Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target
Chao-Hua Yang, Zheng-Xue Quan, Gao-Ju Wang, Tao He, Zhi-Yu Chen,Qiao-Chu Li, Jin Yang, Qing Wang,*
Abstract The currently recommended management for acute traumatic spinal cord injury aims to reduce the incidence of secondary injury and promote functional recovery. Elevated intraspinal pressure (ISP) likely plays an important role in the processes involved in secondary spinal cord injury, and should not be overlooked. However, the factors and detailed time course contributing to elevated ISP and its impact on pathophysiology after traumatic spinal cord injury have not been reviewed in the literature. Here, we review the etiology and progression of elevated ISP, as well as potential therapeutic measures that target elevated ISP. Elevated ISP is a time-dependent process that is mainly caused by hemorrhage, edema, and blood-spinal cord barrier destruction and peaks at 3 days after traumatic spinal cord injury. Duraplasty and hypertonic saline may be promising treatments for reducing ISP within this time window. Other potential treatments such as decompression, spinal cord incision, hemostasis, and methylprednisolone treatment require further validation.
Key Words: blood-spinal cord barrier; decompression; duraplasty; durotomy; edema;hemorrhage; intraspinal pressure; myelotomy; spinal cord injury; therapeutic target
Introduction
In recent years, the number of patients with traumatic spinal cord injury (tSCI) has remained high due to the high incidence of traffic injuries and injuries caused by falling from a height. tSCI often causes long-lasting and irreversible changes in sensory, motor, and autonomic function, but also leads to reduced quality of life and increased paralysis and mortality rates (Hagen et al., 2010; Parra-Villamar et al., 2021; Zhang et al., 2021). An estimated 40 people per million per year are affected by tSCI in the United States alone(Cripps et al., 2011), and tSCI has become a serious social problem.However, no widely accepted therapeutic methods are available to attenuate and reverse tissue injury and enhance functional recovery after severe tSCI. The primary reasons for the poor understanding of tSCI and the failure to develop effective treatments are the complex characteristics of, pathophysiological consequences of, and abundant inconsistencies in, outcomes after tSCI. A deeper understanding of the pathophysiological mechanisms that lead to the damage seen following tSCI is required.
Studies have shown that tSCI involves both the primary injury caused by immediate violence and the secondary injury process caused by amplification of a cascade of multiple cellular and molecular sequelae; the secondary injury process is considered to be reversible,and thus is frequently regarded as a potential therapeutic target.The trigger point of secondary injury is hemorrhage caused by destruction of the vascular structures associated with compression,laceration, distraction, and shearing forces (Leonard et al., 2015;Anjum et al., 2020); red blood cell leakage and invasion of various inflammatory cells (neutrophils, monocytes, macrophages, and T and B lymphocytes) are caused by disruption of the bloodspinal cord barrier (BSCB) membrane structure (Jin et al., 2021).Subsequently, multicellular and multimolecular interactions lead to a Na+/K+ionic imbalance and increased calcium influx, free radical production, excitotoxicity, and glutamate accumulation. All of these factors result in vasogenic edema and cell cytotoxic edema (Figure 1) (Vanzulli and Butt, 2015; Alizadeh et al., 2019). Hematoma and edema lead to swelling of the contused spinal cord and may block normal cerebrospinal fluid flow (Jones et al., 2012). The swollen cord is compressed against the relatively noncompliant dura, leading to elevated intraspinal pressure (ISP) (Maikos et al., 2008). This elevated ISP can have a tamponade effect on the spinal vasculature,initially limiting venous outflow from the spinal cord and ultimately exacerbating the blood supply to the spinal cord parenchyma.Spinal cord hypoperfusion and ischemia further cause cytotoxic and vasogenic edema (Anjum et al., 2020). These molecular chemical events eventually lead to a vicious cycle of “ischemia, edema,elevated ISP, ischemia” and sustained elevation of ISP (Figure 1),which has been documented in rats, pigs, and humans (Werndle et al., 2014; Khaing et al., 2017; Streijger et al., 2017). Cao et al. (2015)described these symptoms as “spinal compartment syndrome (SCS)”.tSCI can be thought of as being analogous to traumatic brain injury(TBI), and increased intracranial pressure (ICP) following TBI yields detrimental outcomes. Indeed, for TBI patients, specific clinical guidelines have been proposed to strengthen the monitoring of ICP and oxygen partial pressure in brain tissue (using a multimodality monitoring device) in combination with craniectomy decompression(with durotomy), internal decompression (partial resection of nonfunctional brain tissue), and pharmacological therapy with the goal of reducing ICP and maintaining adequate perfusion to alleviate secondary injury to the brain tissue (Padayachy et al., 2010; Narotam et al., 2014; Carney et al., 2017). This treatment paradigm has resulted in a 50% reduction in mortality and morbidity and significant improvements in clinical outcomes for TBI patients (Miller et al.,1981). Decompression surgery of the bony canal with laminoplasty or laminectomy within 24 hours following injury results in a minor decrease in ISP (Ghasemi and Behfar, 2016; Aarabi et al., 2019).However, this type of surgery does not sufficiently alleviate elevated ISP caused by dural compression (Werndle et al., 2014; Phang et al., 2015a), which leads to persistent spinal hypoperfusion and secondary injury progression in tSCI patients. Here, we review recent developments regarding the components and pathophysiological mechanisms associated with elevated ISP and the potential contribution to the secondary damage caused by elevated ISP.
