Mn?MOF Derived Mn2O3 Micromotors Applied to Removal of Methyl Blue in Water
HUANG Jing TIAN Xin?Yu YANG Lan FENG Xiao?Miao
(Key Laboratory for Organic Electronics and Information Displays&Institute of Advanced Materials(IAM),School of Materials Science&Engineering,Nanjing University of Posts&Telecommunications,Nanjing 210023,China)
Abstract:A Mn?based metal?organic framework(Mn?MOF)was selected as the precursors to prepare the Mn?MOF derivative(Mn2O3)microspheres.The Mn2O3microspheres had a homogenous size of ca.4 μm,with perfect sphere morphology,rough surface,good crystallinity and high yield.At the same time,the movement behavior of Mn2O3 micromotors under different conditions and the degradation properties of methyl blue(MB)were studied.As?prepared Mn2O3micromotors had excellent autonomous movement ability,and their moving speed can reach 81.32 μm·s-1in 10% H2O2solution.Experimental results show that with the addition of H2O2,the Mn2O3micromotors can effectively remove MB through degradation within 5 min.
Keywords:self?propelled;micromotor;Mn2O3;degradation;methyl blue
0 Introduction
Micro/nanomotors are a class of micro/nanoscale devices which can convert external energy or chemical substances into driving forces to realize autonomous movement.According to the propulsion mechanism[1],they can be categorized into physical?driven(including light energy[2],magnetic field[3],ultrasonic wave[4],elec?tric field[5],and thermal energy[6])and fuel?driven(e.g.,H2O2[7],glucose[8],lactose[9],and urea[10]).Fuel?driven micro/nanomotors are powered by catalytic reactions which present high speed and efficiency.A large num?ber of catalytic materials have been used in the design of fuel?driven micro/nanomotors.Traditional catalysts including noble metals(e.g.,Pt[11],Ag[12]),active metals(e.g.,Mg[13],Zn[14]),metal oxides(e.g.,MnO2[15],Fe2O3[16]),or enzymes[17],have excellent catalytic performances.However,some of these materials have problems of high cost and complicated preparation process,which are not conducive to large?scale production and practi?cal application.Therefore,the development of low?cost,easy?to?prepare and high catalytic activity micro/nano?motor materials still faces major scientific challenges.
The extensive application of micromotors has been made great progress in explosives detection[18],biomedicine[19],environmental restoration[20],nano engi?neering[21]and other fields for the past few years.At present,the issue of environmental restoration,espe?cially sewage treatment,is a huge challenge facing the world[22].Generally,organic pollutants in water are removed by physical adsorption and chemical oxida?tion.Micro/nanomotors have a promising potential application in this field and many works have been reported to date.Liang et al.used silver nano?colloids as the surface?enhanced Raman scattering(SERS)substrate to introduce SERS technology,and designed micro/nanomotors with water quality monitoring func?tions,which could identify the characteristic peaks of pollutants in the SERS spectrum of the resulting solu?tion[23].The combination of micro/nanomotors and sens?ing technology provided a new direction for environ?mental monitoring.Ma et al.prepared Janus micromo?tor by incorporating iron ions into the zeolite frame?work[24].The micromotors used Pt to catalyze H2O2to generate driving force,while iron ions catalyze H2O2to continuously generate hydroxyl radicals,which degrad?ed phenol as a model pollutant.Similarly,Shi et al.used reduced graphene oxide(rGO)to immobilize Fe3O4nanoparticles(NPs)to synthesize heterogeneous Fe3O4?rGO/Pt microjets which could improve the degra?dation capability of Fenton composite microjets and effectively remove methylene blue in a short time[25].In response to recent oil spills,many researchers had applied micro/nanomotors to dynamic oil removal.The canned hollow MnFe2O4micromotor could quickly and efficiently collect oil droplets through the hydrophobic surface?oil interaction of the pre?existing long oleic acid chains[26].For the first time,Luis et al.used the wax printing template?assisted rolled?up method to pre?pare rGO/Pt tubular micromotors[27].The hydrophobici?ty and high specific surface area to volume ratio of rGO provided favorable conditions for reversibly collecting and releasing oil in water.The removal and recycling of heavy metal from sewage are also crucial in environ?mental remediation issues.A multi?functional mesopo?rous silica?based microinjector was immobilized MnO2andγ?Fe2O3NPs on the inner and outer surface,respectively[28].The interaction between Fe2O3NPs and heavy metals could remove heavy metal ions such as Cd2+and Pb2+in wastewater.And the microjets could be easily guided and recycled by a magnetic field.Hou et al.designed a self?propelled tubular micromotor based on a copolymer of aspartic acid and cysteine(PAsp?Cys),which could quickly remove inorganic and organic heavy metals in polluted water[29].This was due to the strong binding force between sulfhydryl groups of PAsp?Cys and metals(especially mercury).Mean?while,the multi?carboxyl and amino groups of PAsp were also used to adsorb various metal ions.
Metal?organic framework(MOF)with large pore size,stable crystal lattice,and functional groups is a kind of porous material assembled by metal ions and bridged organic ligands,which has attracted a lot of attention in recent years[30].Therefore,the introduction of MOF materials into fuel?catalyzed micro/nanomo?tors,which increases the catalytically active sites and active surfaces of the micro/nanomotors,is expected to realize the efficient role of the micro/nanomotors in environmental remediation.Ikezoe et al.proposed the application of MOF materials to micromotors for the first time,which expanded the new research direction of micromotor preparation materials[31].Recently,we have synthesized a spherical and dodecahedron?based ZIF?67 catalytic micromotor by changing the solvent in one step[32].Owing to the higher surface area and hol?low structure of ZIF?67,the micromotors can dramati?cally adsorb the methyl blue(MB)within 5 min.
A series of derivatives can also be obtained by using MOF as a precursor,which not only retains the original porosity and morphology of the precursor,but also exhibits stronger catalytic activity[33].In this work,Mn2O3micromotors were prepared by using Mn?MOF as a precursor through high?temperature annealing.The size of Mn2O3was about 4 μm,with a microporous structure and good crystallinity.At the same time,the movement behavior of Mn?MOF derivative micromotors under different conditions and the degradation proper?ties of MB were studied.The prepared Mn2O3micromo?tors had excellent autonomous movement ability,and their moving speed can reach 81.32 μm·s-1in 10% H2O2solution.The experimental results illustrate that after adding H2O2,the Mn2O3micromotors showed a strong degradation effect on MB.These Mn2O3micro?motors using Mn?MOF as the precursor had lower mate?rial costs and are suitable for mass production.Com?pared with other external stimuli,such as light,ultra?sound,magnetic field and temperature,H2O2and the Mn2O3micromotors had a Fenton?like reaction to efficiently degrade pollutants,and H2O2can drive the micromotors to achieve autonomous movement simulta?neously.
1 Experimental
1.1 Materials
Manganese acetate tetrahydrate(Mn(CH3COO)2·4H2O),MB and ethanol were purchased from Sino?pharm Chemical Reagent Co.,Ltd.Polyvinylpyrrol?idone(PVP),1,3,5?trimellitic acid,and 2?methylimida?zole are commercially available from Aladdin Inc.Hydrogen peroxide(H2O2,30%)was purchased from Shanghai Chemical Reagent Co.,Ltd.Triton X?100 was purchased from Alfa Aesar Chemical Co.,Ltd.The ultrapure water used in the experiment was purified by the Milli?Q system.All the reagents were analytical grade and used as received without further treatment.
1.2 Synthesis of Mn?MOF derivatives
Mn?MOF was prepared according to the reported method[34].First,0.49 g of Mn(CH3COO)2·4H2O was dissolved in 100 mL of the mixture of water and etha?nol(1∶2,V/V).After it was completely dissolved,2 g of PVP was added under vigorously stirring until com?pletely dissolved.It was recorded as solution A.Then,0.9 g of 1,3,5?trimellitic acid was dissolved in the mix?ture of 80 mL of water and ethanol(1∶2,V/V).It was designated as solution B.Solution B was dropwise add?ed to solution A under vigorous stirring and it was kept with stirring for 20 min,and then,the reaction was al?lowed to stand at room temperature for 12 h.After the reaction was completed,the solution was divided into two layers including the supernatant and the underly?ing white precipitate.The solution was centrifuged and the precipitate was washed using ethanol three times.The final product was dried under vacuum at 60℃for 6 h to obtain Mn?MOF.The white powder of Mn?MOF was evenly spread on the bottom of the porcelain boat.The outer layer of the porcelain boat was covered with tin foil.The porcelain boat was placed in a muffle fur?nace.The temperature was programmed at 700℃at a heating rate of 10℃·min-1and held for 3 h at 700℃.After the end of the procedure,it was naturally cooled to room temperature to get black Mn?MOF derivatives.
1.3 Characterization
The composition of Mn?MOF and Mn?MOF deriva?tive micromotors was characterized by X?ray diffraction(XRD,BRUKER D8 Advance,CuKα,λ=0.154 001 nm,2θ=10°?80°,40 kV,40 mA)and FT?IR(Bruker model VECTOR?22 Fourier transform spectrometer).Thermogravimetric analysis(TGA)was performed in air atmosphere using a differential scanning calorime?ter(DSC214 polyma).The microscopic morphology was observed by a scanning electron microscope(SEM,Hit?achi S?4800,3 kV)and a transmission electron micro?scope(TEM,Hitachi HT7700,100 kV).Chemical bond analysis was performed by X?ray photoelectron spectroscopy(XPS,PHI 5000 Versa Probe),and UV?Vis absorption spectroscopy was performed by a UV?Vis spectrometer(PerkinElmer,Lambda 650S).Motion videos were recorded by an inverted fluorescence microscope(Ti?S/L100).The calculation and analysis of motor speed were done by NIS?Elements software.Before the motion experiment,Mn?MOF derivatives were coated with a 10 nm?gold layer by the E?1010 ion sputter.
2 Results and discussion
The morphologies of Mn?MOF and its derivative were characterized by SEM and TEM,as shown in Fig.1.From Fig.1a and 1b,we can see that the Mn?MOF precursor showed a perfectly sphere shape with a smooth surface and an average diameter was about 4 μm.After annealing in the muffle furnace from 400 to 700℃,it still maintained relatively complete spherical morphology without cracking(Fig.1c,1d;Fig.S1a?S1c in Supporting information).However,the surface of the sphere became rough after annealing,which may be due to the release of gas molecules caused by the decomposition of Mn?MOF during annealing.As pre?sented in Fig.S1d,when the annealing temperature reached 800℃,its spherical morphology was obviously broken.Video S1(Supporting information)shows that the motion performance of derivatives of Mn?MOF obtained at annealing temperatures of 700℃was better than those of the samples obtained at 400,500,and 600 ℃.Therefore,we choose the Mn?MOF deriva?tive obtained at an annealing temperature of 700℃for subsequent research.
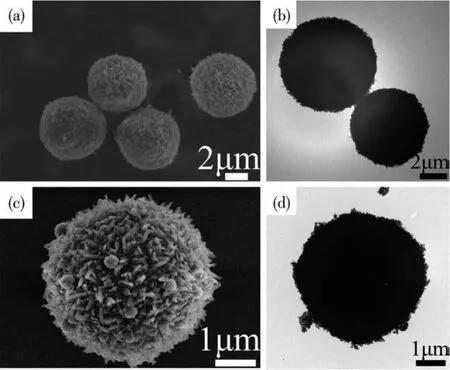
Fig.1 SEM(a,c)and TEM(b,d)images of Mn?MOF(a,b)and its derivative obtained at an annealing temperature of 700℃(c,d)
The structures of Mn?MOF and its derivative were further analyzed by XRD,as shown in Fig.2a.The Mn?MOF precursor exhibited similar characteristic peak with the reference[34].After annealing,the diffrac?tion peaks are consistent with Mn2O3completely(PDF No.41?1442,lattice constanta=0.940 9 nm),indicating the perfect crystallinity and high purity of the product.As shown in the TG curve of Mn?MOF(Fig.2b,top),the first 18.28% weight loss around 200℃is attributed to the loss of lattice water.The second weight loss around 480℃originates from the thermal decomposition of the sample,indicating that Mn?MOF has a stable crys?tal structure above 480 ℃.The TGA curve(Fig.2b,bot?tom)of Mn2O3derived from Mn?MOF showed no change within the temperature range illustrating it has good thermal stability.The XPS results show that there are two elements(Mn and O)in the sample.Fig.2c shows the high?resolution XPS spectrum of Mn2p.The peaks of Mn2p1/2and Mn2p3/2were situated at 652.4 and 641.2 eV,respectively.The peaks appearing at 640.8 and 643.9 eV correspond to Mn3+and Mn4+,respectively[35].As shown in Fig.2d,the peaks of O1sat 529.0 and 531.0 eV correspond to crystal lattice oxy?gen and the surface adsorbed oxygen species in Mn2O3,respectively.The result is well consistent with that of the XRD analysis.
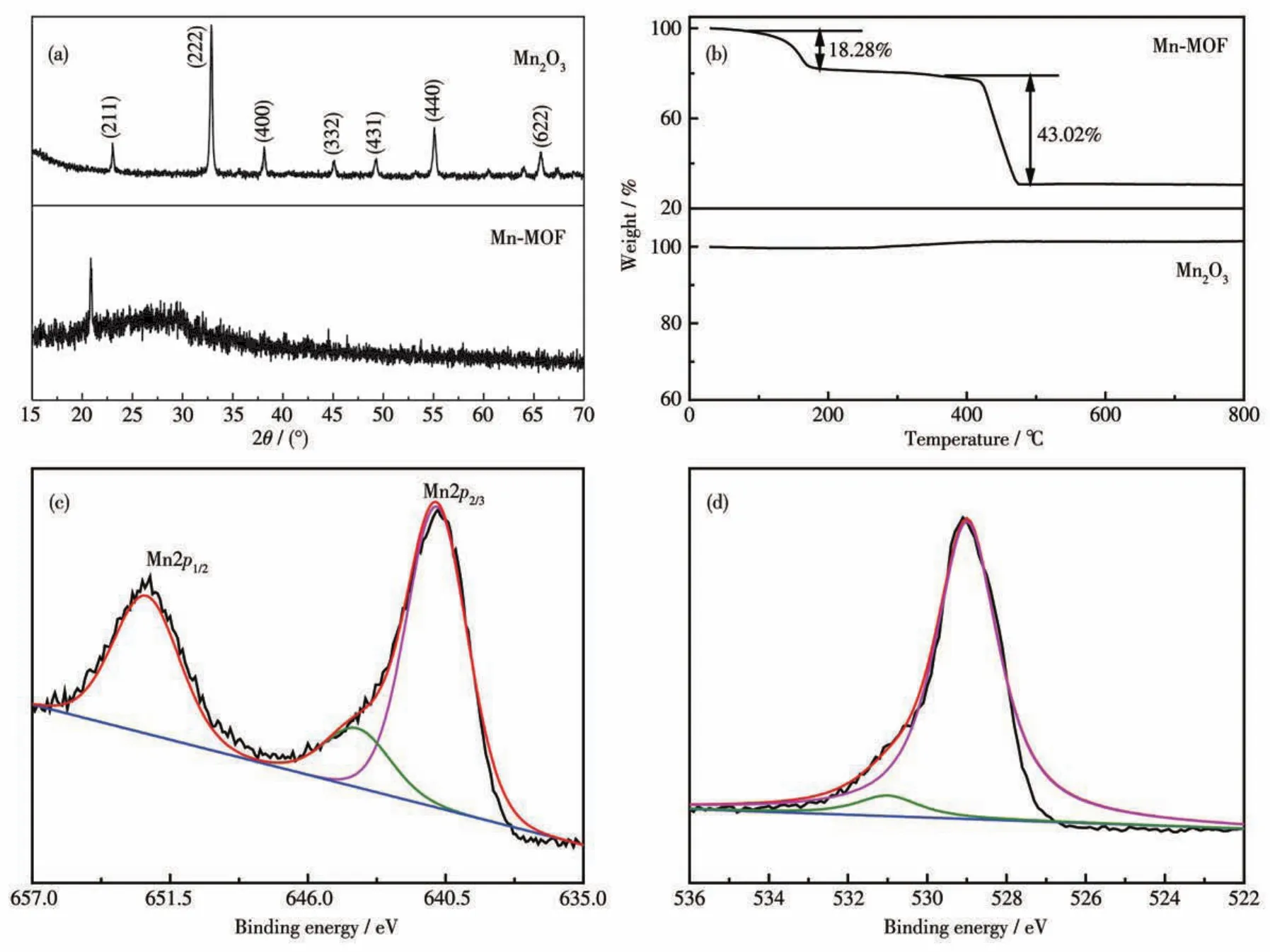
Fig.2 XRD patterns of Mn?MOF and its derivative(a);TGA curves of Mn?MOF and its derivative(b);XPS spectra of Mn2p(c)and O1s(d)of the derivative of Mn?MOF
The Mn2O3micromotors derived from Mn?MOF can move efficiently by decomposing H2O2.Fig.3a and 3b display time?lapse images taken from Video S1 in a 2 s period powered by different H2O2concentrations.These images illustrate a tail of oxygen bubbles gener?ated and released from the Mn2O3surface.Video S1 shows that the Mn2O3micromotors display autonomous locomotion with different speeds in different H2O2concentrations.The concentration of H2O2fuel strongly influences the velocity of the Mn2O3micromotors.Fig.3c shows the relationship between micromotors'speed and H2O2concentrations.When the H2O2concen?tration was increased from 1% to 10%,the speed of the micromotors was correspondingly increased from 21.71 to 81.32 μm·s-1with an average lifetime of about 30 min.
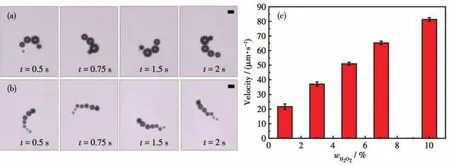
Fig.3 Time?lapse images of Mn2O3micromotors in different concentrations(a:1%,b:7%)of H2O2;Relationship between micromotors'speed and H2O2concentration(c)
In this work,MB was selected as a model pollut?ant to study the removal performance of Mn2O3micro?motors.To analyze the removal process of MB,the absorption peaks of MB in solution were measured by the UV?Vis spectrometer.The maximum absorption wavelength of MB was 598 nm.Fig.4 shows the chang?es of UV absorbance of different samples with time.As shown in curves a and b,the absorbance of MB did not change without or with H2O2,indicating that H2O2has no effect on the removal of MB.It can be seen that the absorbance of MB decreased only after adding Mn2O3micromotors(curve c),which further proves that Mn2O3has an adsorption effect on MB.As shown in curve d,after adding Mn2O3micromotors and H2O2to MB solu?tion simultaneously,the absorbance of MB was signifi?cantly reduced,which indicates that the Fenton?like system composed of Mn2O3micromotors and H2O2exhibits higher degradation efficiency for MB than the adsorption performance of pure Mn2O3.
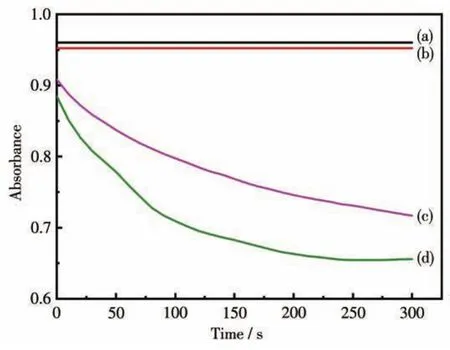
Fig.4 Changes of absorbances of different samples with time:(a)MB,(b)MB and H2O2solution,(c)MB and Mn2O3micromotors,(d)MB,Mn2O3 micromotors and H2O2solution
FT?IR measurements were performed to study the removal mechanism of MB.Fig.5 shows the FT?IR spectra of Mn2O3(a),MB(b),mixed Mn2O3and MB solution without H2O2(c)or with H2O2(d).In curve a,the characteristic absorption peaks at 612 and 529 cm-1correspond to the asymmetric and symmetric Mn—O—Mn stretching vibrations in Mn2O3,respec?tively[36].Curve b shows that the characteristic absorp?tion peaks at 1 575 and 1 496 cm-1correspond to the stretching vibrations of the C=C and C=N groups in MB,respectively.The peak located at 1 169 cm-1corre?sponds to the stretching vibration of the S=O group.Compared with pure Mn2O3(curve a),the characteristic absorption peak of MB appears additionally in curve c,which indicates that in the absence of H2O2,Mn2O3exhibits an adsorption effect on MB.However,the absorption peak of MB was not observed in curve d,which proves that in the presence of H2O2,Mn2O3micromotors exhibit degradation effect rather than adsorption to MB.
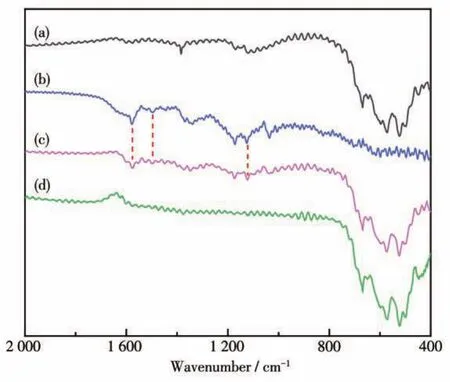
Fig.5 FT?IR spectra of Mn2O3(a),MB(b),mixed Mn2O3 and MB solution without H2O2(c)or with H2O2(d)
Based on the above discussions,the likely catalyt?ic mechanism for the activation of H2O2by Mn2O3micromotors in the degradation of organic pollutants through the Fenton?like effect is proposed as follows.Firstly,Mn3+on the surface of Mn2O3is easily reduced by H2O2to produce Mn2+,H+and HO2· under near?neutral conditions.Then,Mn2+reacts with H2O2to pro?duce Mn3+,HO-and·OH.Therefore,Mn2O3can decompose H2O2cyclically and produce a large number of reactive oxygen species(HO2·,·OH),which realizes the effect of the micromotor to remove MB[37].Moreover,after adding Mn2O3micromotors and H2O2to the MB solution simultaneously,Mn2O3contacts with H2O2in the solution to continuously generate bubbles,which not only promotes the movement of the micromo?tors to achieve mixing,but also accelerates the degra?dation process without the need of external mechanical stirring.
Fig.6 presents that the amount of Mn2O3micromo?tors and time has a direct effect on the degradation of MB.It shows that different amounts of Mn2O3micromo?tors degraded the MB sample with the same concentra?tion within 5 min.With the increase of the amount of Mn2O3micromotors,the absorbance of MB was getting lower and lower.It indicates that the degradation effi?ciency is positively correlated with the amount of Mn2O3micromotors.As shown in the right panel of Fig.6,the absorption peak of MB gradually decreased with time going,which proves that the Mn2O3micromo?tor has high degradation efficiency compared to static adsorption.
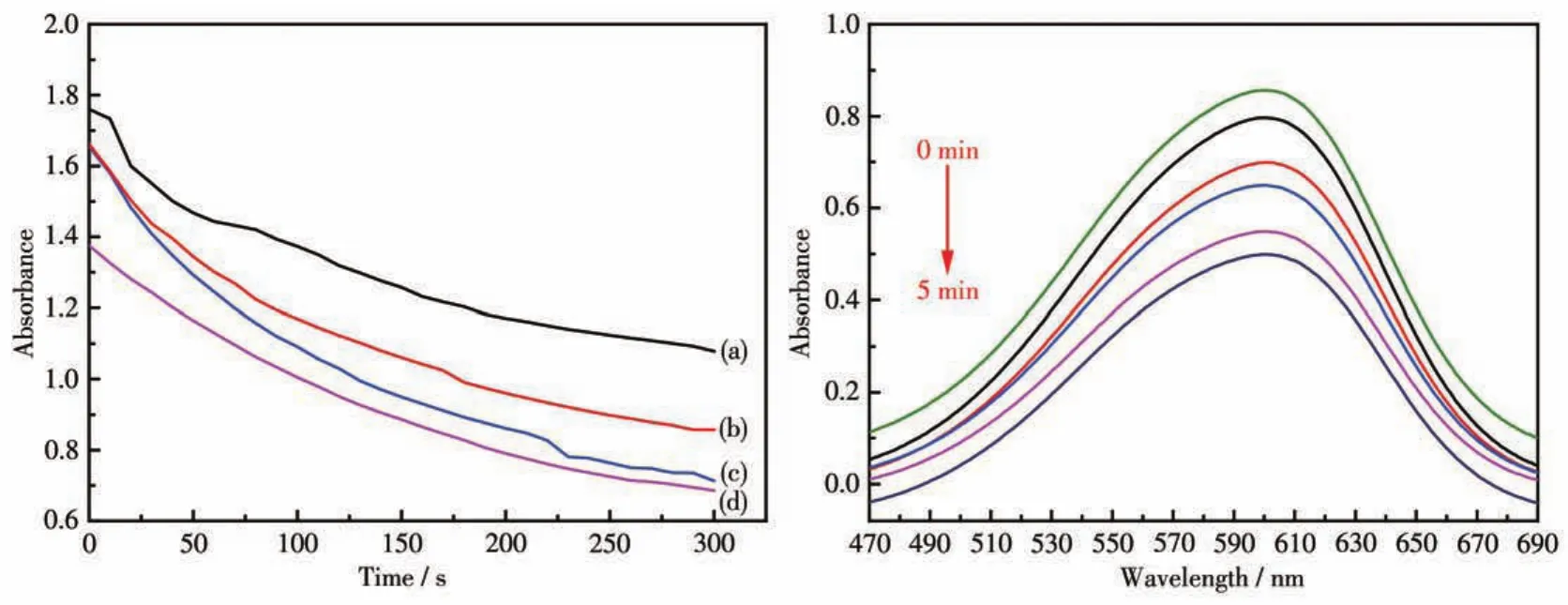
Fig.6 Effect of amount of Mn?MOF derivative micromotors(left)and effect of time(right)on degradation of MB
Harnessing excellent autonomous motion and catalytic performance of MOF derivative micromotors,they hold great potential for environmental applica?tions.Table 1 lists some specific applications of the same type of Mn2O3micromotors in environmental remediation.Compared with them,these Mn2O3micro?motors have the facile design strategy,lower material and production costs,as well as higher degradation effi?ciency for organic pollutants.
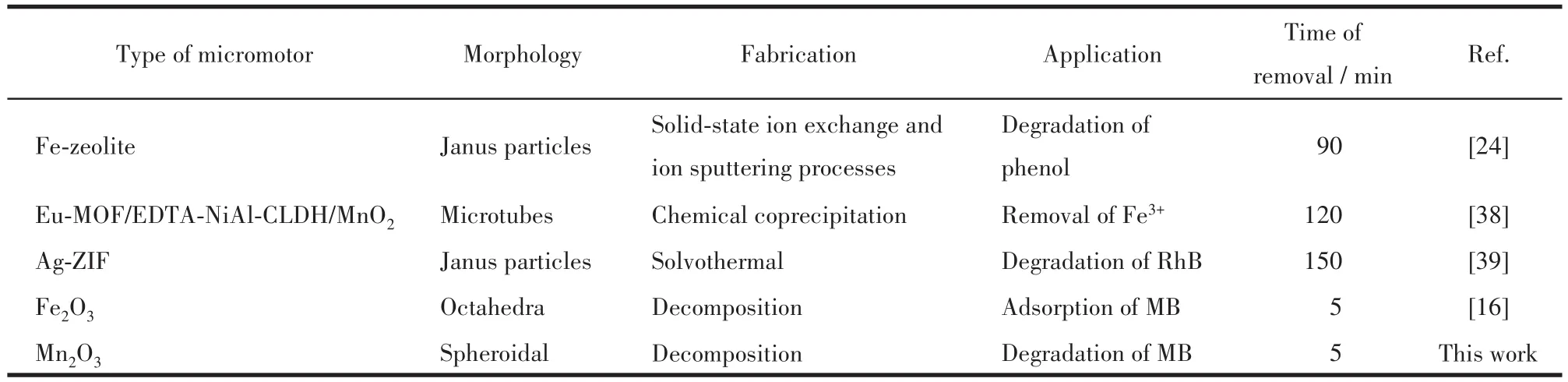
Table 1 Micro/nanomotors based on MOF derivatives in the field of environmental remediation
3 Conclusions
In conclusion,Mn?MOF derivative(Mn2O3)micro?motors were successfully synthesized by thermal decomposition of Mn?MOF.The Mn?MOF derivatives present perfect spherical morphology with rough sur?face and pore characteristics.In addition,Mn2O3micro?motors show excellent catalytic activity and efficient autonomous mobility in H2O2.With the increase of H2O2concentration,the speed of Mn2O3micromotors was increased.It can reach 81.3 μm·s-1in 10% H2O2with an average lifetime of about 30 min.At the same time,Mn2O3micromotors exhibit a Fenton?like effect in H2O2,which can efficiently degrade MB in water.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- 《無機化學學報》投稿須知(NOTICE TO AUTHORS)
- Replacement of Carboxylate Ligand Substituent on Modulation of Structures and Magnetic Properties in Salen?Type Dinuclear Dy Complexes
- Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Two Diphenyl Ether Tetracarboxylic Acid?Co Coordination Polymers
- Crystal Structures and Magnetic Properties of LnⅢ2Complexes Based on a Polydentate Schiff Base Ligand
- Synthesis of a Water?Stable Zn?Based Metal?Organic Framework for Luminescence Detecting Fe3+and 2,6?Dichloro?4?nitroaniline
- Constructing and Photocatalytic Performance of Flower?like CeO2/TiO2 Heterostructures

