In Vitro and in Vivo Effect of Lactobacillus plantarum XCT 1-10 on the Growth of Botrytis cinerea and Its Possible Mechanisms
LI Canying, CHENG Yuan, HOU Jiabao, GE Yonghong*, DU Hong, BAI Fengling
(National and Local Joint Engineering Research Center of Storage, Processing and Safety Control Technology for Fresh Agricultural and Aquatic Products, College of Food Science and Engineering, Bohai University, Jinzhou 121013, China)
Abstract: The effects of Lactobacillus plantarum XCT 1-10 at different concentrations (0, 102, 104, 106, 108, and 1010 CFU/mL) on the colony growth and spore germination of Botrytis cinerea and the decay of apples inoculated with B.cinerea were investigated in this study.The results demonstrated that L.plantarum XCT 1-10 at 106 CFU/mL significantly restrained lesion development in apple fruit inoculated with B.cinerea, and distinctly depressed the spore germination and colony growth of B.cinerea.The in vitro test demonstrated that L.plantarum XCT1-10 treatment increased the contents of malondialdehyde (MDA), soluble sugar and soluble proteins, and nucleic acid release, but decreased the relative conductivity of B.cinerea.These findings suggest that L.plantarum XCT 1-10 can suppress the growth of B.cinerea possibly by destroying the cell membrane.
Keywords: Botrytis cinerea; plasma membrane; lactic acid bacteria; mycelial growth
Postharvest losses of fresh fruits and vegetables caused by different fungal pathogens are huge all over the world.Some fungal invasions occur at all stages of the growth of horticultural products including in the field, harvest, storage and transportation.Some fungi could produce mycotoxins which are toxic and harmful to human health.Botrytis cinerearanks the second place at the top of ten important fungal pathogens worldwide and can infect blueberries, blackberries,tomatoes, strawberries, and grapes[1-2].Therefore, new technologies for control of fungal infection or inhibition of fungal growth have also been widely concerned in recent years.
Physical, chemical and biological agents have developed to control gray mould of fruits and vegetables.Among them, so far, chemical fungicides such as fludioxonil,thiabendazole, benzodime, benzodime, anilinopyrimidine are still the most extensive method[3].However, more attention has attracted to the harm to human health caused by the residue of fungicides in fruits and vegetables, pollution to the environment and enhancement to fungicide resistance.Therefore, many new alternative technologies are proposed by using the biological control based on naturally produced microorganisms including yeasts, bacteria and filamentous fungi[4].Study has shown thatSaccharomyces cerevisiaeinhibited the growth ofB.cinereaon grape fruit[5].Kloeckera apiculatastrain 34-9 andMeyerozyma guilliermondiiH3 had a good control effect on ring rot of apple fruit and postharvest pathogens in citrus respectively[6-7].Bacillus subtilisET-1 effectively inhibited gray mould of strawberries and green mould of lemons[8].Atoxigenic strains ofAspergillus flavushave also been used as biological control agent to inhibit mycotoxin contamination[9].Many lactic acid bacteria (LAB)are generally recognized as safe bio-preservation organisms in foods, often used to preclude the growth of spoilage microorganisms[10].The most recognized reason is that LAB could produce different kinds of low-molecular-weight inhibition substances including organic acids, hydroxyl fatty acids, phenolic compounds, peptide and reuterin.
The application of LAB has increased in all kinds of fermented foods in the past few years[11].Previous studies have reported that LAB could prolong the shelf-life of various dairy products, reduce the pollution of the fungus for bakery products[12].Lactobacillus plantarumNCU116 fermentation could improve the physical and chemical properties, bioactive compounds, antioxidant properties and aroma composition ofMomordica charantiajuice[13].SprayingL.plantarumcould delay the browning of litchi peel and increase total phenol content, reduce the browning and decay of lotus root[14-15].These findings showed that the use ofL.plantarumcould improve the characteristics of food and beneficial to human.However, there are a few researches on using LAB as biological agents to control postharvest decay of fruits and vegetables.The previous studies showed thatL.plantarumUM55 cell-free supernatant could inhibit the growth ofA.flavusand mycotoxin production[16].Lv Xinran et al.[17]have proved that the supernatant ofL.plantarumC10 had a remarkable effect on pink rot caused byTrichothecium roseumin muskmelons.However, there is still little information about thein vitroeffect ofL.plantarumXCT 1-10 on the growth ofB.cinereaand the possible mechanism involved.
Therefore, this study was carried out to investigate the effects ofL.plantarumXCT 1-10 on the colony growth and spore germination ofB.cinerea, to investigate the effects ofL.plantarumXCT 1-10 on lesion development of apple fruit inoculated withB.cinerea, and to study the possible mechanisms involved in inhibiting the growth ofB.cinerea.
1 Materials and Methods
1.1 Materials and reagents
Apple fruits (cv.Golden Delicious) were harvested at commercial maturity from a 20-year-old orchard in Jinzhou, Liaoning, China.The fungal pathogenB.cinereawas originally isolated from decay apple fruit and cultured on potato dextrose agar (PDA) at 26 ℃.Then, the pathogen was purified, pathogenicity test and ITS identification.The identified fungus was kept on PDA at 4 ℃.L.plantarumXCT 1-10 was originally isolated from pickle soup, purified on the MRS solid medium and kept in the laboratory after ITS identification.
PDA, potato dextrose broth (PDB), MRS solid medium andD-glucose were purchased from Beijing Solarbio Technology Co.Ltd.; ethyl acetate and anthranone, were purchased from Shandong Xiya Chemical Industry Co.Ltd.;2-thibabituric acid was purchased from Shanghai Yuanye Biological Technology Co.Ltd..
1.2 Instruments and equipment
UV-2250 Ultraviolet and visible spectrophotometer was purchased from Shandong Arcane Technology Co.Ltd.;H1650 high speed refrigerated centrifuge was purchased from Shanghai Huyueming Scientific Instrument Co.Ltd.;HZQ/THZ thermostatic oscillator was purchased from Shanghai Yiheng Scientific Instrument Co.Ltd.; DDSJ-308Aconductivity meter was purchased from Shanghai Yidian Instrument Science Co.Ltd.; Accuri C6 flow cytometry was purchased from BD Co.Ltd., USA.
1.3 Methods
1.3.1 Preparation of spore suspension
Spore suspension was prepared by the method of Ge Yonghong et al.[18], and the final density was modulated to 1 × 106spores/mL by a hemocytometer.
1.3.2 Isolation and culture ofL.plantarumXCT 1-10
L.plantarumXCT 1-10 was activated by liquid MRS at 37 ℃ for 24 h before using.The absorbance of culture medium was measured by spectrophotometer, and the concentration was calculated by standard calibration curve and adjusted to the required concentration (CFU/mL).
1.3.3 Effect ofL.plantarumXCT 1-10 on mycelia growth ofB.cinerea
The effect ofL.plantarumXCT 1-10 on mycelia growth ofB.cinereawas conducted using the method of Tatsadjieu et al.[19]with slight modifications.A 7-day oldB.cinereadisc(5 mm in diameter) was put in the center of each PDA plate containing various concentrations ofL.plantarumXCT 1-10 at 0, 102, 104, 106, 108, and 1010CFU/mL.Colony diameter and inhibition rate were calculated at 10 d.There were 3 replicates for each concentration and 10 plates for each replicate.
1.3.4 Effect ofL.plantarumXCT 1-10 on spore germination ofB.cinerea
The effect ofL.plantarumXCT 1-10 on spore germination ofB.cinereawas measured following the method of Pane et al.[20]with minor modifications.The freshly prepared spore suspension (1 × 106spores/mL) was mixed with various concentrations ofL.plantarumXCT 1-10 at 0, 102, 104, 106, 108, and 1010CFU/mL, respectively.One hundred microliter of the mixture was then transferred to PDA plates and incubated at 26 ℃.Germinated spores were counted every hour from the beginning of the control spores germination in all concentrations, starting 8 h after inoculation.Three hundred spores were calculated each time for each concentration.
1.3.5 Assay of the content of malondialdehyde (MDA),soluble protein, soluble sugar, nucleotides release and relative conductivity
100 μL spore suspension (106spores/mL) were mixed with 100 mL PDB and incubated at 26 ℃, 120 r/min for 3 d.L.plantarumXCT 1-10 was mixed in one of the flask with a final density of 106CFU/mL, a coordinative volume of distilled water was mixed into another flask as the negative control.Meanwhile, the same volume ofL.plantarumXCT 1-10 was mixed into 100 mL PDB as the positive control.The mycelia were disposed by centrifugation at 4 ℃, 12 000 ×gfor 10 min at 1, 2, 3, 4, 5 h.The supernatant and sediment were collected and used for subsequent determination.
MDA content was assayed by following the method of Sun Jian et al.[21].MDA content was expressed as mg/mL.Relative conductivity was determined according to the method of Li Wusun et al.[22]with slight modifications.The precipitate was mixed with 4.0 mL sterilized distilled water using a conductivity meter (r0), and then boiling for 30 min to determine again (r1).The relative conductivity is the percentage of the conductivity of the living fungus extract (r0)and the conductivity of the killed extract (r1).
Soluble protein content was determined following Bradford’s method[23], and the content of soluble protein was expressed as mg/mL.Quantitative determination of soluble sugars was conducted by anthrone reagent according to the method of Wu Zhilin et al.[24], and soluble sugar content was expressed as mg/mL.Nucleic acids were estimated by measuring the optical density at 260 nm following method of Cai Jianghua et al.[25].
1.3.6 Determination of cell survival
Cell survival was assayed following the method of Wang Kaituo et al.[26].Two milliliter spore suspension ofB.cinerea(1 × 106spores/mL) were mixed with 100 mL of 0.1 mol/L phosphate buffer (pH 7.4), 0.2 gD-glucose, and 200 μLL.plantarumXCT 1-10 (1 × 108CFU/mL), and then incubated at 120 r/min for 0, 3, 6, 12, 24, 48 h at 26 ℃.The mixtures withoutL.plantarumXCT 1-10 were used as the negative control and withoutB.cinereawere used as the positive control.An Accuri C6 flow cytometer (BD Biosciences, Beijing, China) was used to detect the spores.There were 3 replicates for each sample, and each replicate sorted 10 000 spores.
1.3.7 Effects ofL.plantarumXCT 1-10 on lesion development of apple fruit inoculated withB.cinerea
Inoculation of apple fruit was conducted according to Ge Yonghong et al.[18], with slight modifications.Apple fruit with similar size and maturity and without damages were selected and disinfected with 70% ethanol, then wounded with a sterile nail four wounds (each wound 0.2 cm deep ×0.3 cm wide) around the equator.After air-drying, various concentrations ofL.plantarumXCT 1-10 at 0, 102, 104,106, 108, and 1010CFU/mL were injected into each wound.Four hours later, 20 μL spore suspension (106spores/mL) ofB.cinereawas inoculated in each wound.Inoculated fruit were kept at 26 ℃ with 70%–80% relative humidity in plastic trays.The lesion diameter was recorded at 2, 4 and 6 d after inoculation.Each treatment contained 30 fruit, and the whole experiment was repeated twice.
1.4 Statistical analysis
The experiment was carried out 3 times with 3 repetitions at a time.Statistical data were performed by SPSS software (SPSS Inc., Chicago, IL, USA).Duncan’s multiple range test of analysis of variance (ANOVA) was carried out to determine the differences of the means (P< 0.05).
2 Results and Analysis
2.1 Effect of L.plantarum XCT 1-10 on mycelia growth and spore germination of B.cinerea
The colony diameter ofB.cinereaincreased gradually in the control medium, and nearly covered the entire PDA plate at 10 d.However, the growth ofB.cinereawas suppressed byL.plantarumXCT 1-10 treatments (Fig.1A), showing a dose-dependent trend.Colony growth was significantly inhibited byL.plantarumXCT 1-10 at 106, 108and 1010CFU/mL.And the inhibition rates were 26.77%, 42.42%and 51.61%, respectively (Fig.1B).
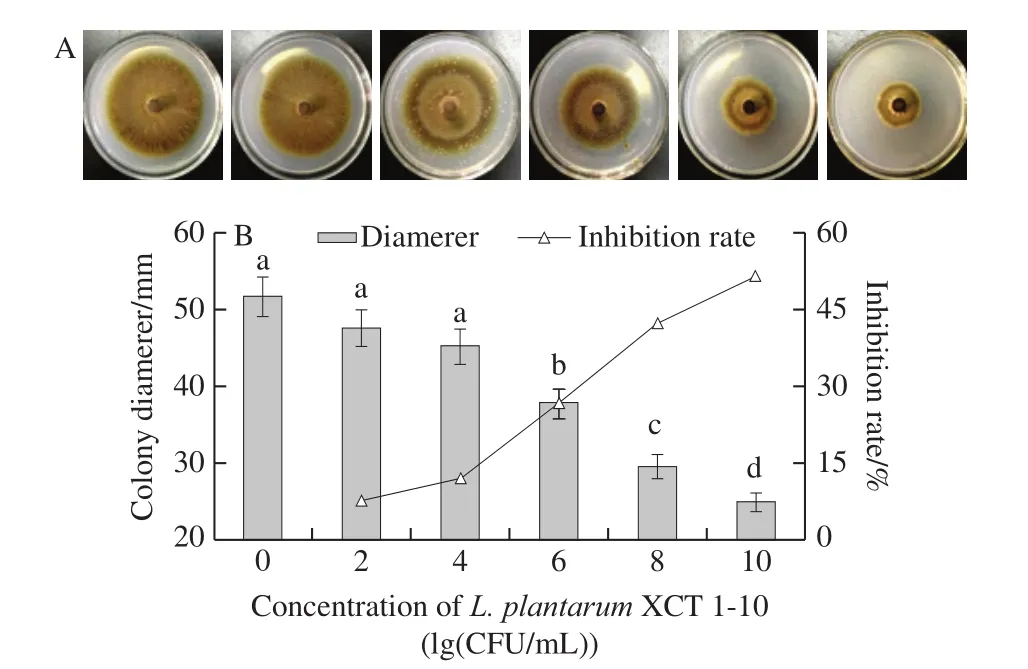
Fig.1 Effect of L.plantarum XCT 1-10 at different concentrations on the morphology (A) as well as colony diameter and inhibition rate (B) of B.cinerea in vitro after incubation at 26 ℃ for 10 days
Fig.2 demonstrates that spore germination rate ofB.cinereaincreased with the incubation time, and was restrained by different concentrations ofL.plantarumXCT 1-10.Compared with the control,L.plantarumXCT 1-10 at 102CFU/mL significantly suppressed the germination of the spores.The spore germination rate of the control was 92.0% at 12 h, which was significantly higher thanL.plantarumXCT 1-10 treatments.The germination rate of 102, 106, 108and 1010CFU/mL was 74.3%, 73.0%, 71.0%,73.0% and 57.7%, respectively.Combining the above results,high concentration ofL.plantarumXCT 1-10 has a strong suppressing effect on the growth ofB.cinerea.
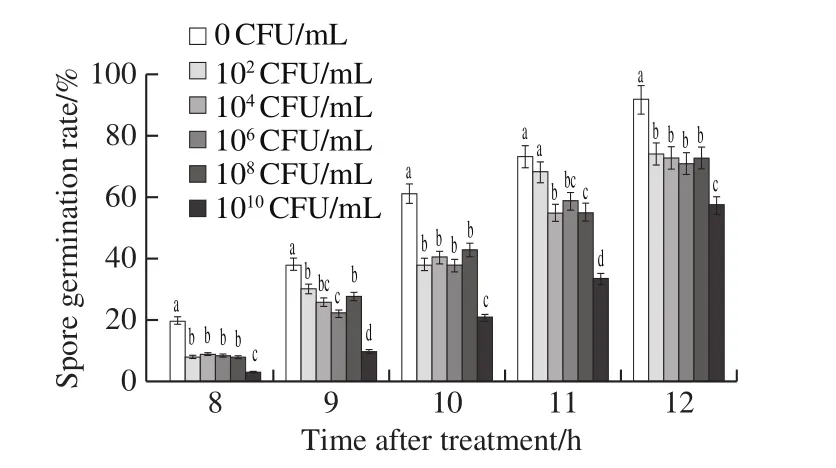
Fig.2 Effect of L.plantarum XCT 1-10 at different concentrations on spore germination of B.cinerea in vitro during incubation at 26 ℃
2.2 Effect of L.plantarum XCT 1-10 on the content of soluble sugar, soluble protein, and nucleotides release of B.cinerea
Soluble sugar content remained stable in both theB.cinereaandB.cinerea+L.plantarumXCT 1-10 media at 0?2 h, reached the maximum at 4 h, and then decreased in the following time (Fig.3A).The content of soluble sugar inL.plantarumXCT 1-10 inoculated media maintained at low level during the entire incubation time.Compared to the media inoculated withL.plantarumXCT 1-10 orB.cinerea,the soluble sugar content in the mediainoculated withB.cinerea+L.plantarumXCT 1-10 increased significantly at 1, 3, 4 and 5 h.
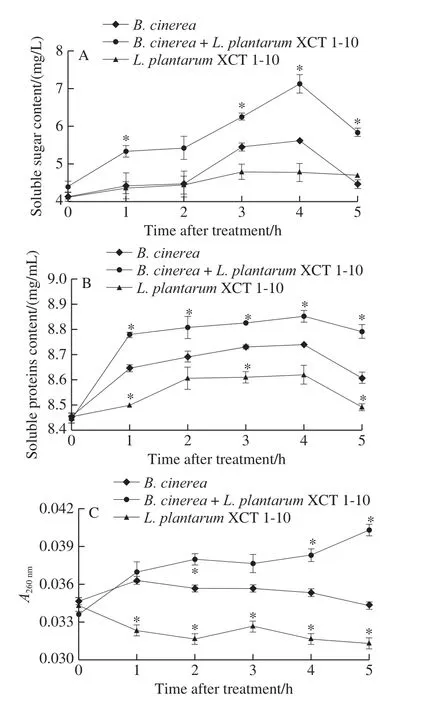
Fig.3 Effect of L.plantarum XCT 1-10 on the contents of soluble sugar (A), soluble proteins (B) and nucleotides (C) in B.cinerea during incubation at 26 ℃
Fig.3B shows that the content of soluble protein in the three treatments was similar, which increased rapidly at 0?4 h,then decreased at 4?5 h.Soluble protein content in the media inoculated withL.plantarumXCT 1-10 was significantly lower than that ofB.cinereaat 1, 3 and 5 h.Soluble protein content in the mediainoculated withB.cinerea+L.plantarumXCT 1-10 was significantly higher than that of the media only inoculated withB.cinereaat 1, 3, 4 and 5 h.
As demonstrated in Fig.3C, nucleic acid leakage in the media inoculated withB.cinereaandL.plantarumXCT 1-10 remained largely stable at 0?5 h.Nucleic acid leakage in theL.plantarumXCT 1-10-treatedB.cinereamedia increased gradually, and was significantly higher at 2, 4 and 5 h than theB.cinerea.At 5 h, the nucleic acid release ofL.plantarumXCT 1-10 was 91% ofB.cinerea, and that ofL.plantarumXCT 1-10-treatedB.cinereawas 17% higher than that ofB.cinerea.
2.3 Effect of L.plantarum XCT 1-10 on MDA content and relative conductivity of B.cinerea
MDA content and relative conductivity in the media inoculated withB.cinereaorL.plantarumXCT 1-10 kept at a low level during the entire incubation time (Fig.4).MDA content in the media inoculated withB.cinereaandL.plantarumXCT 1-10 was significantly higher than the media only inoculated withL.plantarumXCT 1-10 orB.cinereaat 3, 4 and 5 h (Fig.4A).The relative conductivity of theL.plantarumXCT 1-10-treatedB.cinereawas significantly lower than theB.cinereaorL.plantarumXCT 1-10 at 3, 4 and 5 h, which was 54% and 56% at 5 h, respectively (Fig.4B).
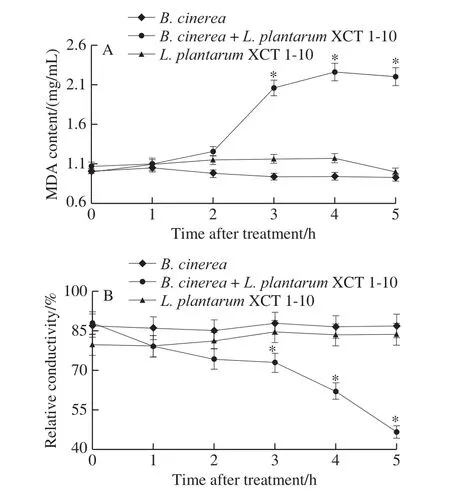
Fig.4 Effect of L.plantarum XCT 1-10 on MDA content (A) and relative conductivity (B) of B.cinerea during incubation at 26 ℃
2.4 Changes in the cell survival of B.cinerea after L.plantarum XCT 1-10 treatment
The survival rate ofL.plantarumXCT 1-10 increased slightly at 0?6 h, and then kept around 92% at 6?48 h (Fig.5).However, the survival rate ofL.plantarumXCT 1-10 was significantly higher than that of theB.cinereaandB.cinerea+L.plantarumXCT 1-10 at 0?24 h.The addition ofL.plantarumXCT 1-10 to the media inoculated withB.cinereadecreased the survival rate ofB.cinerea.
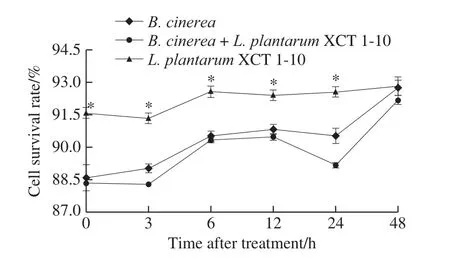
Fig.5 Effect of L.plantarum XCT 1-10 on the cell survival rate of B.cinerea during incubation at 26 ℃
2.5 Effects of L.plantarum XCT 1-10 on lesion development of apple fruit inoculated with B.cinerea
Lesion diameter developed with storage time, and different concentrations ofL.plantarumXCT 1-10 inhibited the growth of lesions on apple fruit inoculated withB.cinerea(Fig.6).On the second day, compared with the control,there was no significant difference in other concentrations ofL.plantarumXCT 1-10 except 1010CFU/mL significantly promoted the increase of lesion diameter.On day 3, 102, 104,106and 108CFU/mLL.plantarumXCT 1-10 significantly inhibited the lesion diameter on the apple fruit.On the fourth day, 104, 106and 108CFU/mLL.plantarumXCT 1-10 remarkably delayed the increase of the lesion diameter.In addition, the inhibitory effect of 106CFU/mLL.plantarumXCT 1-10 was better than that of the other concentrations.
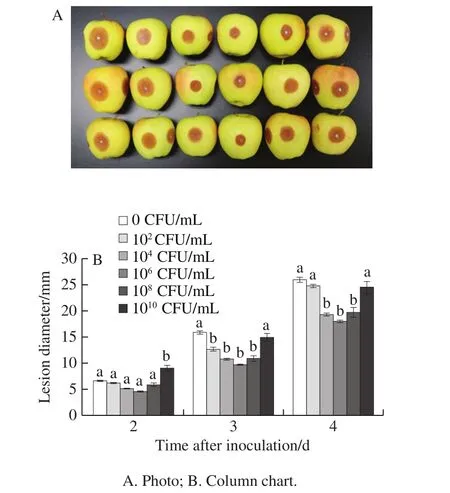
Fig.6 Effect of L.plantarum XCT 1-10 at different concentrations on lesion diameter of apple fruit inoculated with B.cinerea during storage at 26 ℃
3 Discussion
LAB are considered as biological agents for food safety and widely used in various fermented foods to inhibit the growth of mould and prolong the shelf life of the product[27].Interestingly, LAB is also part of the intestinal flora and has a beneficial effect on human health.Twenty-five percent of the food crops in the world are contaminated with toxins which caused economic losses about millions of dollars each year[28].Many studies have shown that LAB cell-free suspension has been used as a safe biological control agent in fruits and vegetables due to its properties of antifungal growth and inhibition of toxins production[29-30].L.plantarumCUK-501 showed a strong inhibitory effect on the growth of grey mould and significantly inhibited cucumber spoilage[31].Our results proved thatL.plantarumXCT 1-10 did have a strong antifungal activity against the growth ofB.cinereain apples.
The production of organic acids by LAB is also the main reason for its antimicrobial activity[28,32].Organic acids diffuse to the cell membrane, causing the disorder of electrochemical proton gradient, reducing the pH in the cell, thus inhibiting the growth and even death of fungi[33].In addition, LAB could degrade or adsorb mycotoxins by producing organic acids mainly lactic acid and phenolic compounds to inhibit fungal growth and toxin production[28].Guimar?es et al.[16]have proved thatL.plantarumUM55 produced lactic, phenyllactic,hydroxyphenyllactic and indole lactic acids to inhibit the growth ofA.flavus.It has been reported that the inhibitory capability of LAB by producing organic acids was used to regulate pH, which inhibited food spoilage microorganisms includingColletotrichum gloeosporioides,B.cinerea,P.expansum, andA.flavus[32].Our results showed thatL.plantarumXCT 1-10 could inhibit the growth of mycelia and spore germination ofB.cinerea, showing a dosedependent trend.It was similar thatL.plantarum21B could efficiently restrain spore germination ofEurotium repens,P.expansum,Endomyces fibuligerandA.niger[34].Lv Xinran et al.[17]reported thatL.plantarumC10 suppressed the mycelia growth ofT.roseumin vitro.
Combined the spore germination and mycelia growth results, 106CFU/mLL.plantarumXCT 1-10 were selected to study the possible mechanisms.The present study demonstrated thatL.plantarumXCT 1-10 could destroy membrane integrity ofB.cinerea, therefore inhibited its growth.Khalaf et al.[35]found thatLactobacillusNC8 could destroy the outer membrane ofPorphyromonas gingivalisthat leaked out a large number of intracellular substances and caused the cell death.MDA is one of the principal products of lipid peroxidation in a plasma membrane that can cause the cross-linking reaction of proteins, polysaccharides, nucleic acids, and other macromolecules.LAB can produce hydrogen peroxide[17]under aerobic conditions, which is a strong oxidant and can form more toxic hydroxyl free radicals,leading to membrane lipid peroxidation.After 2 h incubation,L.plantarumXCT 1-10 showed higher activity, and MDA accumulated rapidly by producing more hydrogen peroxide.MDA content increased after exposure toL.plantarumXCT 1-10 suggests thatL.plantarumXCT 1-10 destroyed cell membrane ofB.cinerea.Similar results have previously reported onB.cinereaafter treating with rapamycin[36]and dicarboximide[37].Simultaneously,L.plantarumXCT 1-10 also broke the electric balance, resulted in reducing relative conductivity.The destruction of cell membrane might lead to the damage of membrane binding protein that is related to the maintenance of ionic homeostasis[38].Therefore, the changes of conductivity could reflect the permeability of cell membrane.Our results showed that the electrolyte was released from the cytoplasm and resulted in a decrease in relative conductivity.
The destruction of cell membrane accelerated the soluble sugar, protein, and nucleotides flow out of the cell.The present study demonstrated that the content of soluble protein,sugar and nucleotides increased in the media inoculated withL.plantarumXCT 1-10 andB.cinerea, indicating the damaging of membrane integrity byL.plantarumXCT 1-10.Carbohydrate, lipid, protein, and nucleic acid are the important biomolecules[39].Sugar does not only provide energy for the cell, but also provide a carbon skeleton for the synthesis of other important biological molecules such as amino acids, nucleic acids, and fatty acids[40].Protein and nucleic acid are the main components of protoplasm in the cells, and the synthesis of proteins depends on nucleic acids[41].Insufficient sugar, protein and nucleic acid could inhibit the growth of living organisms or seriously lead to cell death.The results of flow cytometry also proved thatL.plantarumXCT 1-10 treatment decreased the cell survival ofB.cinerea.These results suggest thatL.plantarumXCT 1-10 treatment caused cell death partially due to inhibiting the growth and metabolism ofB.cinerea.
In vitrostudies demonstrated thatL.plantarumXCT 1-10 could indeed inhibit the growth and affect the survival ofB.cinerea.Our results on apple fruit inoculated withB.cinereaproved thatL.plantarumXCT 1-10 could inhibit gray mould on fruit, and the results also showed that 106CFU/mLL.plantarumXCT 1-10 was the optimal concentration.This result was similar to that of direct inhibition of the growth of grey mould byL.plantarumXCT 1-10in vitro.It has also been reported thatL.plantarumIMAU10014 had a good control effect on tomato leaves infected withB.cinerea[42].The use ofL.plantarumstrains on the surface of litchi and lotus root slices showed that they could effectively improve postharvest quality[14-15].In addition, application of LAB might increase disease resistance of the fruit, thus resisting the further growth of the fungus.Li Junsheng et al.[15]found that application ofL.plantarum(LH-B02) could produce metabolic enzymes to degrade phenols, thus limiting the enzymatic browning of lotus root and extending shelf life.It has been reported that sprayingL.plantarumcould delay the browning of litchi and maintain the contents of cyanidin-3-rutinoside and total anthocyanins[14].Cell-free supernatant ofL.plantarumC10 increased defense enzymes, promoted the accumulation of phenols and flavonoids, and finally inhibited the growth of fungi in muskmelons[17].Optimal concentrations ofL.plantarumXCT 1-10 repressed lesion development in apple fruit inoculated withB.cinerea, but too high concentrations ofL.plantarumXCT 1-10 increased lesion diameter.We speculated that the high content of some metabolites ofL.plantarumXCT 1-10 might be beneficial to the infection of grey mould.Moreover, the anti-fungal mechanism ofL.plantarumXCT 1-10 might also be the competition of nutrients and the secretion of bacteritin.More studies are still needed to do in the future to fully elucidate the mechanisms involved in the inhibition of gray mould in apples byL.plantarumXCT 1-10 and the efficient application methods in the practical application process.
In a conclusion,L.plantarumXCT 1-10 could significantly inhibit the growth of gray mould in apple fruit.Moreover,L.plantarumXCT 1-10 is efficient in suppressing mycelia growth and spore germination ofB.cinereain vitro.The mechanisms involved in the inhibition partially because of destroying the cell membrane and leading to the leakage of soluble sugar, protein and nucleic acid.

