D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure
Jian-Zhu Bo , Ling Xue , Shuang Li, Jing-Wen Yin Zheng-Yao Li Xi WangJun-Feng Wang Yan-Shu Zhang ,
Abstract Although exogenous D-serine has been applied as a neural regulatory intervention in many studies, the role played by D-serine in hippocampal injuries caused by lead exposure remains poorly understood. Rat models of chronic lead exposure were established through the administration of 0.05% lead acetate for 8 weeks. Simultaneously, rats were administered 30 or 60 mg/kg D-serine, intraperitoneally, twice a day. Our results showed that D-serine treatment shortened the escape latency from the Morris water maze, increased the number of times that mice crossed the original platform location, and alleviated the pathological damage experienced by hippocampal neurons in response to lead exposure. Although D-serine administration did not increase the expression levels of the N-methyl-D-aspartate receptor subtype 2B(NR2B) in the hippocampi of lead-exposed rats, 60 mg/kg D-serine treatment restored the expression levels of NR2A, which are reduced by lead exposure. These findings suggested that D-serine can alleviate learning and memory impairments induced by lead exposure and that the underlying mechanism is associated with the increased expression of NR2A in the hippocampus. This study was approved by the Animal Ethics Committee of North China University of Science and Technology, China (approval No. LX2018155) on December 21, 2018.
Key Words: D-serine; hippocampus; lead; neurological function; N-methyl-D-aspartate; poisoning; protection; repair
Introduction
Lead, which is a ubiquitous environmental pollutant, causes a wide variety of long-lasting adverse effects in humans.Epidemiological and toxicological data have shown that lead exposure can impair the central nervous system (Sharma et al., 2015; Assi et al., 2016). A growing body of evidence has demonstrated that declining cognitive capacity and behavioral dysfunctions are the most common outcomes of lead exposure (Zeng et al., 2018; Santa Maria et al., 2019).Previous studies have shown that lead can induce learning and memory deficits associated with selective accumulation in the hippocampus, which is a basic anatomical structure associated with learning and memory (Zhou et al., 2018).
Evidence has suggested that damage to long-term potentiation (LTP) mechanisms, associated with N-methyl-D-aspartate receptor (NMDAR) injury, may represent the underlying mechanism that is responsible for neurologic damage observed in lead-exposed animals. Lead is a non-competitive NMDAR agonist capable of affecting the production and induction of LTP through the activation of the NMDAR, resulting in learning and memory impairments in rodents. Electrophysiological studies performed on isolated hippocampal neurons have shown that lead exposure can reduce the frequency of NMDAR single-channel opening.However, the mechanism through which lead causes NMDAR-mediated damage during the development of LTP in the hippocampus remains unclear. NMDAR is well-known to act as a crucial excitatory amino acid receptor in learning and memory processes. A previous study showed the removal of the NMDAR NR1 gene from the CA1 region in mice resulted in the development of learning and memory deficits associated with deficits in LTP (Wise and Lichtman, 2007). Lin et al. (2007)found that the disruption of the NMDAR downstream signaling pathway can prevent the induction of LTP and the formation of hippocampal-dependent memories. NMDAR is a heterooligomer, composed of seven subunits that belong to the NR1,NR2, and NR3 gene families (Rondi-Reig et al., 2006). The NR2 subunit represents a regulatory subunit for the NMDAR and is primarily distributed in the hippocampus (Scherzer et al., 1998). NR2 acts to modify NMDAR channel function, and at least one NR2 subunit is necessary to form a complete,functional NMDAR (Fodor and Nagy, 2007). Previous reports have shown that the various NR2 subunits are associated with different contributions to learning and memory functions(Sprengel et al., 1998; Fox et al., 2006; Gilmartin et al., 2013).Learning and memory deficits have been associated with reductions in NR2B gene expression during late-stage cerebral ischemia/reperfusion injuries. Repeated injections of ketamine in rats resulted in impaired learning and memory function and decreased NR2A and NR2B contents in the hippocampus(Wako et al., 1995). Evidence has suggested that NR2 may be an essential subunit during the process of learning and memory.
In the human brain, D-serine is converted from L-serine by serine racemase, which can act as a selective agonist of the glycine binding site of postsynaptic NMDARs (Klass et al.,2017). Previous studies have demonstrated that D-serine has significant prophylactic actions against learning and memory deficits in rat and mouse models (Mothet et al., 2006; Taylor et al., 2014; Han et al., 2015). By enhancing the activity of glutamate-activated NMDARs, D-serine can produce a series of effects, including depolarization, calcium influx, the release of neurotransmitters, and neuronal death (Montes., 2018).D-amino acid oxidase has been shown to selectively degrade D-serine in the rat brain, resulting in decreased NMDAR activity, which was completely reversed by the administration of exogenous D-serine (Rahman et al., 2012). D-serine can enhance fear extinction by increasing GluA2-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor endocytosis and may serve as a potential therapeutic agent against learning and memory disorders (Bai et al.,2014). However, few studies have examined the influence of lead exposure on D-serine in the central nervous system and the effects of D-serine interventions on lead exposure models.Therefore, this study aimed to investigate the potential neuroprotective effects of D-serine against lead exposureinduced injury in the rat hippocampus.
Materials and Methods
Animals and treatments
This study was approved by the Animal Ethics Committee of North China University of Science and Technology, China(approval No. LX2018155) on December 21, 2018. The animals were cared for in accordance with the guidelines issued by North China University of Science and Technology for the Care and Use of Laboratory Animals.
Sixty specific-pathogen-free, male, Sprague-Dawley rats, aged 21 days, were purchased from Beijing Hua Fukang Biological Polytron Technologies Inc. [License No. SCXK (Jing) 2014-0004]. The rats were fed a standard diet, allowed free access to double-distilled water, and were acclimatized for 1 week prior to experimentation, under a 12-hour light/dark cycle at 25 ± 2°C. The rats were randomly divided into four groups,with 15 rats in each group. The rats in the control group were treated with 500 mg/kg sodium acetate (Aladdin, Shanghai,China), administered in the drinking water. The rats in the lead, 30 mg/kg D-serine, and 60 mg/kg D-serine groups were treated with 0.05% lead acetate (Aladdin), administered in the drinking water, for 8 weeks. The rats in the 30 and 60 mg/kg D-serine groups were also intraperitoneally injected with D-serine (Sigma-Aldrich, St. Louis, MO, USA) at the indicated dose, twice a day for 8 weeks. The rats in the control and lead groups were intraperitoneally injected with the same amount of saline (500 mg/kg).
Morris water maze test
To test whether learning and memory abilities were impaired,rats were subjected to the Morris water maze test, 24 hours after the last D-serine injection. Briefly, both place navigation tests and space probe trials were performed (Zhao et al.,2018). In the place navigation test, rats underwent one trial per day for 4 consecutive days. Rats were placed in each of four quadrants and learned to find a hidden platform, which was located 2 cm below the water surface in a pool (1.8 m in diameter). Escape latency was defined as the time required to find and climb onto the platform. If the animal was unable to find the platform within 2 minutes, the time recorded for this test was 120 seconds. On day 5, the space probe trial test was performed. Briefly, after the hidden platform was removed, the rats were placed in the water, in a quadrant other than where the platform had been, and a 60-second trial was performed.The number of times of the rats crossed the previous platform location was recorded. The cumulative time, cumulative distance, and speed were recorded using a Water Maze Video Tracking System (Zheng Hua Biological Instrument Equipment Co., Huaibei, China). All cognitive tasks were performed between 10:00 and 17:00 during the light-off phase.
Inductively coupled plasma-mass spectrometry
After performing the learning and memory tasks, the rats were sacrificed by sodium pentobarbital injection (120 mg/kg,Sigma-Aldrich), and serum and brain tissue were collected.The lead contents in the hippocampus and blood were determined by inductively coupled plasma-mass spectrometry(Li et al., 2015). Tissue samples were digested with spectrapure nitric acid, in a MarsX microwave mineralizer (CEM Corp.,Charlotte, NC, USA). Then, samples were filtered through a 0.22-μm polyethersulfone syringe filter. Next, the lead contents of the tissue samples were determined using an inductively coupled plasma-mass spectrometry spectrometer(Agilent Technologies, Palo Alto, CA, USA).
Hematoxylin and eosin staining and Nissl staining
Hematoxylin and eosin staining was performed, according to the standard procedure, after an 8-week D-serine intervention(Zhang et al., 2015). The hippocampal samples were fixed in 4%paraformaldehyde, dehydrated with 75% alcohol, embedded in paraffin, and cut into 4-μm thick slices. Paraffin-embedded slices were stained with hematoxylin and eosin, dewaxed with xylene, and dehydrated with an alcohol gradient. Pathological reactions were observed using a high magnification lens on a light microscope (Leica, Wetzlar, Germany).
Nissl staining was performed according to the standard procedure. Hippocampal samples were post-fixed in 4%paraformaldehyde. Paraffin sections were coronally cut at a thickness of 5 μm. The sections were stained with Nissl stain. Nissl staining solution was purchased by Beyotime Biotechnology (Cat# C0117; Shanghai, China). Pathological reactions in hippocampal neurons were observed in Nissl-stained tissue sections, using a high magnification lens on a light microscope.
Immunohistochemistry
Brain samples were obtained after an 8-week D-serine intervention, fixed in 4% paraformaldehyde (Aladdin), and placed in a 20% sucrose solution overnight at 4°C. Brain tissues were treated in xylene and embedded in paraffin,and sections (4-μm) were cut using a paraffin wax slicing machine (Leica, Heidelberg, Germany). Antigen retrieval was performed in citric acid buffer (pH 6.0), using a microwave oven. The sections were cooled to room temperature and transferred to 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase. The sections were washed three times with 0.01 M phosphate-buffered saline (5 minutes for each wash) and blocked with appropriate goat serum (blocking solution; 1:50; Beijing Boosen Biological Technology Co., Ltd.,Beijing, China), for 1 hour at 37°C. The slices were incubated with primary antibodies, overnight at 4°C. The primary antibodies used included rabbit anti-NR2A antibody (1:50;Cat# bs-3304R; Beijing Boosen Biological Technology Co.,Ltd.) and rabbit anti-NR2B antibody (1:200; Cat# bs-0222R;Beijing Boosen Biological Technology Co., Ltd.). Subsequently,the sections were equilibrated to room temperature, washed with phosphate-buffered saline, 3 times for 5 minutes each,and incubated with biotin-labeled goat anti-rabbit IgG (1:200;Cat# bs-0295G-Bio; Beijing Boosen Biological Technology Co.,Ltd.), for 30 minutes at 37°C. The sections were washed with phosphate-buffered saline, three times for 5 minutes each.Next, horseradish peroxidase-labeled avidin (1:1000; Cat# bs-0437P-HRP; Beijing Boosen Biological Technology Co., Ltd.)was added, and the sections were incubated for 30 minutes at 37°C. Subsequently, the sections were developed with freshly prepared 3,3′-diaminobenzidine working fluid (1:1000; Cat#C-0003; Beijing Boosen Biological Technology Co., Ltd.). The samples were counterstained with hematoxylin. After being treated with alcohol and xylene, mounted on slides, and sealed with neutral balsam, the samples were observed under a light microscope (Nikon, Tokyo, Japan).
Real-time polymerase chain reaction
Total RNA was extracted from the hippocampus, after an 8-week D-serine intervention, using Trizol reagent(Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into complementary DNA. Real-time polymerase chain reaction was performed to determine the expression of genes using a Roche FastStart DNA Green Master kit (Roche LightCycler96,Basel, Switzerland). Polymerase chain reaction primers were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,USA). The primer sequences were as follows: NR2A, 5′-GAA CAC AGA GCT CAT CCC CAA-3′ and 5′-AGA TCC CAA GAC CGT CTC TCA-3′; NR2B, 5′-TTG ATG AAA TCG AGC TGG CCT-3′ and 5′-AA GTC TCG GAG CCC TTC TTTG-3′; and glyceraldehyde-3-phosphate dehydrogenase, 5′-CCT GGA GAA ACC TGC CAA GTAT-3′ and 5′-AGC CCA GGA TGC CCT TTA GT-3′. The threshold cycle (Ct) value for each test gene was normalized to that for glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD)for each group. Differences were analyzed using a one-way analysis of variance, followed by Tukey’spost hoctest, using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). A value ofP< 0.05 was considered significant.
Results
D-serine enhances the learning and memory performance of lead-exposed rats
To evaluate the effects of the D-serine intervention, we used the Morris water maze to analyze the spatial learning and memory abilities of the treated rats. On day 4, the mean escape latency for the lead group was significantly longer than that for the control group (P< 0.05). The mean escape latencies in the 30 and 60 mg/kg D-serine groups were shorter than those for the lead group (P= 0.028 andP= 0.001,Figure 1A). As shown in Figure 1B, the number of times that the animals in the 30 mg/kg D-serine and 60 mg/kg D-serine groups crossed the expected location of the hidden platform was significantly higher than that in the lead group (P<0.0001). The results of the Morris water maze test showed that the D-serine intervention reversed the decline in learning and memory abilities induced by lead exposure. The effects of D-serine on the performance of lead-exposed rats in the place navigation test are shown in Additional Tables 1 and 2.
D-serine does not affect the lead contents in either the hippocampus or blood of lead-exposed rats
The lead contents in the hippocampus and blood samples were determined using inductively coupled plasma-mass spectrometry. The lead contents in hippocampus samples from rats in the lead group were significantly higher than those in the control group (P= 0.001). No significant difference was observed in the lead contents between the lead and D-serine treatment groups (P> 0.05). The evaluation of lead contents in the blood samples showed the same trend as was observed in the hippocampus (P< 0.001; Figure 2).
D- serine reduces the pathological damage observed in the hippocampus of lead-exposed rats
Alterations in hippocampal morphology were assessed by hematoxylin-eosin staining (Figure 3A). The cells in the hippocampus of control rats were arranged closely,with complete and plump structures, a clear nucleus, and homogeneous cytoplasm. In the lead-exposed rats, the number of cells in the hippocampus decreased, the cell arrangement was disordered, and a large number of cells had died, indicated by nuclear condensation and unclear cell boundaries. Compared with lead-exposed rats, the number of cells in the hippocampal samples from D-serine treated rats increased, and the disordered cell arrangement improved.The administration of 60 mg/kg D-serine resulted in a marked decrease in the number of dead cells when compared with the number of dead cells following the administration of 30 mg/kg D-serine. The Nissl staining method was used to observe the morphology of hippocampal neurons (Figure 3B).In the control group, the neurons of the hippocampus were arranged closely and neatly, with clear structures. For the leadexposed rats, hippocampal neurons were arranged loosely and appeared disordered, whereas, in D-serine-treated rats,the neuronal arrangement was improved. The administration of 60 mg/kg D-serine resulted in a marked decrease in the disruption of the spatial arrangement of neuronal cells compared with that observed for the administration of 30 mg/kg D-serine.
Effects of D-serine on the expression of NMDAR in the hippocampus of lead-exposed rats
The expression patterns for NR2A and NR2B in the hippocampus were observed by immunohistochemical staining. The level of NR2A immunopositivity in hippocampal samples from rats in the lead group was lower than that in the control group (P< 0.05). Compared with the lead group,NR2A immunopositivity in the hippocampus of the 60 mg/kg D-serine group was significantly increased (P= 0.003), but no significant difference was observed for NR2A immunopositivity between the 30 mg/kg D-serine and lead groups (P> 0.05).No significant differences in NR2B immunopositivity were observed among the four groups (P= 0.081). Real-time polymerase chain reaction was used to detect the mRNA expression levels of NR2A and NR2B in the hippocampus. The resultant mRNA expression levels were consistent with the protein immunopositivity results (Figure 4).

Figure 1 | Effects of D-serine on the cognitive performance of lead-exposed rats.

Figure 2|Effects of D-serine on lead content in the hippocampus (A) and blood (B) samples from lead-exposed rats.

Figure 3| Effects of D-serine on the hippocampal structure of lead-exposed rats.
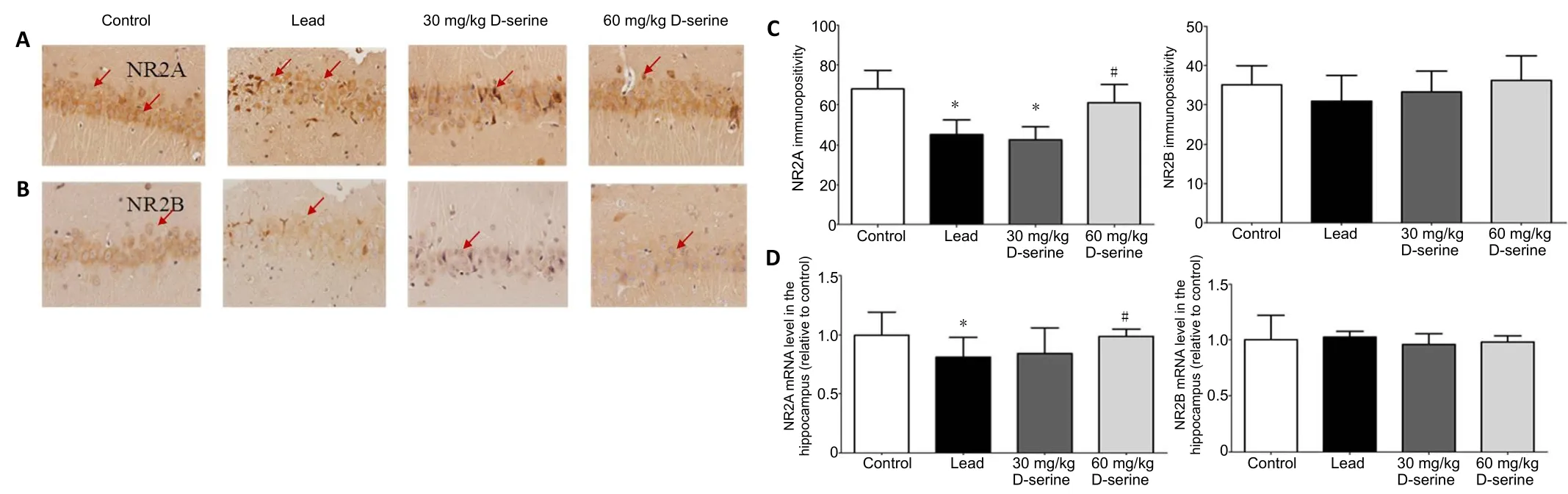
Figure 4| Effects of D-serine on the expression of NR2A and NR2B in the hippocampus of lead-exposed rats.
Discussion
To confirm whether D-serine intervention was sufficient to improve learning and memory abilities in lead-exposed rats,we conducted an animal study using a D-serine intervention.Our data showed that lead exposure could induce damage to hippocampal cells, associated with learning and memory deficits. After D-serine injection, the learning and memory abilities of treated rats were significantly enhanced, and the injury to hippocampal neurons was partially alleviated,which is likely related to the observed increase in NR2A expression in the hippocampus of D-serine-treated rats.These findings indicated that exogenous D-serine treatment was able to repair damage to the hippocampus induced by lead exposure in rats, which improved learning and memory abilities. Our results showed that the lead contents in the hippocampal and blood samples from treated rats increased significantly after 8 weeks of lead exposure. The data also showed that D-serine did not affect either the lead contents in either hippocampal or blood samples of lead-exposed rats. These results suggested that D-serine antagonizes lead neurotoxicity, possibly by affecting the lead contents in other parts of the body. A previous study showed that exogenous D-serine administration relieved chronic lead exposureinduced deficits in synaptic plasticity via NMDAR activation(Jiang et al., 2017). NMDAR activation is necessary for LTP induction in the central synapse (de Lima et al., 2005). LTP is believed to represent the cellular and molecular mechanism that underlies hippocampus-dependent learning and memory functions (Baudry et al., 2015). Therefore, NMDAR regulation may represent an important target for the treatment of nerve injuries associated with lead exposure.
NMDARs are primarily composed of NR1 and NR2 subunits,and NMDAR activation is necessary for cognitive functions and memory formation in animals (Boomhower and Newland,2017; Wang et al., 2019; Liu et al., 2020). NR2 serves as the regulatory subunit of the NMDAR (Bickler et al., 2003). The present study indicated that D-serine can reverse the lead exposure-induced decrease in NR2A expression in a dosedependent manner. A previous study showed that NMDAR structure and function did not mature until the third week after birth, and during the process of NMDAR maturation, the NMDAR structure transformed from an NR1/NR2B receptor into and NR1/NR2A receptor (Yashiro and Philpot, 2008).
Evidence has suggested that NMDAR-dependent currents and LTP were decreased in the hippocampus of NR2A knockout mice, which also displayed cognitive deficits. Mice with the targeted deletion of NR2A subunit C presented visual-spatial working memory impairments (Boyce-Rustay and Holmes,2006; Brigman et al., 2008).
In addition, NR2B plays an important role in the structure and function of NMDARs (Morrone et al., 2007). In a transgenic mouse model that overexpressed NR2B in the forebrain,specific voltage-dependent stimulation of NMDAR activity and synaptic transfer were enhanced (Neal et al., 2011).These results suggested that NR2B may play an important role in the modulation of synaptic transmission and electrical activity. Lead is a potent, specific, non-competitive antagonist of the NMDAR, which interacts at the Zn regulatory site of NMDAR complexes that contain the NR2A subunit but not those that contain the NR2B subunit (Usuda et al., 2018).However, a previous study found that the protein and mRNA levels of NR2A and NR2B were decreased in the hippocampal neurons of lead-exposed rats (Han et al., 2015). In the present study, the expression of NR2B in the hippocampus did not change following either lead exposure or exogenous D-serine administration.
D-serine is a D-amino acid that can be found at high levels in the mammalian brain, where it is required for excitatory neurotransmission. A previous study showed that D-serine is the dominant endogenous co-agonist for NMDAR neurotoxicity(Bai et al., 2014). D-serine can enhance the glutamateinduced activation of NMDAR by binding to the glycine site,which is essential for NMDAR-dependent potentiation and the depression of synaptic transmission. Exogenous D-serine has also been shown to rescue the impairment of hippocampal LTP associated with sodium fluoroacetate exposure (Duffy et al., 2008).
A previous study has shown that increasing D-serine levels,through pharmacological or genetic means, can reverse learning deficits (Matsuda et al., 2010). In the present study,we found that exogenous D-serine administration dramatically improved spatial learning and memory, as assessed by the Morris water maze test, and had a positive effect on hippocampal impairments associated with lead exposure. In addition, the increased expression of NR2A was observed in rats subjected to 60 mg/kg D-serine injection. Although the rats injected with 30 mg/kg D-serine showed the reduction of learning and memory impairment, they did not show any significant changes in NR2A or NR2B expression levels in the hippocampus. Therefore, determining the mechanism through which 30 mg/kg D-serine alleviates the learning and memory impairments of rats after lead exposure requires further research. Low levels of D-serine may rescue some signaling pathways. These results implied that D-serine may enhance spatial learning and memory abilities after surpassing a threshold concentration, through the upregulation of NR2A expression. Our results were not consistent with those reported by Andersen and Pouzet (2004), which may be due to the use of different toxicants or neurobehavioral tests that measure different aspects of spatial learning and memory(Andersen and Pouzet, 2004).
This study has certain limitations. Although this study suggests that D-serine may represent a candidate drug with certain recovery effects for memory impairment and neuronal damage caused by lead exposure, whether it can be used to cure center nervous system diseases still requires further research. For example, D-Serine should be used to treat rats after lead exposure to verify whether it can rescue leadinduced damage as well as prevent it. In addition, NR2A knockdown mice can be used to identify the mechanisms through which D-serine functions to alleviate hippocampal damage. Additionalin vivostudies and possibly even clinical trials are necessary to clarify whether D-serine can be applied to improve memory impairments (Liu et al., 2009; Fuchs et al.,2012).
Taken together, our results showed that interventions using certain doses of D-serine were able to prevent learning and memory deficits induced by lead exposure. NR2A mRNA and protein levels were up-regulated, which may alleviate damage to hippocampal cells, suggesting that D-serine may improve the learning and memory abilities and alleviate the damage caused by lead exposure through the increased expression of NR2A in the hippocampus. Moreover, our findings also indicated that D-serine administration may represent a useful clinical application for alleviating central nerve damage caused by environmental pollutants in the future.
Acknowledgments:We thanked Mrs Qing-Zhao Li (College of Public Health, North China University of Science and Technology, China) for animal intervention and Mrs Chun-Fang Zhao (College of Basic Medicine,Hebei Medical University, China) for pathological analysis.
Author contributions:Study design: YSZ, LX; experiment implementation:jZB, XW, jFW; result analysis: jZB, ZYL, jWY; manuscript writing: jZB, LX,ZYL. All authors approved the final version of the manuscript. All authors approved the final version of this paper.
Conflicts of interest:The authors report no potential conflict of interests.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81673208 (to YSZ), and 31802194(to SL). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:This study was approved by the Animal Ethics Committee of North China University of Science and Technology, China (approval No. LX2018155) on December 21, 2018.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Suman Chowdhury, Guru Gobind Singh Indraprastha University, India; Michael Veldeman, Universit?tsklinikum Aachen, Germany; Luís García García, Universidad Complutense de Madrid, Spain.
Additional files:
Additional Table 1:Effect of D-serine on the performance of lead-exposed rats in place navigation test of Morris water maze.
Additional Table 2:Original data of Additional Table 1.
Additional file 1:Open peer review reports 1–3.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study
- Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy
- Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury:a prospective self-controlled study
- Recognition of moyamoya disease and its hemorrhagic risk using deep learning algorithms: sourced from retrospective studies
- An integrative multivariate approach for predicting functional recovery using magnetic resonance imaging parameters in a translational pig ischemic stroke model
- AAV8 transduction capacity is reduced by prior exposure to endosome-like pH conditions