Monodelphis domestica: a new source of mammalian primary neurons in vitro
Jelena Ban, Miranda Mladinic
In vitromodels have tremendously revolutionized cell biology and biomedical research, reducing the need forin vivoexperiments, as well as offering the simplified models for easier investigations at molecular and cellular level (for example genetic manipulations, electrophysiological measurements, drug screening etc.).However, the major challenge is to develop long-survivingin vitropreparations of postmitotic cells such as neurons or cardiocytes.To overcome the problem of neuronal inability to proliferate, the immortalized cell lines derived from neuronal tumors have been prepared. Such secondary neuronal cultures are restricted to neuroblastomalike cells, but their biological relevance is often questionable due to genetic drift and lack of mature, differentiated neuronal phenotypes, which makes primary cultures the better choice. Mammalian central nervous system (CNS)in vitroprimary cell cultures are mostly prepared from the late embryonic or early postnatal mice and rats.Other mammalian species have been less used, meaning that inter-species diversity is not sufficiently investigated and that the additional comparative analyses are required to avoid misinterpretations in translating the knowledge to humans (Bonfanti and Peretto,2011).
We have recently established and extensively characterized the long-term primary dissociated cortical neuronal cultures derived from the neonatal grey South American short-tailed opossums[Monodelphis (M.) domestica] that we propose as a new source of mammalian CNS cells forin vitrodevelopmental and regeneration studies (Petrovi? et al., 2021).These cultures are unique for the possibility to obtain embryonic-like CNS cells from the postnatal animals, at wide range of postnatal days. For example, the radial glia cells(RGCs), with both neurogenic and gliogenic potential, can be obtained in co-culture with neurons from the cortex of the postnatal day (P) 3–5 opossums and maintained in proliferative conditions for weeks and even monthsin vitro. RGCs cultures can be replated several times, offering a continuous source of new RGCs andin vitro-generated neurons. Alternatively, if cultured in serumfree medium, these cells differentiate into almost pure neuronal cultures (more than 90% of neurons in the first few days after plating and more than 80% of neurons during the first two weeks). Furthermore,neuronal cultures can be prepared also from P16–18 opossum cortex that, in addition to neurons, contain also around 19% of the glial fibrillary acidic protein (GFAP)-positive astrocytes and around 2% of the ionized calcium-binding adaptor molecule 1-positive microglia cells, as well. The percentage of astrocytes is comparable to rat hippocampal neuronal cultures obtained from P2–3 rat hippocampus (around 23%) (Pozzi et al., 2017). The possibility to prepare neuronal cultures from animals at several weeks of postnatal age offers an additional opportunity to investigate specific developmental events and transitions that occur during cortical neurogenesis.
The special characteristics of the opossum CNS cultures come from the fact that opossums are born at a very immature state(Nicholls et al., 1990; Mladinic et al., 2009)which makes them an excellent model forin vitrodevelopmental studies. Opossums belong to marsupials, mammalian infraclass diverged from placental mammals around 180 million years ago (Mikkelsen et al., 2007).Their short gestation of 14 days, i.e., 1 week shorter than rodents, allows fast and easy breeding. According to recent developmental transcriptome analysis (Cardoso-Moreira et al., 2019), P3–5 opossums correspond to E15.5–18.5 rat or E14–16 mice embryos,while P16–18 opossums are developmentally similar to neonatal (P1–P2) rat or mice.Their CNS development, that include both neurogenesis and gliogenesis, occurs almost completely postnatally and therefore it offers exceptionally wide time window for developmental studies. In addition, unlike the other marsupials,M. domesticalack pouch which makes pups easily accessible.The use of postnatal animals reduces the number of animals needed for studies and reduces their suffering, contributing to the 3Rs (reduce, refine, replace). Shorter gestation and embryonic-like tissue available using postnatal animals with extended postnatal development makesM. domesticaappealing and ethically improved cell source.Most importantly, opossums are unique among mammals in their ability to postnatally completely and fully functionally regenerate their spinal cord after injury(Nicholls and Saunders, 1996). This capacity persists until the P12 in the upper cervical part, and until the P17 in the lower, less mature thoracic-lumbar spinal cord segments. The postnatal spinal cord regeneration in opossums has been studiedin vivoandin vitro(Varga et al., 1996) using the intact spinal cord preparation in which the regenerative potential retains the time and space distribution asin vivo. However,both approaches do not allow easy molecular or pharmacological manipulations, unlike cell cultures, which are easier to maintain,manipulate and analyse.
Thus, using developing cortex of P3–5 opossums, we have successfully established long-term and nearly pure neuronal cultures(Petrovi? et al., 2021). These cultures recapitulatein vitromany developmental processes, remarkably similar to what has been observed with well-known rodent models, for example neurite outgrowth,neuronal growth cones (GCs) formation, axon and dendrite specification, synaptogenesis,neurotransmitter synthesis, network formation etc. (Kaech et al., 2012).
For instance, we reported for the first time the efficientin vitrogeneration of neuronal GCs derived from opossum cortex, with an average size of around 40 μm2, comparable to what is observed in the rat hippocampal GCs cultured under similar conditions (Pozzi et al., 2017).M. domestica-derived GCs are formed at the tips of the growing neurons at the first dayin vitro(DIV)1 and are identified by actin staining. It will be interesting to further investigate to what extent the cytoskeletal dynamics and expression of receptors for axon guidance molecules,growth factors or neurotrophins that occur in early mammals such as opossums correlate to what is observed in rodents.
Extensive characterization of the cell morphology, composition, differentiation as well as maturation of opossum cortical cultures was done by immunocytochemistry(Petrovi? et al., 2021). The expression of the neuron-specific β-tubulin III, was confirmed throughout the observation period (up to DIV30) with 4 different β-tubulin III antibodies, having between 90–99.8% sequence similarity between opossum protein and the immunogen of interest. High sequence similarity with rat,mouse or human proteins allows the use of commercially available and widely-used antibodies, such as TUJ1. This marker was used to quantify the percentage of neurons in cultures prepared from two postanal age groups, P3–5 and P16–18. Both cultures were composed of more than 80% of β-tubulin III-positive neurons during the first weekin vitro. However, at DIV15 the cultures derived from P16–18 cortex showed significantly lower percentage of neurons(around 55 %) and the higher proportion of non-neuronal cells. These observations were comparable to rat hippocampal cultures,in which higher number of glial cells was found in postnatal versus embryonic primary cortical cells. To further study neuronal cells in opossum cortical cultures, two additional neuronal markers were used, namely microtubule-associated protein (MAP) 2 and neuronal nuclei (NeuN, Figure 1). Triple staining indicated that the expression of NeuN starts slightly earlier at DIV1, followed by MAP2 and β-tubulin III, with great majority of neurons (more than 86% at DIV1 and more than 93% at DIV4) expressing all three neuronal markers.
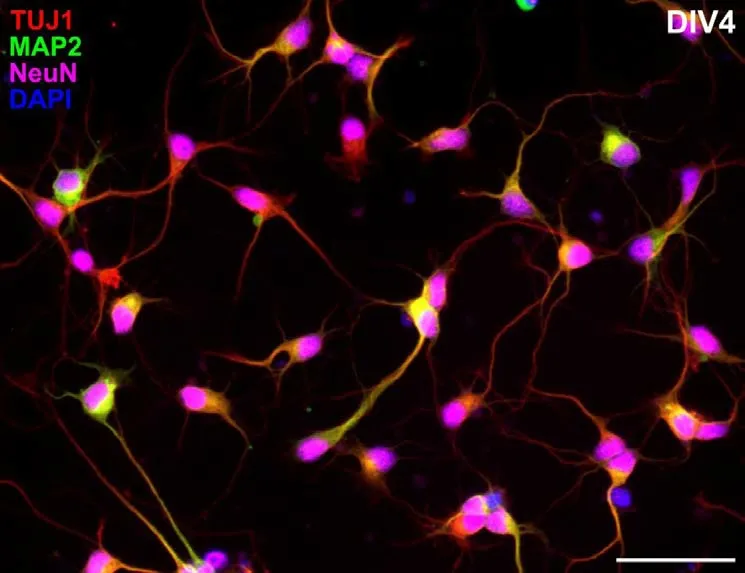
Figure 1 |Neuronal markers expression in nearly pure cortical cultures derived from P5 opossum.Cells were prepared as described in a previous study (Petrovi? et al., 2021),fixed at the fourth day in vitro and stained for β-tubulin III (TUJ1, red), microtubuleassociated protein 2 (MAP2, green), neuronal nuclei (NeuN, magenta) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (blue).More than 93% of cells at the fourth day in vitro (DIV4) are triple-positive, TUJ+/MAP2+/NeuN+. Scale bar: 50 μm. Unpublished data.
To the best of our knowledge, these are the first reports for the expression of MAP2 and NeuN neuronal markers inM. domesticaprimary dissociated cortical cultures.These observations suggest that NeuN can be reliably used as the early neuronal marker and not only as a marker for mature neurons, as it was considered for decades.In addition, we have shown for the first time the expression of synaptic (synapsin)as well as neuronal subtype (VGLUT2 and GAD65) specific markers inM. domesticaderived cortical primary cultures, with which is possible to follow neuronal differentiationin vitro, as well as the formation of the neuronal networks.
Next, since the gliogenesis starts in opossums around P18, with the morphological switch from bipolar RGCs to multipolar astrocytes(Puzzolo and Mallamaci, 2010), we analysed the expression of glial cell markers (GFAP,vimentin, ionized calcium-binding adaptor molecule 1) in opossum cortical cultures.Our findings are in agreement with the gene expression analysis done by Cardoso et al. (2019), in which developmental correspondences between different mammalian species are reported. We have identified astrocytes and their subtypes,protoplasmic-like and stellate forms, by GFAP and vimentin staining, showing that their morphology was strikingly similar to rodent primary astrocytes, indicatingM. domesticaalso as the new source of mammalian astrocytes forin vitroinvestigations. In addition, classification of astrocytes and glial cells in general is still incomplete and it would be interesting to verify if the glial subtypes in opossums resemble more rodent or human-specific glial repertoire.
Perhaps the main advantage that neonatal opossums’ cortical cultures offer is the possibility to expand RGCs and generate new neurons and gliain vitro. To do that,the cultures can be maintained in specific conditions for several weeksin vitro, similar for neuroblastoma cell lines, but with the advantage of having non-transformed,i.e., wild-type neuronal and glial precursor cells in culture, with obvious biological relevance.M. domestica-derived stem/progenitor/RGC were identified by their elongated bipolar morphology, GFAP and vimentin expression, proliferative state and by the potential to form neurospheres.Neurospheres were obtained in suspension,using non-adherent conditions, where they reached the diameters of around 100 μm and were positive for early progenitor marker paired box gene 2. When replated on adhesive substrate in serum-free conditions,they extended neurites and expressed neuronal markers MAP2 and β-tubulin III.This neurogenic potential offers virtually unlimitedin vitrosource of neurons,particularly useful for stem cell research.
Moreover, the long-term survival of the opossum cortical cultures allowed the formation of the secondary organoidlike structures after several weeksin vitro,that consisted of spherical aggregates of neuronal cell bodies from which the thick neurite bundles grew. These results open the possibility to investigate the formation of 3D-cultures combined with 3D biomaterial scaffolds that would more closely mimic the complexity of the CNSin vivo.
Thus, theM. domestica-derived primary CNS cultures (Petrovi? et al., 2021) are novel and robustin vitroplatforms to use in a variety of studies, including development and regeneration. Since theM. domesticagenome (Mikkelsen et al., 2007; Samollow,2008) show high degree of proteincoding sequence homology with placental mammals, the opossum cortical cultures represent an excellent new tool to overcome the lacuna in the diversity of the mammalian preparations available for neurobiology studies.
The present work has been conducted on equipment financed by the European Regional Development Fund (ERDF) within the project“Research Infrastructure for Campus-based Laboratories at University of Rijeka”(RC.2.2.06-0001), the Croatian Science Foundation (CSF) grant IP-2016-06-7060, the financial support from the University of Rijeka (18.12.2.1.01, 18-258-6427 and 18-290-1463) and from the International Centre for Genetic Engineering and Biotechnology(ICGEB), Grant/Award Number: CRP/CRO14-03 (to MM).
The authors declare that there is no potential conflict of interest.Editor note: MM is an Editorial Board member of
Neural Regeneration Research. She was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and their research groups.
Jelena Ban, Miranda Mladinic*Laboratory for Molecular Neurobiology,Department of Biotechnology, University of Rijeka,Rijeka, Croatia
*Correspondence to: Miranda Mladinic, PhD,mirandamp@biotech.uniri.hr.https://orcid.org/0000-0002-3985-6629(Miranda Mladinic)
Date of submission: April 7, 2021
Date of decision: April 19, 2021
Date of acceptance: June 18, 2021
Date of web publication: January 7, 2022
https://doi.org/10.4103/1673-5374.332139
How to cite this article:Ban J, Mladinic M(2022) Monodelphis domestica: a new source of mammalian primary neurons in vitro. Neural Regen Res 17(8):1726-1727.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.?Article author(s) (unless otherwise stated in the text of the article) 2022. All rights reserved.No commercial use is permitted unless otherwise expressly granted.
 中國(guó)神經(jīng)再生研究(英文版)2022年8期
中國(guó)神經(jīng)再生研究(英文版)2022年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Ocular therapies for neuronal ceroid lipofuscinoses: more than meets the eye
- Designing nanocarriers to overcome the limitations in conventional drug administration for Parkinson’s disease
- The second brain in Parkinson’s disease: fact or fantasy?
- Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target
- Construction and imaging of a neurovascular unit model
- Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases
