Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases
Fatemeh Zahedipour, Seyede Atefe Hosseini, Neil C. Henney,George E. Barreto, Amirhossein Sahebkar
Abstract Inflammatory processes and proinflammatory cytokines have a key role in the cellular processes of neurodegenerative diseases and are linked to the pathogenesis of functional and mental health disorders. Tumor necrosis factor alpha has been reported to play a major role in the central nervous system in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis and many other neurodegenerative diseases. Therefore,a potent proinflammatory/proapoptotic tumor necrosis factor alpha could be a strong candidate for targeted therapy. Plant derivatives have now become promising candidates as therapeutic agents because of their antioxidant and chemical characteristics, and anti-inflammatory features. Recently, phytochemicals including flavonoids, terpenoids,alkaloids, and lignans have generated interest as tumor necrosis factor alpha inhibitor candidates for a number of diseases involving inflammation within the nervous system. In this review, we discuss how phytochemicals as tumor necrosis factor alpha inhibitors are a therapeutic strategy targeting neurodegeneration.
Key Words: brain; central nervous system; cytokine; herbal medicine; inflammation;neurodegenerative diseases; phytochemicals; tumor necrosis factor-alpha
Introduction
Neurodegeneration is a destructive process involving the progressive loss of function or death of neurons, including those of the central nervous system (CNS). A number of well-known diseases occur as a result of central neuronal loss, including Alzheimer’s disease(AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis(ALS), but there is no effective curative treatment for any of these conditions. In many neurodegenerative disorders, causation has not yet been identified. However, inflammatory processes and proinflammatory cytokines have a key role in the initiating processes of neurodegenerative disorders and are linked to the pathogenesis of functional and mental impairment (Gitler et al., 2017).
Several groups have demonstrated the pleiotropic effects of tumor necrosis factor alpha (TNF-α) and its involvement in various physiological inflammatory processes. TNF-α has been demonstrated to play a major role in neurodegenerative CNS disorders including AD, PD and ALS and many others (Chen et al., 2016). Thus, a potent proinflammatory/proapoptotic TNF-α provides a strong candidate for targeted therapy. Unfortunately, many otherwise promising therapeutic agents are largely unable to cross the blood-brain barrier (BBB), which severely hinders their clinical use in treating neuroinflammation in the CNS.
Natural phytochemicals can, in some cases, be less toxic than novel synthetic drugs. Some of these plant derivatives have now become promising candidates as therapeutic agents because of their chemical characteristics, antioxidant and anti-inflammatory properties (Kumar and Singh, 2015). Recently, phytochemicals with TNF-α blocking properties have emerged as interesting candidates for a variety of diseases underpinned by neuroinflammation. They may be promising lead compounds for the development of other derivatives offering boosted pharmacological features (Shal et al.,2018). This review addresses the pathological and physiological functions of TNF-α together with an overview of plant extracts that can interfere with TNF-α activity and production. We also focus on the therapeutic potential and effects of phytochemicals including polyphenols, terpenoids, alkaloids and steroids as TNF-α inhibitors toward amelioration and prevention of neuroinflammation as a novel treatment strategy for neurodegenerative diseases.
Search Strategy and Selection Criteria
The articles used in this review were conducted via the online library.These databases included PubMed, ScienceDirect, Google Scholar,Web of Science and Clinical Trials for literature describing human and animal model studies of neurodegenerative diseases from 2001 to 2020. The following search terms were used to locate articles specific to this study: “TNFα and neuroinflammation”, “phytochemicals and TNFα Inhibitors”, “phytochemicals and neurodegenerative diseases”.Variations of these terms were used to ensure exhaustive search results.
An Overview of Tumor Necrosis Factor Alpha Signaling Pathways Involved in Neuroinflammation
TNF-α is a proinflammatory cytokine involved in many neuroinflammatory and neurodegenerative disorders, including AD,Huntington’s disease, multiple sclerosis (MS), PD, stroke and spinal muscular atrophy (Moriwaki et al., 2015; Siebert et al., 2015). TNF-α has a weight of 26 kDa and is synthesized as a type II transmembrane protein. It can undergo proteolytic cleavage by TNF-α converting enzyme (TACE/ADAM17) to a 17 kDa monomeric protein (Hartl et al.,2020).
TNF-α can bind to two receptors. While TNF-α receptor I is expressed under normal conditions in many types of cells and tissues, TNF-α receptor II is expressed in immune and endothelial cells at low levels.TNFR1 has a death domain in its cytoplasmic domain and can activate an apoptosis pathway via the TNF receptor-associated death domain,Fas-associated death domain, and Fas-associated death domainlike interleukin-1β-converting enzyme (also called caspase-8).Caspase-8 activation in turn results in caspase-3 activation, and induces apoptosis via degradation of several proteins. TNF receptorassociated factor TRAF2 is also used by TNF receptor-associated death domain, which activates IκBα kinase (IKK-α) via receptorinteracting protein, resulting in IκBα phosphorylation, ubiquitination,and degradation. IκBα degradation leads to nuclear factor-κB (NFκB) activation. Following the NF-κB activation, TNF induces the expression of genes involved in apoptosis. The activated TNFR1 could induce activation of apoptosis if the NF-κB pathway fails to activate(Pegoretti et al., 2018; Brás et al., 2020). Ras/mitogen-activated protein kinases (MAPK) and c-jun N-terminal kinases (JNK), and PI-3K/Akt signaling pathways (cell-survival signaling pathways) are other pathways that can be activated by TNFR1. Therefore, both apoptosis and cell survival mechanisms are triggered simultaneously by TNFR1 (Kang et al., 2021). TNFR2 can trigger MAPK, JNK, and NF-κB signaling pathways and it also activates apoptosis but the underlying mechanism is currently unknown (Shi and Sun, 2018).
The proinflammatory effects of TNFs associated with most diseases are mainly due to their ability to trigger the NF-κB signaling pathway.More than 200 genes have been recognized to be regulated by NFκB activation (Kumar et al., 2004) and therefore inhibitors of NF-κB are able to alleviate TNF-linked disorders. Conclusively, we speculate that phytochemical drugs, which in some cases may have favorable safety profiles and yet remain efficient in inhibiting both production and action of TNF-α, are good therapeutic choices in the treatment of TNF-linked neurodegenerative diseases. This is discussed in more detail below.
Neurodegenerative Diseases and Phytochemicals as Tumor Necrosis Factor Alpha Inhibitors
Proinflammatory cytokines are essential determinants in the pathophysiology of neurodegenerative diseases. Thus, the role of neuroinflammation in neurodegeneration must be fully explained. With the important role of TNF-α in mediating a wide variety of neuroinflammatory disorders, it has become an important target for many types of drug development including phytochemicals (Velmurugan et al., 2018). The TNF-α pathway and its neuroinflammatory role in the following neurodegenerative disorders are summarized in Figure 1.
AD and TNF-α inhibitors
There are three major neuropathological hallmarks of AD have been fully characterized: (i) the extracellular accumulation of amyloid beta(Aβ) oligomers and other materials into dense senile plaques; (ii)intracellular tau-containing neurofibrillary tangles in the brain; and (iii)chronic CNS inflammation (Hosseini et al., 2018). In AD development and related dementia, cytokine-mediated neuroinflammation plays a major role. TNF-α inhibits learning as a process critical for memory by dampening long term potentiation (Donzis and Tronson, 2014). Aβ peptides are particularly neurotoxic as key components of amyloid plaques. Aβ plaques in the brain activate macrophages which in turn lose their ability to phagocytose and remove this protein and the subsequent release of cytokines results in chronic inflammation,with TNF-α being the main culprit in this downward spiral leading to neuronal damage (Shamim and Laskowski, 2017). Dysregulation of the kynurenine pathway, mainly associated with an overexpression of indoleamine dioxygenase (IDO), has been implicated in the pathogenesis of neuroinflammatory and neurodegenerative disorders, such as MS and AD. It has been shownin vitrothat primary human microglia and, in turn, IDO expression are activated by Aβ and that kynurenine pathway overactivation and IDO overexpression are associated with the pathogenesis of AD. Microglia are also activated by other proinflammatory cytokines, such as TNF-α, and at raised concentrations in the brain these have an additive effect further contributing to Aβ-activation of microglia and expression of IDO(Rodríguez-Gómez et al., 2020).
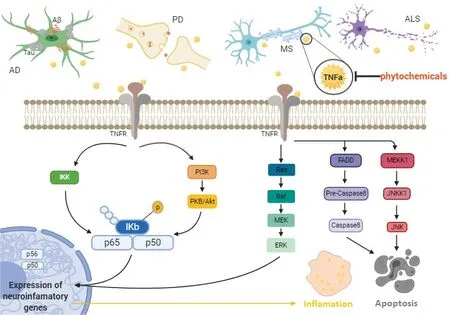
Figure 1 |The role of the TNF-α pathway in neuroinflammation and neurodegeneration in vivo and in vitro.AD: Alzheimer’s disease; ALS: amyotrophic lateral sclerosis; Aβ: amyloid-β peptide; ERK: extracellular signal-regulated kinase; FADD: fas-associated death domain protein; IKK: IκB kinase; JNK: c-Jun N-terminal kinases; JNKK1: c-Jun N-terminal kinase kinase 1; MEK: mitogen-activated protein kinase kinase;MEKK1: mitogen-activated protein kinase kinase kinase 1; MS: multiple sclerosis; PI3K: phosphoinositide 3-kinase; PKB: protein kinase B; TNFα: tumor necrosis factor alpha; TNFR: tumor necrosis factor alpha receptor.
TNF-α also upregulates vascular cell adhesion molecule-1 in the endothelium, allowing the immune cells to move and adhere to regions under attack (Kinney et al., 2018). Therefore, vascular cell adhesion molecule-1 is also considered to be a putative drug target.Among the neuroinflammatory cytokines, TNF-α is probably the most studied since it has an essential role in the cytokine cascade during the inflammatory response. TNF-α is a major proinflammatory cytokine that is upregulated in patients with AD (Wang et al., 2015b).Although TNF-α in the peripheral and central nervous systems of healthy adults is maintained at very low basal levels (Perry et al.,2001), there is an overexpression of TNF-α in AD patient brains, as observed by elevated levels of TNF-α in the CSF and blood plasma of AD patients (McCaulley and Grush, 2015). Further evidence is derived from multiple clinical and animal studies demonstrating the correlation between high TNF-α level and beta amyloid and tau protein expression in AD (Kinney et al., 2018).
Elevated TNF-α levels in AD appear to be associated with increased neuronal loss and dysfunction, enhanced Aβ production, decreased Aβ clearance, loss of synaptic function, all of which are related to the cognitive decline characteristic of AD (Chang et al., 2017). It has been shown that TNF-α may drive the neuroinflammatory process by potentiating the astroglial response. TNF-α, along with interleukin-1 and interferon-γ, can induce amyloid precursor protein cleavage via activation of the mitogen-activated protein kinase pathway. It is also capable of stimulating the NF-κB signaling that in turn leads to increased production of Aβ (Ekert et al., 2018).
As a TNF-α target, brain nerve growth factor (NGF) is an important neurotrophic factor crucial to the development and maintenance of brain cholinergic neurons. It appears there is an inverse relationship between TNF-α expression and brain NGF since elevated TNF-α is associated with reduced hippocampal NGF expression (Golan et al.,2004). TNF-α may have a role in regulating synaptic transmission and plasticity and therefore also Aβ-induced synaptic dysfunction.Moreover, TNF-α is associated with Aβ generation by regulating β-secretase 1 activity. The intensity of AD symptoms is correlated with the serum level of TNF-α in AD patients (Fu et al., 2018). In this regard, the overexpression of TNFR1 in the hippocampal tissue of mouse was sufficient to activate neuronal apoptosis prompted by NFκB and Aβ. In addition, the upregulation of β-secretase production and increased γ-secretase activity by TNF-α may result in increased Aβ (Alasmari et al., 2018). This suggests that cytokines might be suitable targets for new AD interventions. Because of its key role in inflammation, TNF-α is an attractive pharmacological target and experimental findings have insinuated that increased TNF-α levels exacerbate amyloidogenesis. Hence, different approaches have been harnessed to reduce TNF-α signaling in rodent models of AD, showing significant reductions in AD-like brain pathology accompanied by an amelioration of cognitive dysfunction (Fu et al., 2018).
The FDA-approved biological tumor necrosis factor inhibitors may provide potential treatment opportunities for AD (Boado et al.,2010); however, a key problem with TNF-α inhibition via monoclonals or synthetic TNF receptors is the large size of the molecules which are as a result unable to cross the blood brain barrier (McCaulley and Grush, 2015). Currently, U.S. FDA-approved drugs for AD are limited to acetylcholinesterase inhibitors and the N-methyl-Daspartate receptor antagonist memantine (Marotta et al., 2020).However, because of the potential for adverse effects and prescribing restrictions related to efficacy data, these drugs are prescribed only in limited circumstances (Kumar and Singh, 2015). These are effectively types of palliative care, attempting to decelerate the development of further cognitive symptoms and maintain a functional quality of life for longer. There remains no curative option for AD despite enormous effort to identify various disease-modifying therapies and to discover drugs that target molecular pathways to block AD progression (Kurz and Perneczky, 2011).
One further possibility in the pipeline is the development of a successful vaccine against AD. A large number of attempts have been made to produce effective vaccines against amyloidosis and tauopathy over the past two decades (there have been 140 promising strategies against Aβ deposition and 25 against Tau reported in the literature), but despite big investment in experimental models and/or in clinical trials none of these has successfully achieved FDA approval (Cacabelos, 2020). Lack of effective therapies has prompted attempts to screen natural compounds that could serve as pharmaceutical leads for drug development purposes (Naoi et al., 2019). Therefore, pharmacologically active natural components with anti-neuroinflammatory potential may be good candidates for the development of therapeutic options in AD. A number of preclinical and clinical trials have been completed on nutritional and botanical agents. Analysis of phytochemicals as anti-inflammatory and neuroprotective agents, such as phenolic derivatives, terpenoids,alkaloids, steroidal saponins, and glycosides, shows therapeutic potential (Shal et al., 2018). Importantly, a number of identified alkaloids, flavonoids, and terpenes can cross the BBB and are shown to have high bioavailability and good affinity to relevant receptors in the brain known to be important therapeutic targets in AD (Prasanna and Upadhyay, 2021).
MS and TNF-α inhibitors
MS is a chronic demyelinating CNS disease, characterized by axonal injury, a weakened BBB and disrupted nerve signaling (Filippi et al., 2018). The demyelination is probably due to the death of myelin-forming protective neuroglia, which can be caused in part by inflammation and immune reactions. Overall, inflammatory demyelination is the main cause of both symptoms and progression of MS (Bando, 2015).
A number of studies have investigated the role of TNF-α in MS.Proinflammatory cytokines such as TNF-α and interleukins (IL-17, IL-22, IL-23) appear to promote the development of MS and increased TNF-α concentrations have been found in MS lesions, whereby in patients with active MS, levels of TNF-α correlate with damage to the blood-brain barrier (Wang et al., 2018c). Also, peripherally increased concentrations of TNF-α have been associated with CNS synaptic instability, and may contribute to impairments of cognitive and sensory function (Dong et al., 2015). B cells also contribute to the pathophysiology of MS by secreting inflammatory cytokines including IL-6, IL-12, and TNF-α (Arneth, 2019).
Current treatments for MS may successfully suppress the immune system thus alleviating some of the symptoms; but, the drugs used have various unpleasant adverse effects, are prone to therapeutic failure and severe toxicity, and tend to be high-cost (Qureshi et al.,2018). Presumably partly because of this there has been a strong increase in the use of complementary and alternative medicine in MS patients, with between 33 and 80% of patients trialing various complementary and alternative medicine options, among which herbal remedies are the most common (Moawad et al., 2015). At the same time, evidence of the effectiveness of herbal medicines in MS is growing. Curcumin is one of the common plant-derived drugs in use. Curcumin may be neuroprotective in MS through multiple mechanisms. For example, Qureshi et al. showed that curcumin suppressed neuroinflammation by inhibiting cyclooxygenase-2(COX-2), lipoxygenase, phospholipases, NF-κB, and activated protein-1. Curcumin also suppressed the production of TNF-α, IL-1β,macrophage inflammatory protein 1β, monocyte chemoattractant protein (MCP-1), and IL-8 in microglial and astrocytes cells (Qureshi et al., 2018). From a clinical perspective, patients with MS were enrolled in a study to examine the potential role of medicinal plant extracts or derivatives, such as a green tea, in the improvement of impairment associated with MS. It was shown that the effects of this extract on inflammation is associated with the suppression of key inflammatory cytokines, such as IL-1β, interferon γ, TNF-α, and inducible nitric oxide synthase (iNOS) (Farzaei et al., 2017).
PD and TNF-α inhibitors
PD is ranked as the second most common chronic neurodegenerative disease, affecting approximately 6 million people around the world with the characteristic and slowly physically disabling symptoms of resting tremor, rigidity, bradykinesia and postural instability, along with the less visible but equally disabling autonomic dysfunction,depression, anxiety, cognitive dysfunction, and sleep disturbance(Gopalakrishna and Alexander, 2015). The prevalence of PD is between 1% and 4% over 60 years of age. Motor symptoms appear with progressive damage of nigrostriatal dopaminergic neurons(Bassani et al., 2015; Moon and Paek, 2015). Lewy bodies are hallmark lesions of PD resulting from the cytoplasmic accumulation of misfolded α-synuclein and ubiquitin protein inclusions in neurons.This phenomenon results in neuronal death via necrosis and/or apoptosis (Shahpiri et al., 2016). Neuroinflammation also plays an major role in PD, adding to the degeneration and death of dopaminergic neurons in the substantia nigra (Hirsch and Hunot,2009).
The proinflammatory cytokines TNF-α and IL-1β are increased in PD patients (Chan et al., 2021) and TNF-α concentrations are markedly augmented in the CSF and the brain of PD patients. TNFR1 is also increased in the substantia nigra (Mogi et al., 1994). Recent research illustrates that TNF-α and its receptors have similar roles in PD as previously described for AD. Therefore, pharmaceuticals with anti-TNF-α activities in PD could be effective in other neurodegenerative diseases. Although dopamine replacement therapy is the main effective treatment for PD, long-term utilization of this compound results in the accumulation of toxic metabolites and ROS. This, along with changes in dosing needs associated with disease progression,means such medications are only effective for around 10 years.Besides, these drugs are unable to reduce PD progression. Recently,studies showed considerable efforts to identify novel therapeutic options such as plant-derived compounds, especially polyphenols,with neuroprotective potential for the treatment of PD (de Andrade Teles et al., 2018; Carrera and Cacabelos, 2019).
Amyotrophic lateral sclerosis and TNF-α inhibitors
ALS is a complex, multifactorial, multisystem condition involving inflammation and an immune response in the neuromuscular,glial, and peripheral immune systems as part of the progression and development of the disease. A common feature of ALS is neuroinflammation, which can be detected in both the nervous system and in peripheral biological fluids (Vu and Bowser, 2017).Alterations of TNF-α concentration are common in nerve tissue,cerebrospinal fluid and blood in both ALS patients and animal models. TNF-α is key in governing mechanisms of ALS. However, the cell type and the receptors involved determine its role in influencing the pathophysiology either positively or negatively through all stages of ALS (Tortarolo et al., 2017) and there is a correlation between TNF-α activity and ALS (Guidotti et al., 2021).
Despite the poor prognosis it brings, there are limited drug options for treating ALS. Drugs such as riluzole may increase survival in ALS patients by about 2–3 months by delaying the need for tracheostomy and artificial ventilation due to its effects on slowing muscle atrophy and weakness, wasting, dysarthria, muscle spasticity, dysphagia,and improving overall patient quality of life (Andrews et al., 2020).But the limitations of the therapeutic options have driven attention towards alternatives such as natural products that could be used in novel therapeutic strategies to manage the symptoms of ALS.Generally, studies have shown that natural products may have useful effects on ALS patients, with few side effects and multiple receptor targets. Some natural antioxidant substances, which may have useful properties to be exploited in treating ALS, are resveratrol,Ginkgo biloba, ginseng, genistein, and epigallocatechin gallate (Nabavi et al.,2015).
Other diseases
Ischemic stroke often occurs when atherosclerotic and thrombotic processes block the arteries that carry blood to the brain (Anrather and Iadecola, 2016). Secondary brain damage happens when an uncontrolled neuroinflammatory cascade is triggered following ischemic and traumatic injury (Nakka et al., 2016), mainly due to the release of proinflammatory cytokines such as TNF-α.
TNF-α is present in every stage of neuronal damage in stroke such as inflammatory and prothrombotic events. TNF-α acts as a chemotactic agent toward leukocytes and induces production of intercellular adhesion molecule, vascular cell adhesion molecule, monocyte adhesion protein and E-selectin. Additionally, TNF-α exerts a key function in thrombogenesis by increasing plasminogen activating inhibitor-1 tissue factor and also platelet-activating factor, while suppressing activity of tissue plasminogen activator. Resveratrol is a polyphenolic phytochemical that appears to prevent TNF-α signaling via its action on NF-κB; this was shown following lipopolysaccharide(LPS) exposure in N9 microglial cells which were in co-culture with rat primary microglia (Bi et al., 2005; Bureau et al., 2008). Resveratrol treatment was also demonstrated to suppress TNF-α and IL-1β in adult mice following ischemic stroke (Shin et al., 2010). Resveratrol also prevented attenuation of NF-κB signaling associated with p65 nuclear translocation, and inhibited transcription of TNF-α, IL-1β, IL-6, and matrix metalloproteinase-9 (Shao et al., 2014; Lopez et al.,2015).
Another disease in which TNF-α plays a role is epilepsy. Epileptic seizures have been reported to cause neurodegenerative changes that may eventually lead to memory loss (Kamali et al., 2020).Neuroinflammation is a major factor that contributes significantly to the pathophysiology of epilepsy. Animal studies have shown that epileptic seizures activate CNS TNF-α expression (Kama?ak et al.,2020). Kaur et al. (2015) showed that curcumin supplementation to the convulsant drug pentylenetetrazole administered to Wistar rats resulted in decreased levels of mRNA and protein of IL-6, TNF-α, IL-1β and MCP-1.
Phytochemicals as Tumor Necrosis Factor Alpha Inhibitors
Beyond conventional medicine, there is evidence showing phytochemicals and traditional herbs can help preserve neurons and delay or slow development of neurodegenerative disorders. The anti-inflammatory, anti-oxidative, and anti-amyloidogenic characteristics of these compounds permit recovery through targeting different pathological causes of these diseases. Furthermore, neurotrophic factors, apoptotic factors, free radical scavenging processes and mitochondrial stress are modulated by a number of phytochemicals(Wang et al., 2018a).
Uncontrolled microglia activation in neurodegenerative disease generates neuronal damage due to the overproduction of TNF-α and nitric oxide (NO), leading to oxidative stress induction and apoptosis(Islam, 2017). With due attention to the role of TNF-α and targeting this as part of a suitable strategy for the treatment of ND, the identification of novel potent drugs from nature for prevention and/or treatment of neurodegenerative diseases is becoming a hot topic(Di Paolo et al., 2019). Many of the conventional drugs inhibiting TNF-α cause extremely unpleasant adverse effects (Kunnumakkara et al., 2019), and therefore it is extremely desirable to develop safer,less toxic anti-TNF-α drugs. An extensive source of TNF-α inhibitors is herbal compounds and already many herbal extracts and derivatives,belonging to different chemical classes, have been identified to block molecules upstream of TNF-α expression (Iqbal et al., 2013).Furthermore, lipophilic flavonoids, alkaloids, and terpenes can cross the BBB and have high bioavailability (Naoi et al., 2019), conferring a clear advantage and boosting clinical potential.
Polyphenols
Polyphenols are present in many plants and consist chemically of aromatic rings with multiple hydroxyl groups. These are subcategorized into flavonoids, flavones, flavanones, isoflavones,anthocyanins, catechins, phenolic acids (such as gallic and ferulic acid), stilbenes (such as resveratrol), curcumin, phytoestrogens,astaxanthin, diferoxymethane and tannins (Abbas et al., 2017).
ROS-scavenging enzymes such as catalase and superoxide dismutase are upregulated by polyphenols (Naoi et al., 2019). They also suppress macrophages by reducing COX-2 iNOS, and inhibit expression of IL-1β, TNF-α and IL-6 (González et al., 2011). Several polyphenols exert their influence on the balance between the development of pro and antiinflammatory cytokines, such as catechins and quercetin, such that they increase the release of IL-10 while inhibiting TNF-α and IL-1β (Yahfoufi et al., 2018). By modulating MAPK pathway at different levels of the signaling pathway, polyphenols can inhibit TNF-α release. Luteolin (a flavonoid polyphenol) reduces the release of TNF-α by LPS-activated macrophages, which blocks ERK1/2 and p38 phosphorylation (Xagorari et al., 2002).
Resveratrol
Resveratrol is a stilbenoid compound found in peanuts, grapes, tea and wine. It is named a miracle molecule, because of its antioxidant and anti-inflammatory effects with regards to neurodegenerative disorders (Meng et al., 2021). Following resveratrol treatment,various cognitive deficiencies induced in rat models were reversed by inhibiting TNF-α and IL-1 concentrations and increasing hippocampus brain-derived neurotrophic factor (BDNF) levels. Another study also showed resveratrol increased IL-10 concentration, promoting antiinflammatory effects by inhibiting NF-κB and TNF-α levels (Ma et al.,2014). In yet another study, resveratrol suppressed the expression of the proinflammatory mediator TNF-α along with NF-κB while promoting the anti-inflammatory molecule IL-10 in microglial cells(Song et al., 2014).
The faithful creature trotted20 off, and soon returned with a table-napkin full of the most delicious food, and the napkin itself was embroidered21 with a kingly crown
Tannins
Tannins are a varied family of phytochemicals which may have a number of beneficial effects on health. Tannins are present in vegetables and fruits and are commonly consumed as part of the daily diet. The key neuroprotective effects of tannins stem from their ability to scavenge free radicals and activate the body’s endogenous antioxidant pathways conferring some protection against neurotoxins,neural inflammation and oxidative-stress-induced neuronal damage(Hussain et al., 2019).
Hydrolysable tannins (HTs) are found in terrestrial plants such as bananas and grapes. This is an important group with these tannins containing polyhydric alcohol within their core structure. A diverse class of HTs are ellagitannins from pomegranate fruit, which have shown some neuroprotection against PD. Ellagitannins act as a blocker of inflammatory mediators such as proinflammatory cytokines including TNF-α and IL-6 and tumor necrosis factor receptor 6 (TRAF-6). Thus, it appears to help improve the pathogenesis of PD by inhibiting neuroinflammation (Garcia et al., 2020). We summarize the CNS anti-inflammatory effects and presumed mechanisms of a number of polyphenols in Table 1.
Flavonoids
Flavonoids are the largest groups of polyphenols with > 6000 compounds identified. ROS production is prevented by the flavonoids apigenin and kaempferol inhibiting xanthine oxidase which produce ROS. Other flavonoids including quercetin, baicalein, epigallocatechin gallate and myricetin and alsonon-flavonoids including gallic and protocatechuic acids have been reported to chelate iron and copper ions, this reducing the production of free radicals (Naoi et al., 2019).There is a close relationship between EGCG and inflammatory neurodegenerative diseases. Inflammation may be inhibited by reducing the expression of the inflammatory molecules IL1, TNF and TGB. However, studies have shown than when high concentrations of EGCG were administered, conflicting findings were observed. EGCG increased the expression of inflammatory TNF and IL6 at higher concentrations (Li et al., 2004; Wei et al., 2016).
Finally, the flavonoid curcumin, lycopene (found in tomatoes)and stilbenoids inhibit the proinflammatory NF-κB and activated protein-1, this suppressing the synthesis of IL-6, TNF-α, MCP-1 and iNOS (Spagnuolo et al., 2018).
Curcumin
Curcumin is a major components of turmeric and has different pharmacological properties such as anti-inflammatory effects that are relevant to the treatment of various diseases (Iranshahi et al.,2010; Sahebkar, 2010; Ghandadi and Sahebkar, 2017; Hewlings and Kalman, 2017; Teymouri et al., 2017; Bianconi et al., 2018; Panahi et al., 2018a, b; Bashang and Tamma, 2020; Ghasemi et al., 2019;Mollazadeh et al., 2019; Bagheri et al., 2020). Curcumin, by inhibiting NF-κB, was found to have high affinity for amyloid plaques thus potentially reducing the pathogenesis of AD (Reddy et al., 2018).Curcumin may also defend against PD through destabilization of α-synuclein protein (Liu et al., 2014). In addition, curcumin has been shown to alleviate neurodegenerative disorders by disrupting amyloid plaques, scavenging ROS, and exhibiting anti-apoptotic and anti-inflammatory effects (Darvesh et al., 2012). As acting on neurons, curcumin was reported to boost regenerating neurons by activating Trk/PI3K signaling pathways, which in turn elevated BDNF levels in a PD model. It may reduce caspase and TNF-α levels while simultaneously elevating BDNF concentration (Liu et al., 2014).

Table 1 |A summary of polyphenols as inhibitors of TNF-α and neuroinflammatory responses in neurodegenerative diseases
Nowadays, research is focused on finding more lipophilic derivatives of to facilitate crossing of the BBB and greater affinity for amyloid plaques (Lee et al., 2019). A recent study showed that curcumin decreased amyloid plaque formation bothin vitroandin vivoin a rodent model (Wang et al., 2018b). Also, in another study, curcumin appeared to decrease expression of IL-6 and TNF-α (Rogers and Lue,2001). Furthermore, in the JNK pathway, TNF-α, PGE2, IL-1β, iNOS,caspase, Bax, and cytochrome C inhibit Bcl2 and Bcl-xL so induce apoptosis in neurodegenerative disorders. Curcumin inhibited the JNK pathway in MPP- and MPTP-induced neurotoxicity in animal models (Yu et al., 2010).
Terpenoids
The largest family of natural compounds is the terpenoids (terpenes)and this group is particularly biologically active having antibiotic,antiparasitic, antitumor and anti-inflammatory properties (Nguyen Ngoc et al., 2019; Tu et al., 2020). This group of compounds has been evaluated for its ability to suppress numerous inflammatory cytokines and appears to inhibit production of IL-1β, NO and TNF-α by LPS-stimulated macrophages and microglia cells (Iqbal et al.,2013). For example extract of red ginseng reduces Aβ42-induced toxicity by suppressing TNF-α, IL-1β, NF-kB, and iNOS in BV-2 cells(Joo et al., 2008). Indeed, pretreatment of mouse macrophages with cyano-3,12 dioxooleana-1,9 dien-28-imidazolide (CDDO-Im) inhibits the phosphorylation of intrinsic bound inhibitor of NF-kB (IkBα) by IKK in response to TNF-α. This IkBα phosphorylation is the final step in NF-kB activation (Wardyn et al., 2015). A summary of terpenoids actions on various CNS diseases is displayed in Table 2.
Alkaloids
Similar to terpenoids, plant alkaloids are the second largest group of secondary plant metabolites. “Alkaloid” is a term often used to describe cyclic compounds containing one or more basic nitrogens. At low pH they are water soluble, but at high pH in the neutral form they are in the lipohilic. This property means they can pass readily through cell and tissue membranes, making them ideal drug candidates (Zou et al., 2017) .
Huperzine A is a naturally occurring sesquiterpene alkaloid found as a potent reversible acetylcholinesterase inhibitor in the extracts ofHuperzia serrata(Li and Shi, 2019). Huperzine A reduces cognitive defects in streptozotocin-induced diabetic rats by increasing the levels of BDNF, ChAT, SOD, catalase, and glutathione peroxidase while also inhibiting acetylcholinesterase, TNF-α, NF-κB, IL-1β, IL-6, CAT, MDA, and caspase-3 (Mao et al., 2014). In a similar way, the isoquinoline alkaloid berberine is the major component ofCoptis chinensis. Its pharmacological effects include cholinergic-induced NGF, activity-mediated neurite outgrowth, BDNF secretion, and the inhibition of TNF-α, IL-1β, NF-κB, Cox2, and iNOS (Jia et al., 2012)(Table 3).
Lignans
Lignans are a group of plant metabolites frequently found in their free form in roots, stems, rhizomes, seeds, leaves and fruits and they may also be present as glycosides. Lignans have complex pharmacological profiles and share anti-inflammatory, antitumor,antioxidant, cardiovascular, immunosuppressive and antiviral properties (Barker, 2019). In previous studies, these compounds were found to inhibit the induction of TNF-α and NO synthesis in LPS-activated RAW 264.7 cells (Iqbal et al., 2013). In addition, lignans appear to suppress TNF-induced NF-kB activation by inhibiting IKKs(Tse et al., 2005) (Table 4). Like the flavonoids, lignans also have pharmacological activity in the μM range.
Phytochemicals for Neurodegenerative Diseases in Clinical Trials
Because of the many beneficial therapeutic effects observedin vitroandin vivo, recently, several phytochemicals aimed at treating neurodegenerative disorders have entered into phases of clinical trial - many of these are listed in Table 5. It has also been reported in a number of studies that TNF-α release in human whole blood and serum is blocked by some phytochemicals. Quercetin, a member of the flavonoid family, exerts immunomodulatory effects that might be useful in treating MS. In a study on peripheral blood mononuclear cells (PBMC) in 23 MS patients, quercetin led to a dose-dependent reduction in IL-1β and TNF-α (5–50 μM) (Sternberg et al., 2008). Luteolin, another flavonoid, has also been shown to have an immunomodulatory role that could be applicable in treating MS and other neurodegenerative diseases. In an experiment on PBMCs isolated from 14 relapsing-remitting MS patients, luteolin dose-dependently (0.2–50 μM) decreased PBMC proliferation and modulated concentrations of IL-1β and TNF-α in the culture supernatants released by PBMCs (Sternberg et al., 2009). The dietary phytosterol β-sitosterol has been shown to reduce TNF-α release by PBMCs of 11 MS patients at 4 μM. It also reduced the IL-5, IL-10, and IL-12 levels (Desai et al., 2009). Lemon verbena extracts contain the phenylpropanoid glycoside verbascoside, which has been reported to have antioxidant/anti-inflammatory effects in a number of pre-clinical and clinical trials. Mauriz et al. (2015) showed that verbascoside treatment (600 mg/day for 28 days) in 30 MS patients reduced serum concentrations of C reactive protein and inflammatory cytokines including IFN-γ, IL-4, IL-6, IL-10, IL-12, IL-23, TNF-α and TGF-β (Mauriz et al., 2015).
Conclusions
In this review, we have discussed a number of phytochemicals that could potentially mitigate neuroinflammation caused by TNF-α in neurodegenerative diseases. These agents may prevent neuronal damage caused by the proinflammatory activity of TNF-α.
The exact pathophysiology of many neurodegenerative diseases is still not fully understood, partly because of the immense complexity of the human CNS, and also because good understanding of these complicated interactions requires large quantities of good quality data to be collected at all levels of experimental and clinical research.Determining the exact molecular mechanism of pathological subsystems of the CNS would clearly contribute to the agenda of developing appropriate targeted treatments.
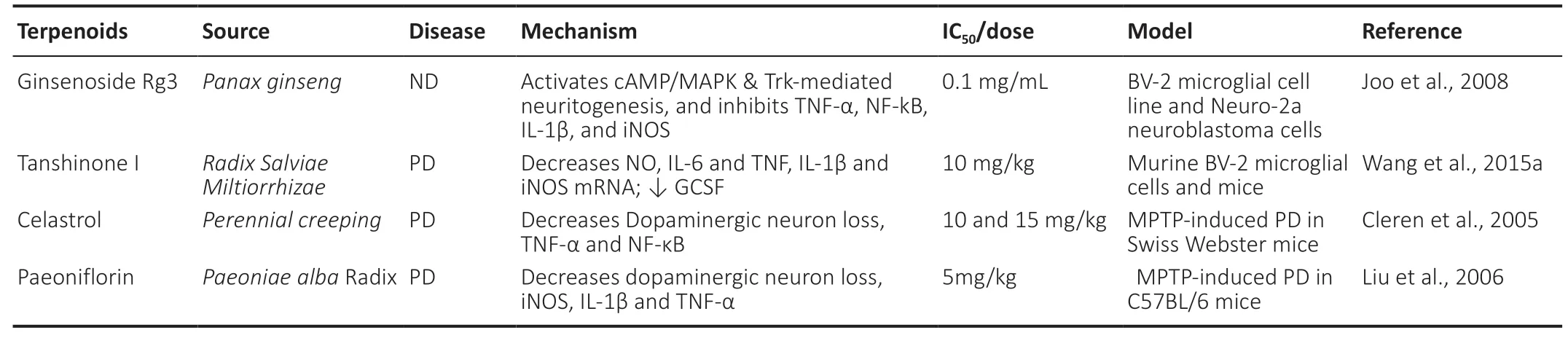
Table 2 |Terpenoids as inhibitors of TNF-α and neuroinflammatory responses in neurodegenerative diseases

Table 3 |Alkaloids as inhibitors of TNF-α and neuroinflammatory responses in neurodegenerative diseases

Table 4 |Lignans as inhibitors of TNF-α and neuroinflammatory responses in neurodegenerative diseases

Table 5 |Phytochemicals for neurodegenerative diseases in clinical trials
The enormous diversity of plants offers plenty of untapped potential for identifying and developing novel drugs. Many natural agents such as terpenoids, alkaloids, flavonoids and lignans possess anti-TNF-α activityin vitroby suppressing upstream signaling at low micromolar concentrations comparable to conventional medicines such as adalimumab, infliximab and etanercept. But one of the key problems is that many phytochemicals, including curcumin, have poor CNS bioavailability as a result of digestion, liver enzyme degradation,poor absorption across intestinal membranes and across the BBB,and active CNS efflux mechanisms. In an attempt to address this problem, various drug delivery strategies may hold the key to better bioavailability through the use of liposomes, nanoparticles,the formation of conjugation molecules and complexes with phospholipids and amphiphilic polymers. Some success has been reported with resveratrol liposome in preventing oxidative damage and neuronal death in a rat model of PD, along with some restoration of motor control, by improving bioavalability compared to free resveratrol.
Inflammation is a complex process and a silver bullet for a universal drug target is extremely unlikely to be identified. But a combinatorial approach including natural product extracts alongside conventional medicine may offer part of the solution in future preventative and curative strategies.
Author contributions:AS, FZ and SAH conceived the subject matter. FZ and SAH performed literature search and drafted the original version of the manuscript. AS, NCH and GEB critically revised the original draft. All authors approved the final version.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
?Article author(s) (unless otherwise stated in the text of the article) 2022.All rights reserved. No commercial use is permitted unless otherwise expressly granted.
 中國(guó)神經(jīng)再生研究(英文版)2022年8期
中國(guó)神經(jīng)再生研究(英文版)2022年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Ocular therapies for neuronal ceroid lipofuscinoses: more than meets the eye
- Designing nanocarriers to overcome the limitations in conventional drug administration for Parkinson’s disease
- The second brain in Parkinson’s disease: fact or fantasy?
- Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target
- Construction and imaging of a neurovascular unit model
- Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease