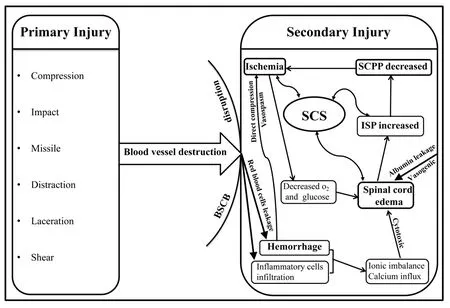
Figure 1 | Pathophysiological mechanism of spinal compartment syndrome (SCS).BSCB: Blood-spinal cord barrier; ISP: intraspinal pressure; SCPP: spinal cord perfusion pressure.
Literature Search
In this narrative review, an electronic search was performed using the Web of Science and PubMed databases to identify any studies published before May 2021. The Medical Subject Headings (MeSH) that were used in the search included “intraspinal pressure, intramedullary pressure, intrathecal pressure, spinal cord compartment syndrome, edema, spinal cord injury, blood spinal cord barrier, hemorrhage, and hematoma”. The references in the studies obtained from this search were also manually screened to identify any other relevant studies. The identified articles were then used to conduct our review.
Components Causing Elevated Intraspinal Pressure
Hemorrhage and hematoma
Mechanical disruption of the microvasculature causes initial bleeding following primary tSCI. Subsequently, secondary petechial hemorrhage formation occurs in the spinal parenchyma surrounding the primary lesion, and these hemorrhages have been shown to be related to aggravation of the lesion (Gerzanich et al., 2009). Spinal cord hemorrhage was found in 85% of subjects with complete motor deficits and 21% of subjects with incomplete motor injuries(Flanders et al., 1996). Furthermore, blood infiltration caused by destruction of the BSCB is an important cause of hematoma in the spinal parenchyma. Petechial hemorrhage is thought to be one of the triggers of secondary tSCI (Fleming et al., 2006), leading to toxic events including elevated thrombus formation, increased extracellular glutamate levels, iron toxicity, and red blood cell lysis (Hua et al.,2006; Wagner et al., 2006). Leonard et al. (2015) demonstrated that hemorrhage significantly increased at 5 hours after tSCI and is the major contributor to lesion volume. Weirich et al. (1990) found scattered hemorrhages in spinal gray matter at 2 hours after injury that quickly spread and reached their peak at 12 hours. Zhang et al.(2019) found that hemorrhage was most severe at 1 hour post-spinal cord injury (SCI) and then gradually decreased. Therefore, volumetric expansion caused by hemorrhage and hematoma may contribute to the early increase in ISP (Leonard et al., 2015).
Intramedullary hemorrhage could also cause secondary damage by direct compression of adjacent structures with a downstream mass effect, and by vasospasm caused by microvascular exposure to the degradation products of heme (an effective oxidant, even at low molecular concentrations) (Regan and Panter, 1993; Regan and Guo,1998). These events, combined with intravascular microthrombosis,are thought to contribute to reduced blood flow and exacerbated ischemia (Tator and Fehlings, 1991). Low blood flow perfusion will result in different levels of insult to gray and white matter, which may lead to delayed cell death. Owing to the high metabolic demands of neurons, the gray matter is particularly sensitive to ischemic injury.
Blood-spinal cord barrier disruption
The BSCB is formed by a significant difference in structure between the spinal cord and peripheral vasculature. Endotheliocytes,intercellular connections, pericytes, the basement membrane, and astrocytic foot process cells and structures, which form the BSCB, are responsible for regulating molecular exchanges and protecting the spinal parenchyma from toxins of the BSCB (Jin et al., 2021). Among these elements, endotheliocytes play a key barrier role because of their nonfenestrated cell membranes, high level of cytosolic mitochondria, absence of pinocytic vacuoles, and formation of tight and adherens junctions (Bartanusz et al., 2011).
Although the structures of the BSCB and blood-brain barrier (BBB)are analogous, the permeability of the BSCB is different from that of the BBB. Research based on assessing the uptake of radioactive tracers in the central nervous system (CNS) showed that the BSCB is more permeable than the BBB (Prockop, 1995). A study by Pan et al. (1997) suggested that the cervical region had the highest permeability for radiolabeled interferons (interferons α and γ and tumor necrosis factor α), while the permeability was lowest in the brain region. Interestingly, any given region exhibited different levels of permeability for different cytokines, and this selective permeability may contribute to complex CNS injuries (Daniel et al.,1981). The destruction of the BSCB is accompanied by abnormal leakage of blood-borne molecules and cells, albumin dextran, and water molecules and infiltration of immune cells into the spinal cord parenchyma, which leads to vasogenic edema, reactive astrogliosis,demyelination, and scar formation (Wagner and Stewart, 1981;Ankeny and Popovich, 2009).
Goodman et al. (1976) demonstrated that endothelial tight junctions in the gray matter were impaired as early as 15 minutes following primary injury. Matsushita et al. (2015) used imaging and spectrophotometric quantification of Evans blue (EvB) content to show that the permeability of the BSCB was greatly increased at 1 and 3 days post-injury in the injury epicenter of a contused SCI model and reached the peak at 1–2 weeks; however, the permeability began to increase at 2 weeks after SCI in rostral and caudal segments and peaked at 6 weeks, leading to a biphasic increase in total permeability and leakage lasting until the 10thweek after SCI (Matsushita et al.,2015; Morita et al., 2016). This phenomenon may be explained by the delayed degeneration of ascending and descending tracts after SCI, which may be related to the increased permeability of the vasculature far from the injured epicenter or the self-repair process of the vasculature (Matsushita et al., 2015). Abnormal permeability was shown to be persistently high for at least 28 days based on C14-marked acid testing (Popovich et al., 1996), whereas horseradish peroxidase testing indicated that it was restricted to the first 2 weeks after injury (Noble and Wrathall, 1987, 1988). This difference may be due to variation in the BSCB permeability for molecules of different sizes.
Edema
Spinal cord edema includes cytotoxic (cell swelling) and vasogenic(leaky capillaries) edema characterized by excessive accumulation of water intracellularly and interstitially in tissue. Cytotoxic edema is mainly due to Na+/K+ionic imbalance, increased calcium influx, free radical production, excitotoxicity caused by blood cell infiltration,ischemia, and hypoxia (Alizadeh et al., 2019). Angiogenic edema is mainly related to the disruption of the BSCB, leading to leakage of water molecules, albumin, and dextran. Furthermore, accumulating evidence has shown that AQP4, a molecular water channel that is expressed at high levels in astrocytic endfeet in the spinal cord,which are triggered by posttraumatic ischemia (Frigeri et al., 1995;Nielsen et al., 1997; Nesic et al., 2010), is the main regulator of water flow into and out of the injured cord and can cause vasogenic (Kimura et al., 2010) and cytotoxic edema (Saadoun et al., 2008).
To date, several studies have reported that excessive AQP4 expression within 72 hours of tSCI is associated with increased spinal edema (Liu et al., 2015; Yu et al., 2015), and that decreased AQP4 function correlates with improved functional recovery after tSCI (Jing et al., 2014; Sato et al., 2014; Hu et al., 2015). Spinal cord edema starts as early as 30 sec after injury, markedly increases within several minutes, peaks at 24 hours, and lasts up to 15 days (Nolan,1969; Demediuk et al., 1987, 1990; Lemke et al., 1987; Lemke and Faden, 1990). However, in a rat model of severe thoracic contusion SCI, researchers demonstrated that the water content of the spinal cord was significantly elevated as early as 1 hour post-injury, peaked at 72 hours, with a value of 78.7 ± 0.67%, and remained elevated 28 days after injury (Hale et al., 2020). The reasons for this difference may depend on the species selected, the SCI model used, and the experimental time points chosen.
Edema formation was most prominent in the gray matter. The study demonstrated that the volume of edema and swelling increased by 127% in the gray matter near the impact site, but only by 24% in the white matter, 1 hour post-injury in a cat model tested using a specific gravity gradient column (Anderson and Hall, 1993; Sharma, 2005).Owing to confinement by the noncompliant dura mater, the swelling spinal cord herniates cranially and caudally along the midline of the posterior funiculi (Tomko et al., 2017). This leads to the edematous region comprising not only the lesion site but also the adjacent segments.
Research has suggested that, at segments 5 mm rostral or caudal to the lesion epicenter, the water content of the spinal cord is elevated 1 day post-injury, peaks at 72 hours, and returns to baseline by 7 days after SCI (Hale et al., 2020). Interestingly, the caudal segments had more pronounced edema development than the rostral segments,related to the release of neurochemicals, as well as BSCB breakdown following SCI influenced edema formation (Sharma, 2005).Additionally, Leonard et al. (2015) suggested that intraparenchymal hemorrhage was the primary cause of elevated ISP within 5 h postinjury, whereas edema became the primary cause of elevated ISP at 3–7 days in a balloon compression model in rabbits.
Dura and pia mater
Analogous to the closed spaces that contribute to TBI and osteofascial compartment syndrome, the tough and nondilated dura mater is the basis of maintaining elevated ISP. Studies have shown that ISP remains high after only anterior and posterior bony decompression (Werndle et al., 2014; Saadoun and Papadopoulos,2020), and spinal cord herniation occurred immediately after the dura was opened in a dog model (Saadoun and Jeffery, 2021) and in a patient with traumatic thoracic SCI (Grassner et al., 2017).In a prospective clinical trial, bony supplementary dura mater decompression significantly reduced ISP and increased spinal cord perfusion pressure (SCPP) compared with bony decompression alone(Phang et al., 2015a). These results suggest that the dura contributes to cord compression.
The role of the relatively firm pia mater in elevated ISP, however,remains controversial. In patients with severe tSCI, the swollen spinal cord dilated and occluded the subarachnoid space after adequate bone decompression on MRI (Aarabi et al., 2019). Furthermore,simultaneously measured pressures in the subarachnoid space and spinal parenchyma were elevated and equal (Phang and Papadopoulos, 2015b). These findings suggest that, after severe tSCI in humans, the pia mater no longer confined the swollen spinal cord. However, in a rabbit model of compressive SCI, durotomy with myelotomy significantly reduced ISP and improved histological outcomes compared with durotomy without myelotomy (Khaing et al., 2021). This result suggests that the pia mater may restrict spinal cord expansion and increase pressure within the spinal cord parenchyma.
Epidural components
In addition to intradural factors, the contribution of epidural components, including the narrow bony spinal canal, ossified ligamentum flavum, posterior longitudinal ligament, and herniated intervertebral disc, to elevated ISP should not be ignored. These components often cause dural sac compression and cerebrospinal fluid circulation block as shown by T2-weighted MRI, especially in some elderly patients with traumatic cervical SCI. Although there is no pressure-monitoring comparison for this type of patient,some studies have shown that early epidural decompression can achieve significant radiological results and functional recovery for such patients, which may indicate that epidural decompression can partially reduce ISP (Piazza et al., 2018; Aarabi et al., 2019).
Monitoring intraspinal pressure
Elevated ISP monitoring was first mentioned by Allen in 1991 and was first used in a dog model in 1995 and in human patients in 2014. Since then, 50 related articles have been published (Figure 2),including 16 animal studies, 23 clinical studies, eight reviews, and three case reports. In particular, the Saadoun and Papadopoulos teams from London University have published 46 articles pertaining to this research field, including one animal study, 18 clinical studies,and four reviews.
Elevated ISP was first mentioned by Allen (Figure 2), who described how, after a median longitudinal incision was made after the early stage of tSCI in dogs, there was “great outpouring of blood from the injured area”. Furthermore, he reported that, in a patient with tSCI who underwent surgery 4 hours after injury, “when the spinal cord was incised, the blood spurted out as if subjected to great pressure”(Allen, 1911). The first use of ISP monitoring was in a hybrid dog model and reported a physiological ISP of 30 ± 12 mmHg at C5 (Iida and Tachibana, 1995). Saadoun et al. (2008) used a Millar pressure catheter to show that the normal intramedullary pressure was 8± 3 mmHg at the T6 level in mice. Later, Soubeyrand et al. (2013)inserted a pressure transducer catheter into the sacral subarachnoid space of rats and found that the ISP was 5.5 ± 0.5 mmHg in normal ratsvs. 8.6 ± 0.4 mmHg in rats with a contusion at T10. In addition,they demonstrated that the ISP increased when the head was elevated (Soubeyrand et al., 2013).
Subsequently, in a clinical study, Werndle et al. (2014) reported an elevated ISP of approximately 20 mmHg at 24 hours following injury compared with approximately 10 mmHg in control patients without spinal cord pathology measured by Codman probe monitoring. They suggested that ISP waveforms are similar to ICP waveforms, which are comprised of three peaks corresponding to arterial pulsation, intracranial compliance, and aortic valve closure(Table 1) (Werndle et al., 2014; Saadoun and Papadopoulos, 2016).Furthermore, the researchers found four compartments at the injury epicenter, including three subdural compartments (the injury site and cerebrospinal fluid (CSF) above and below the injury) and one extradural compartment (Werndle et al., 2014). The ISP at the injury site was higher than the pressure simultaneously recorded in the extradural compartment or from the CSF below the compartment(Werndle et al., 2014; Phang and Papadopoulos, 2015). The authors suggested that the pressure exerted by the swollen injured cord against the dura was different from the pressure exerted on the CSF and extradural compartment (Werndle et al., 2014).However, the pressures in the subarachnoid space can approximate intraparenchymal spinal pressures only when the severely contused spinal cord swells and occludes the subarachnoid space (Phang et al.,2015). Under physiological conditions and in the context of mild SCI,the use of subarachnoid pressure to estimate parenchymal pressure may underestimate the intraparenchymal pressure where CSF fills the subarachnoid space (Mendelow et al., 1983; Ostrup et al., 1987).In humans, the sagittal diameter of the subarachnoid space is, on average, 5.5 ± 0.2 mm in the cervical spine (Ulbrich et al., 2014) and 7.6 ± 0.2 mm in the thoracic spine (Gellad et al., 1983). Obliteration of the subarachnoid space requires expansion of the cervical spinal cord diameter by approximately 72.2% (Ulbrich et al., 2014) and of the thoracic spinal cord diameter by approximately 124.1% (Gellad et al., 1983). The degree of spinal cord swelling corresponds to injury severity (Jones et al., 2012). Additionally, ISP monitoring is an invasive operation that needs to be performed in the operating room; currently, it cannot be performed in patients through lumbar puncture.
Studies have shown that elevated ISP is mainly influenced by hemorrhage and edema in a time-dependent manner. According to a closed balloon compression study in rabbits, elevated ISP is initially driven by hemorrhagic components, whereas edema becomes the primary contributor 3 days post-injury (Leonard et al., 2015).The authors found that ISP began to rise at 5 hours (7.36 ± 0.79 mmHg), reached a peak at 3 days (9.00 ± 1.32 mmHg), and gradually decreased at the first week post-injury, whereas pressures remained elevated compared with those in sham-operated animals (2.59 ± 1.01 mmHg), as determined by Codman probe assessment (Leonard et al.,2015). Khaing et al. (2017) found that, in a moderate contusion SCI rat model assessed using a 1 F Millar pressure probe, ISP increased three-fold 30 minutes after injury (from 2.7 ± 0.5 mmHg to 8.9 ± 1.1 mmHg) and remained elevated (4.3 ± 0.8 mmHg) for 7 days (Khaing et al., 2017). Using the same model and monitoring instruments,Dong et al. (2016) found that intramedullary pressure curves were bimodal over time in the mild and moderate injury groups, peaking at 1 hour (12.51 ± 2.32 mmHg and 22.36 ± 4.39 mmHg) and 48 hours(16.97 ± 4.33 mmHg and 21.73 ± 7.73 mmHg) after SCI, whereas the pressure curve in the severe group was relatively flat (Dong et al.,2016). The intramedullary pressure was positively correlated with the degree of injury (Dong et al., 2016). In a recent rabbit model of aneurysm clip injury assessed using a telemetry system, Zhang et al. (2019) found that changes in ISP were divided into three stages:stage I (steep rise) from 1 to 7 hours, stage II (steady rise) from 8 to 38 hours, and stage III (descending) from 39 to 72 hours, peaking at 38 hours. They found that ISP in conscious rabbits (sham group:5.93 ± 1.28; SCI group: 7.96 ± 2.01 mmHg) was higher than that in anesthetized rabbits (sham group: 3.43 ± 1.33 mmHg; SCI group: 5.76± 1.80 mmHg), and that different probe insertion angles (30°, 45°,and 90°) had no influence on ISP (Table 2). We propose that these differences could be attributable to the physiological and anatomical differences between different species; different types, segments, and severities of injury; and different measurement methods.

Figure 2 |Timeline of ISP monitoring use in research.ICP: Intracranial pressure;ISP: intraspinal pressure;MR: magnetic resonance;SCPP: spinal cord perfusion pressure.
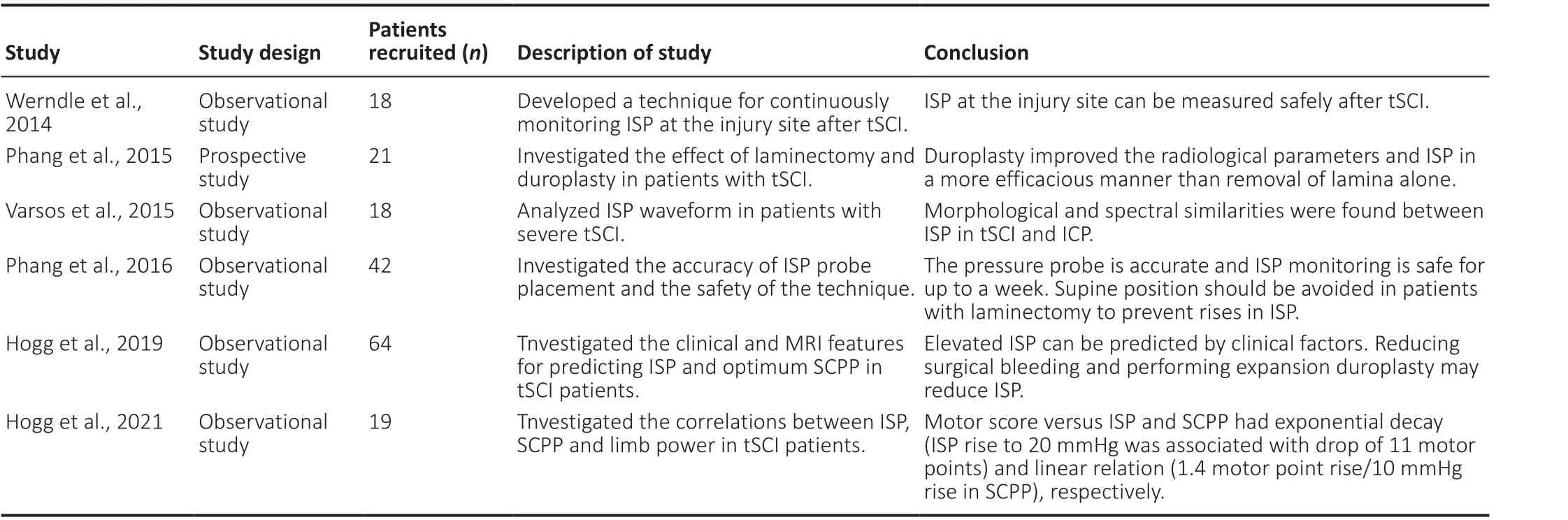
Table 1 |A summary of ISP monitoring in clinical studies
Elevated ISP impairs vascular autoregulation and decreases perfusion of the contused spinal cord (Soubeyrand et al., 2013). Analogous to brain perfusion, SCPP is computed as the mean arterial pressure(MAP) minus ISP. The effect of elevated ISP on spinal cord blood flow (SCBF) is usually attributed to a tamponade effect on the small vessels. This suggests the concept of spinal compartment syndrome,analogous to osteofascial compartment syndrome in the leg (Bourne and Rorabeck, 1989). In a clinical study, Werndle et al. (2014) found that, when the ISP increased by 10 mmHg, the SCBF compensatory reserve became exhausted, and that increases by 15–20 mmHg led to progressive loss of autoregulation. Ischemia and hypoxia are major contributors to secondary injury that worsen spinal cord edema and lead to a vicious cycle of “ischemia, edema, elevated ISP,and ischemia”. Owing to dural restriction, elevated ISP can spread only rostrocaudally. Tomko et al. (2017) found that, in a rat balloon compression model, there was a quiescent phase lasting at least 1 hour, followed by significant enlargement of the lesion; the length of the lesion increased from 15 to 40 mm between 1 and 48 hours,and the spindle-shaped lesion formation was driven by increased ISP. Furthermore, after tSCI, patients exhibit excessive spreading of T2 signals to more than four rostral segments. This condition is also known as subacute post-traumatic ascending myelopathy and is unrelated to syrinx formation or mechanical instability (Miller et al.,2016).
ISP can be accurately measured, and ISP fluctuations visualized,in rats and rabbits after tSCI using microsensor and telemetry monitoring systems (Table 2). Studies have shown that elevated ISP is mainly caused by hemorrhage, edema, and BSCB destruction in atime-dependent manner. However, most of the SCI models used in these studies involve inducing contusion injury to the spine using a weight-drop device or impactor after laminectomy, which opens the spinal canal, leaving it decompressed. Thus, these SCI models do not truly reflect patient injuries, and better animal models are needed for future studies.
Therapies Based on Elevated ISP
In this section, we will summarize the current state of therapeutic strategies, including surgical and nonsurgical interventions that target elevated ISP after tSCI (Figure 2). The experimental and clinical studies reviewed above showed that ISP reaches maximal levels within 3 days after injury, suggesting that there may be a substantialwindow of opportunity for therapeutic intervention to reduce this pressure. The role of surgical and nonsurgical interventions in reducing ISP is discussed below.
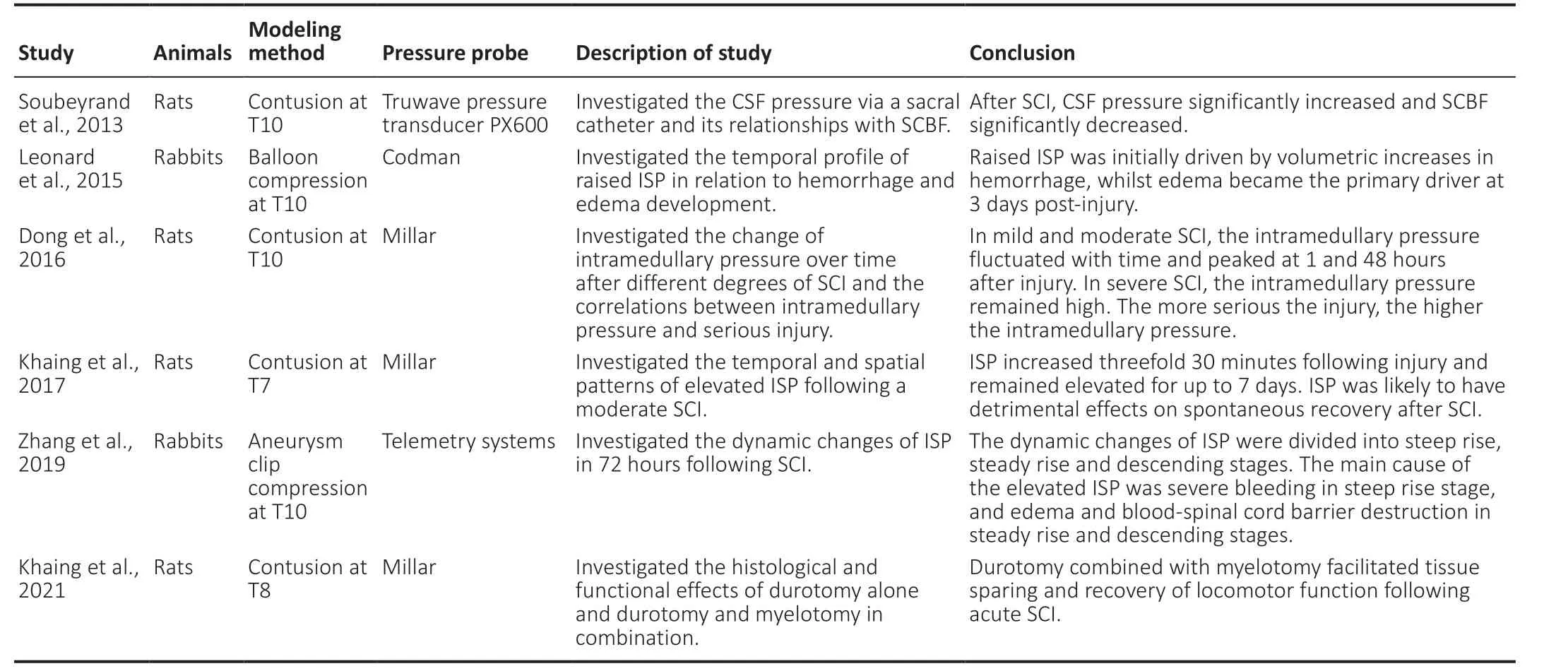
Table 2 |A summary of ISP monitoring in animal studies
Osseous decompression
Numerous clinical studies of tSCI have suggested that early surgical decompression may improve neurological and functional outcomes(Furlan et al., 2011; Jug et al., 2015). These beneficial effects are partially due to extensive osseous decompression of the spinal cord,which should theoretically improve SCBF. In a recent study, Piazza et al. (2018) reported that posterior laminectomy results in better radiological decompression of the posterior CSF space than anterior cervical discectomy. Aarabi et al. (2119) demonstrated that the rates of radiological decompression in patients who underwent anterior discectomy and corpectomy without laminectomy were 46.8% and 58.6%, but in those in patients who underwent laminectomy at one,two, three, four, and five levels were 58.3%, 68%, 78%, 80%, and 100%, respectively.
The effect of bone decompression alone has, however, been questioned and some researchers doubt that it can reduce ISP and improve SCBF. After sufficient decompression of the spinal cord through laminectomy, no CSF was present between the edematous spinal cord and dura on MRI (Werndle et al., 2014). This result suggests that, despite sufficient bony decompression, the edematous spinal cord still exerts pressure against the nonyielding dura mater,causing sustained elevated ISP and low spinal perfusion. In an exploratory clinical study, SCPP after sufficient bony decompression alone was less than 60 mmHg approximately 40% of the time, which is likely to cause spinal ischemic damage (Phang et al., 2015a).RCTs and animal experiments with ISP monitoring are needed to assess whether bone decompression alone improves physiological parameters and functional outcomes.
Durotomy, duraplasty, and myelotomy
Recommended management of TBI involves reducing ICP by decompressive craniectomy, during which part of the skull is removed, and the dura is opened to allow outward herniation of the brain. Similarly, opening the dura to allow the spinal cord to expand is important in tSCI treatment. Perkins and Deane (1988) performed durotomies in six patients with tSCI in whom the dura appeared tense after bony decompression. Three patients had a complete recovery, and three had a partial recovery. In stretched ex vivo pig spinal cords, durotomy reduced ISP from 35 to 10 mmHg (Awwad et al., 2014). In a rat weight-drop SCI study, duraplasty resulted in more white matter sparing than laminectomy alone (Jalan et al., 2017). In a study of 21 patients with cervical or thoracic tSCI, Phang et al. (2015)found that duraplasty reduced ISP by 12.7 ± 0.4 mmHg and increased SCPP to less than 60 mmHg for less than 5% of the time, compared with laminectomy alone (18.0 ± 0.5 mmHg and approximately 40%of the time). Follow-up data suggested a tendency toward greater improvement in American Spinal Cord Injury Association scores,walking, and bladder function in patients who underwent durotomy and duraplasty compared with patients who underwent laminectomy only (Phang et al., 2015a). The expansion duraplasty took 10–15 minutes to perform and had fewer complications, such as CSF leakage, pseudomeningocele, and CNS infection. The above studies show that opening the dura after tSCI is beneficial for humans and animals.
Whether durotomy requires duraplasty has also attracted researchers’ attention. A comparison of durotomy with duraplasty in rat contusion (Smith et al., 2010; Zhang et al., 2016) and compression (Fernandez and Pallini, 1985) SCI models suggested that better functional recovery and reduced lesion volume were obtained through duraplasty, as this procedure led to less inflammation,collagen scarring, and spinal cord cavitation. Duraplasty is beneficial after durotomy because duraplasty reduces the risk of CSF leakage and wound infection. Furthermore, maintaining the continuity of the dura following durotomy maintained a pattern of CSF flow closer to physiological conditions and prevented extradural factors from inhibiting neuroregeneration and promoting inflammation(Iannotti et al., 2006). An RCT investigating the effects of duraplasty is currently underway at the University of London (ClinicalTrials.gov identifier: NCT04936620).
Owing to the possible contribution of the pial lining to elevated ISP,myelotomy as a treatment for tSCI has been the topic of several animal studies. In a rodent model of moderate contusion SCI,piotomy contributed to 53.5% of the pressure reduction during the acute phase, whereas it contributed only 14.6% during the subacute phase of the injury (Khaing et al., 2017). This suggests that early myelotomy may be more effective in promoting functional recovery of the spinal cord. Furthermore, in a similar SCI model, the researchers found that durotomy plus myelotomy more effectively mitigated elevated ISP (3.14 ± 0.40 mmHg) than durotomy alone(4.17 ± 0.30 mmHg) at 3 days post-injury and significantly promoted recovery of hindlimb locomotor function by facilitating tissue sparing(Table 2) (Khaing et al., 2021). This study reported an interesting phenomenon in which durotomy alone led to increased recovery of bladder function compared with durotomy plus myelotomy, which may explain why surgical opening of the pia, involving additional disruption of the injury core, resulted in irritation of the spinal cord,leading to transient urinary retention in animals that underwent durotomy and myelotomy.
A review study revealed that durotomy implemented in tSCI patients resulted in improved neurological function in 92.3% of studies, while durotomy in animals resulted in improved neurological function in 83.3% of studies. However, although myelotomy procedures had positive effects in 80% of animal studies, only one clinical study reported positive results in patients (Telemacque et al., 2020). On the basis of these studies, the functional advantage of implementing durotomy plus myelotomy remains unclear, and there is some concern that myelotomy may itself cause spinal cord damage in humans. However, in a recent clinical study of 19 participants with complete thoracic tSCI, the American Spinal Injury Association impairment scale (AIS) grade conversion rate exceeded that of historical controls at the 6-month follow-up after myelotomy and implantation of a neuro-spinal scaffold (NSS) (Kim et al., 2021).Because there was no control group for myelotomy alone in this study, the therapeutic effect may have been attributable to the myelotomy rather than the implanted bioresorbable polymer materials.
CSF drainage
The benefit of CSF drainage in the treatment of tSCI is controversial.Theoretically, elevated ISP can be alleviated by reducing the contents of the spinal canal (CSF), similar to treatment for TBI. In aortic cross-clamping SCI, CSF drainage significantly improved SCBF and decreased the rate of paraplegia resulting from spinal cord ischemia(Bower et al., 1989; Griepp and Griepp, 2007). In a rabbit SCI model,early CSF drainage through a lumbar catheter resulted in a decrease in the size of histological lesions post-SCI (Horn et al., 2008).Furthermore, some researchers have suggested that CSF leakage induced unintentionally when monitoring ISP reduces ISP. We believe that, if the dura remains intact, CSF drainage may improve CSF communication between the injury site and the upper and lower compartments, but that when the spinal cord is swollen and CSF flow is completely blocked, CSF drainage may not be effective.
Specific positions
In patients who have undergone laminectomy, post-operative body positioning may have an important influence on ISP and SCPP. Owing to the lack of lamina protection and CSF buffering, external forces are transmitted to the swollen cord in the supine position, leading to an increase in ISP and a decrease in SCPP, and thus potentially inflicting spinal cord lesions (Werndle et al., 2014; Saadoun and Papadopoulos,2020). Phang et al. found that, in laminectomy patients, ISP was significantly increased in the supine position compared with the lateral position. The pressure difference was 8 mmHg after cervical laminectomy and up to 18 mmHg after thoracic laminectomy (Phang et al., 2016a). This is probably because, when the patient is supine,the cervical spinal cord is subjected to less compression due to the lordosis of the spinal column, whereas the kyphotic curve of the thoracic spinal column results in increased compression of the spinal cord in this region (Saadoun and Papadopoulos, 2016). Therefore,the authors suggested that, for the first 4 days post-operatively,patients should be cared for in the lateral or supine position, with a ring-shaped pillow used to protect the wound (Phang et al., 2015a).After 5 days, the supine position had no effect on ISP or SCPP.
Drug-mediated ISP reduction
Severe hemorrhage contributes to early increases in ISP, and therefore hemostasis has been attempted to treat tSCI in the early stage following injury. Indeed, studies have demonstrated that administration of glibenclamide after SCI can promote sparing of white matter tracts and improve behavioral outcomes by reducing hemorrhage (Simard et al., 2007; Popovich et al., 2012). After moderate contusion injury in rats, intraperitoneal injection of protocatechuic acid (PCA) and mithramycin A resulted in improved functional recovery by inhibiting BSCB disruption and hemorrhage(Lee et al., 2018; Park et al., 2019).
Hemorrhage is limited to the early stage of tSCI, whereas edema occurs throughout the entire duration of the secondary injury phase.Therefore, alleviating spinal cord edema has attracted the attention of many researchers. Hypertonic saline has been used to treat cerebral edema to reduce ICP after TBI (Ziai et al., 2007; Forsyth et al., 2008). More recent studies have attempted to establish an osmotic gradient between the intracellular and intravascular spaces with hypertonic saline to reduce ISP. Some studies have suggested that hypertonic saline therapy after tSCI can improve histopathological and behavioral outcomes (Tuma et al., 1997; Spera et al., 2000; Legos et al., 2001). Recently, Hale et al. (2020) used a surgically mounted osmotic transport device to continuously remove excess fluid at the site of injury throughout edema progression,which significantly improved recovery following contusion SCI.
The use of high doses of methylprednisolone has been recommended to reduce inflammation and glutamate release and ameliorate edema within the first 8 hours after injury (Fehlings et al., 2017), but this approach has been limited by complications(gastrointestinal hemorrhage and respiratory tract infection) and limited time windows. Furthermore, a recent meta-analysis found no significant difference between the methylprednisolone and control groups in terms of sensory scores and pooled motor function at the last follow-up (Liu et al., 2020).
A possible explanation for this is the low permeation of drugs into the injury site when administer systemically. The disruption of the BSCB seen after acute tSCI allows drug entry into the injury site,but this entry is restricted by elevated ISP and low SCPP. One study reported that an increase in SCPP of 10 mmHg resulted in a threefold increase in dexamethasone penetration into the injury site, based on microdialysis monitoring (Phang et al., 2016b). Therefore, maximizing drug delivery to the injury site requires optimization of SCPP and individualized management of MAP and ISP, rather than following the recommended guidelines by maintaining the MAP at 85 to 90 mmHg for 1 week after SCI (Phang et al., 2016b; Saadoun and Papadopoulos, 2016).
Limitations
This review had several limitations. First, this is a nonsystematic review. Second, articles on using stem cell engineering and materials to improve edema and BSCB were not included. In the future,combining different treatment strategies may lead to a cure for SCI.
Conclusions
In this article we reviewed the factors that increase ISP, as well as the impact of elevated ISP on pathophysiology after SCI. Therapeutic strategies, including both surgical and nonsurgical interventions,targeting elevated ISP after tSCI were also reviewed. Taken together,studies have shown that ISP increases to a maximal level within 3 days after tSCI due to the contribution of hemorrhage and edema.Duraplasty and hypertonic saline may be promising treatments for reducing ISP within this time window. Other treatments,such as osseous decompression, myelotomy, hemostasis, and methylprednisolone, require further verification in animal studies and clinical RCTs.
Acknowledgments:We would like to thank Ke Tang, The First Affiliated Hospital of Chongqing Medical University, for revising the manuscript.
Author contributions:Manuscript design: QW, ZXQ, CHY; data collection:
CHY, GJW, TH, ZYC and QCL; manuscript writing: CHY; manuscript revision and review: CHY, TH and JY. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of
interest associated with this manuscript.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
?Article author(s) (unless otherwise stated in the text of the article) 2022. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
- 中國神經再生研究(英文版)的其它文章
- Ocular therapies for neuronal ceroid lipofuscinoses: more than meets the eye
- Designing nanocarriers to overcome the limitations in conventional drug administration for Parkinson’s disease
- The second brain in Parkinson’s disease: fact or fantasy?
- Construction and imaging of a neurovascular unit model
- Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases
- Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease