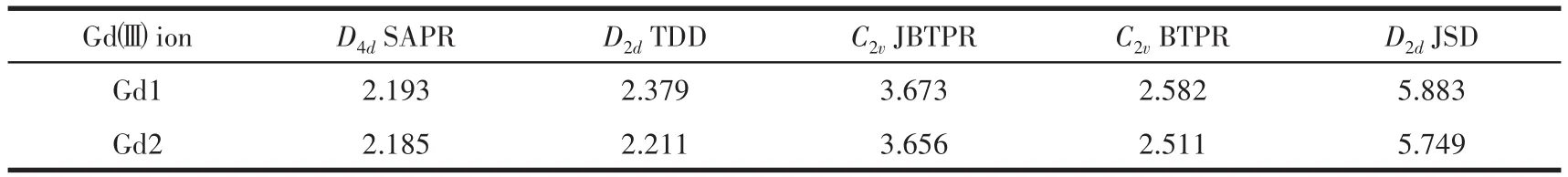
Table 2 Gdgeometry analysis by SHAPE 2.0 for complex 2
SAPR?8=square antiprism;TDD?8=triangular dodecahedron;JBTPR?8=biaugmented trigonal prism J50;BTPR?8=biaug?mented trigonal prism;JSD?8=snub diphenoid J84;The number 8 represents eight?coordinated geometrical configuration.
D2dJSD 5.883 5.749 Gdimages/BZ_159_1521_3006_1571_3050.pngion Gd1 Gd2 D4dSAPR 2.193 2.185 D2dTDD 2.379 2.211 C2vJBTPR 3.673 3.656 C2vBTPR 2.582 2.511
2.2 PXRD pattern and TGA
To prove the phase purities of complexes 1?3,the crystalline products of these complexes were measured by PXRD.As shown in Fig.S2,the PXRD patterns of the crystalline samples of 1?3 were in good agreement with their simulated ones,which proves the high phase purity.
To investigate the thermal stabilities of complexes 1?3,TGA was performed under an air atmosphere.As shown in Fig.S3,the TG curves of 1?3 showed a similar variation trend.Hereon,we selected the TG curve of complex 1 for a detailed description.The weight loss of 6.31%(Calcd.6.02%)between 40 and 294℃can be attributed to the loss of two free CH3OH.After that,complex 1 decomposed gradually.
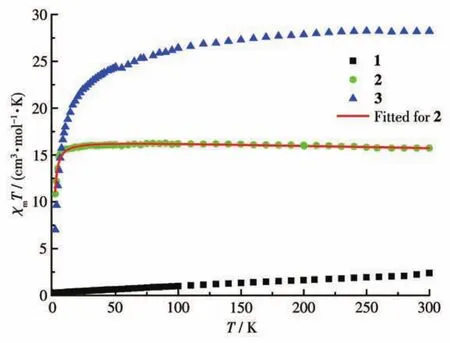
Fig.3 Temperature dependence of χMT at 1.0 kOe for 1?3
2.3 Magnetic properties
The magnetic susceptibility data for the LnⅢ2com?plexes(1?3)were measured with the polycrystalline samples during a temperature range of 2.0 to 300.0 K and under an external magnetic field of 1.0 kOe.TheχΜTvsTplots for complexes 1?3 are shown in Fig.3.At 300.0 K,theχΜTvalues of 1?3 were 2.40,15.73,and 28.20 cm3·mol-1·K,respectively.The expected values for two free Lnions are shown as follows:two isolat?ed Smions(6H5/2,g=2/7)gave 0.95 cm3·mol-1·K for 1;two isolated Gdions(8S7/2,g=2)gave 15.76 cm3·mol-1·K for 2;two isolated Hoions(5I8,g=4/5)gave 28.14 cm3·mol-1·K for 3.For 1,as the temperature decreased,theχΜTvalue slowly declined and reached the minor value of 0.26 cm3·mol-1·K at 2.0 K.For 2,during the temperature range of 20.0?300.0 K,theχΜTvalue almos remained constant;whereafter,theχΜTvalue dropped to a minimum of 10.83 cm3·mol-1·K at 2.0 K.The downtrend suggests that there is an antifer?romagnetic interaction between adjacent Gdions in complex 2[28].For 3,theχΜTvalue decreased slowly from 300 to 50 K,then it decreased quickly to reach the minimum of 7.01 cm3·mol-1·K at 2.0 K.This behavior may be attributed to the thermal depopulation of the HoStark sublevels or/and the antiferromagnet?ic interactions between the adjacent Hoions in complex 3[29].
The Curie?Weiss law was used for fitting the magnetic susceptibility of 2(Fig.S4).Two parametersC(15.83 cm3·mol-1·K)andθ(-0.73 K)were obtained(R=0.999 78).The small and negativeθvalue of 2 further suggests that there is an antiferromagnetic interaction between adjacent Gdions in 2[30].For further explor?ing the magnetic interaction between the adjacent Gdions in complex 2,we fitted theχMTvsTcurve of 2 by using the Hamiltonian:and the two significant param?eters,J=-0.06 cm-1andg=2.05,were obtained.The negativeJvalue further proves that there is an antifer?romagnetic coupling between the neighboring Gdions in 2.
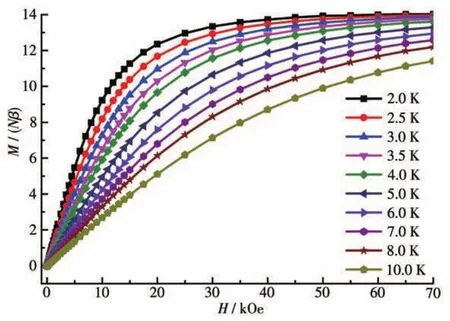
Fig.4 Plots of M vs H at 2.0?10.0 K for 2
The magnetization data for complex 2 were studied at 2.0?10.0 K in a 0?70 kOe field.As depicted in Fig.4,the magnetizationMfor complex 2 rapidly increased blow 20 kOe and then steadily increased to 14.02Nβat 70 kOe,which is very close to the satura?tion value of 14Nβfor two free Gd(S=7/2,g=2)ions.
According to the previous literature[32?34],due to the presence of the isotropic Gdion with a high?spin ground state,hereon,the magnetocaloric effect of com?plex 2 was investigated.The maximum magnetic entro?py change(-ΔSmax)could be calculated by using the Maxwell equation:ΔSmax(T)= ∫[?M(T,H)/?T]HdH[35].As shown in Fig.5,at ΔH=70 kOe andT=2.0 K,the observed-ΔSmaxwas 31.9 J·K-1·kg-1,which was small?er than the theoretical value of 34.2 J·K-1·kg-1based on the equation:ΔSmax=2Rln(2S+1)(SGd=7/2 andR=8.314 J·mol-1·K-1).The difference between experimen?tal and theoretical magnetic entropy change(-ΔSmax)may be due to the weak antiferromagnetic interactions between Gdions in complex 2[36].It is worth mention?ing that the-ΔSmaxof complex 2 was larger than those of mostly reported Gd2complexes[37?39].With the larger-ΔSmaxvalue,complex 2 may be a good candidate for potential application in magnetocaloric materials.
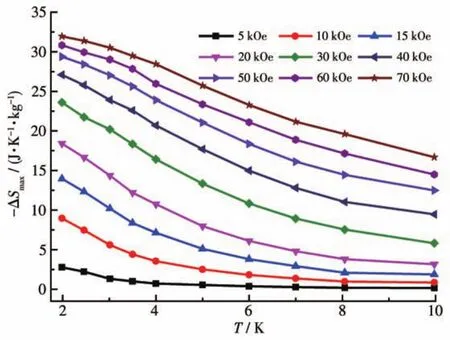
Fig.5 Plots of-ΔSmaxvs T for 2
In order to study the dynamic magnetic behavior of complex 3,the alternating current(ac)magnetic susceptibility measurements were performed during a temperature range of 2.0?15.0 K at various frequencies.Clear frequency dependence of out?of?phase(χ″)signals was observed which suggests that slow magnetic relaxation occurred in 3[40].However,noχ″peaks were observed untilT=2.0 K,andχ″values gradually increased in the lower temperature region,which can be ascribed to the quantum tunneling effect(QTE)[41].This phenomenon commonly occurred in most Ln?based complexes[42].
To check the quantum tunneling of magnetization(QTM)effect above 2.0 K in complex 3,underHdc=2 500 Oe,the variable?temperature ac susceptibilities were determined.As shown in Fig.S5,remarkable and peak shapes were observed,which show that the QTE in complex 3 was pronounced,and the QTM effect was basically suppressed when it was under an external 2 500 Oe dc field.The lnτvsT-1plot is shown in Fig.7.The relaxation time τ obeys the Arrhenius law:τ=τ0exp[ΔE/(kBT)].Two key parameters,energy barrier ΔE/kB=17.99 K and pre ?exponential factorτ0=9.55×10-7s,were obtained.Theτ0of complex 3 was consis?tent with the reported values of 10-6?10-12s for Ln?based SMMs[43?44].
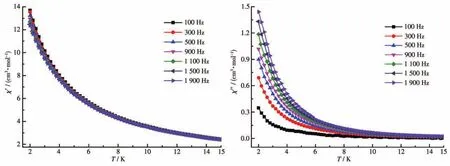
Fig.6 Temperature dependence of in?phase(χ')and out?of?phase(χ″)components of ac magnetic susceptibility for 3 in 0 Oe field with an oscillation of 3.0 Oe
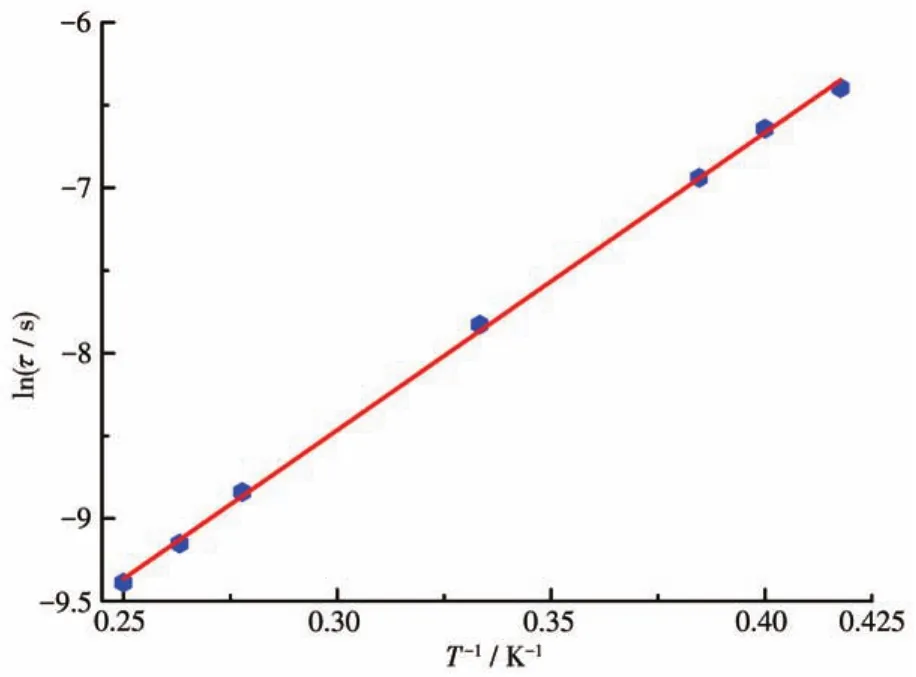
Fig.7 ln τ vs T-1plot for complex 3
3 Conclusions
In summary,we have synthesized three new LnⅢ2complexes[Ln2(L)2(acac)2(CH3OH)2]·2CH3OH(Ln=Sm(1),Gd(2),Ho(3)).Complexes 1?3 are all isostructural and contain a parallelogram[Ln2O2]core.Magnetic measurements imply that Gd2complex 2 displayed significant magnetic refrigeration property with a larger-ΔSmaxof 31.9 J·K-1·kg-1(ΔH=70 kOe andT=2.0 K);while Ho2complex 3 shows slow magnetic relaxation behavior.
Supporting information is available at http://www.wjhxxb.cn
 無(wú)機(jī)化學(xué)學(xué)報(bào)2022年1期
無(wú)機(jī)化學(xué)學(xué)報(bào)2022年1期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- 《無(wú)機(jī)化學(xué)學(xué)報(bào)》投稿須知(NOTICE TO AUTHORS)
- Replacement of Carboxylate Ligand Substituent on Modulation of Structures and Magnetic Properties in Salen?Type Dinuclear Dy Complexes
- Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Two Diphenyl Ether Tetracarboxylic Acid?Co Coordination Polymers
- Mn?MOF Derived Mn2O3 Micromotors Applied to Removal of Methyl Blue in Water
- Synthesis of a Water?Stable Zn?Based Metal?Organic Framework for Luminescence Detecting Fe3+and 2,6?Dichloro?4?nitroaniline
- Constructing and Photocatalytic Performance of Flower?like CeO2/TiO2 Heterostructures
