Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury:a prospective self-controlled study
Yansheng Liu , Jia-Xin Xie, Fang Niu , Zhexi Xu , Pengju Tan , Caihong Shen ,Hongkun Gao , Song Liu , Zhengwen Ma, Kwok-Fai So , Wutian Wu, ,Chen Chen, Sujuan Gao, Xiao-Ming Xu, , Hui Zhu ,
Abstract Although a large number of trials in the SCI field have been conducted, few proven gains have been realized for patients. In the present study, we determined the efficacy of a novel combination treatment involving surgical intervention and long-term weight-bearing walking training in spinal cord injury (SCI) subjects clinically diagnosed as complete or American Spinal Injury Association Impairment Scale (AIS) Class A (AIS-A). A total of 320 clinically complete SCI subjects (271 male and 49 female), aged 16–60 years, received early (≤ 7 days, n = 201) or delayed (8–30 days, n = 119) surgical interventions to reduce intraspinal or intramedullary pressure. Fifteen days post-surgery, all subjects received a weight-bearing walking training with the “Kunming Locomotion Training Program (KLTP)” for a duration of 6 months. The neurological deficit and recovery were assessed using the AIS scale and a 10-point Kunming Locomotor Scale (KLS). We found that surgical intervention significantly improved AIS scores measured at 15 days post-surgery as compared to the pre-surgery baseline scores. Significant improvement of AIS scores was detected at 3 and 6 months and the KLS further showed significant improvements between all pair-wise comparisons of time points of 15 days, 3 or 6 months indicating continued improvement in walking scores during the 6-month period. In conclusion, combining surgical intervention within 1 month post-injury and weight-bearing locomotor training promoted continued and statistically significant neurological recoveries in subjects with clinically complete SCI,which generally shows little clinical recovery within the first year after injury and most are permanently disabled. This study was approved by the Science and Research Committee of Kunming General Hospital of PLA and Kunming Tongren Hospital, China and registered at ClinicalTrials.gov (Identifier: NCT04034108) on July 26, 2019.
Key Words: American Spinal Injury Association Impairment Scale–A; functional recovery;human; intramedullary decompression; spinal cord injury; surgical intervention; walking training
Introduction
Traumatic spinal cord injury (SCI) is a severe medical problem experienced by humans with high mortality and longterm morbidity (Li et al., 2014; Ahuja et al., 2017a; Tang et al., 2019). While the majority of the injuries occur to the cervical spinal cord, they were also found at all spinal levels including the thoracic, lumbar and sacral levels resulting in devastating motor, sensory and autonomic dysfunctions below the level of injury (Onifer et al., 2007). In addition to the enormous financial burdens for necessary surgeries, care and rehabilitation, there exists profound social and emotional costs to patients and their families. Despite all efforts,neurological recovery remains very limited particularly for patients admitted with clinically complete SCI or classified as American Spinal Injury Association Impairment Scale–A (AIS-A)(Hadley et al., 2002; Zhu et al., 2008; Hadley and Walters,2013; Li et al., 2014). Notably, patients with a SCI generally show little clinical recovery within the first year after injury and most are permanently disabled (Fawcett et al., 2007;Freund et al., 2013). Although a large number of trials in the SCI field have been conducted, few proven gains have been realized for patients (Tator, 2006).
Following SCI, there is a primary mechanical injury followed by a complex series of secondary injury events resulting in an expanding of secondary degeneration beyond the initial trauma (Liu et al., 1997; Li et al., 2014; Ahuja et al.,2017b). Although cellular mechanisms leading to secondary injury remain poorly understood, several pathophysiological changes such as inflammation, necrotic and apoptotic cell death, vascular disruption, tissue swelling, and alterations in cerebrospinal fluid (CSF) hydrodynamics have been implicated(Tator and Fehlings, 1991; Tator and Koyanagi, 1997; Fitch et al., 1999; Liu and Xu, 2010). These events could increase the intramedullary pressure, exacerbating the expansion of secondary injury far beyond the original damage and leading to destruction of originally intact axons, neurons, and glial cells resulting in functional deficits (Crowe et al., 1997; Liu et al., 1997; Liu and Xu, 2010). Therapeutic strategies aimed at reducing intraspinal or intramedullary pressure and/or removing intraspinal degenerative tissue would likely reduce secondary degeneration and improve neurological recoveries.
Physical therapy and rehabilitation can lead to a significant impact on overall recovery following SCI in both experimental animal models (Wang et al., 2015; Loy and Bareyre, 2019)and humans (Raslan and Nemecek, 2012). Locomotor rehabilitation holds promise in spinal cord repair (Magnuson and Dietrich, 2017). A basic mechanism underlying locomotor rehabilitation is thought to “retrain” the spinal circuitry responsible for functional locomotion after SCI. Animal studies also support the importance of rehabilitation alone or in combination with other repair strategies in promoting locomotor recovery (Courtine et al., 2009). In human subjects with incomplete SCI, treadmill training improved functional locomotor recovery possibly through increasing the function of the spared corticospinal tract (Thomas and Gorassini,2005). However, according to a recent survey, the majority of SCI participants indicated that exercise was important to functional recovery, yet more than half either did not have access to exercise or did not have access to a trained therapist to oversee that exercise (Anderson, 2004).
In the present study, we determine the efficacy of a novel combinatorial approach involving surgical intervention and long-term weight-bearing walking training in SCI patients clinically defined as complete or AIS-A (showing no evidence of perianal sensation or voluntary anal sphincter contraction).In contrast to the conventional surgical intervention involving stabilization of the spine and extradural decompression(Fehlings and Arvin, 2009), our surgical intervention involves the intradural decompression (via durotomy), and, in some cases, intramedullary decompression (via myelotomy).In addition, we have developed a weight-bearing walking training program named “Kunming Locomotion Training Program (KLTP)” to train the subjects to walk for a duration of 3 hours per day, 5 days per week for a minimum of 6 months(3-5-6 KLTP). We reasoned that early surgical intradural and/or intramedullary decompression would release the intramedullary pressure and, therefore, spare surroundingtissues that would otherwise degenerate during the course of secondary injury. We further reasoned that long-term weightbearing walking training would “retrain” the residual spinal pathways facilitating recovery of locomotor function, and that a combination of the two would result in even greater functional recoveries.
Subjects and Methods
Subjects
A total of 320 patients (271 male and 49 female) aged 16–60 years with early (defined as ≤ 7 days, 201 cases, 62.8%) or delayed (8–30 days, 119 cases, 37.2%) severe traumatic spinal cord injuries between cervical (C2) and lumbar (up to L2) were enrolled in this prospective self-controlled study(Figure 1). All patients were admitted in the Clinical Center for Spinal Cord Injury of the People’s Liberation Army (PLA) and Kunming International Spine and Spinal Cord Injury Treatment Center at Kunming Tongren Hospital, Kunming, Yunnan,China between 2000 and 2016. All patients were informed in detail about the surgical procedure and rehabilitation plan and attested to informed consent for study participation.A signed written consent was also obtained from subjects shown in the publication of identifying information/images.All procedures were approved by the Science and Research Committee of Kunming General Hospital of PLA and Kunming Tongren Hospital. The study was registered at ClinicalTrials.gov(Identifier: NCT04034108) on July 26, 2019.
All included patients were neurologically examined on admission according to standards established by the American Spinal Injury Association (ASIA) (Li et al., 2014) to confirm that their ASIA Impairment Scale (AIS) was Grade A (AIS-A), which indicated that the patients’ injuries were clinically “complete”and they had no perianal sensation and no voluntary contraction of the anal sphincter. Imaging assessments included X-ray and magnetic resonance imaging (MRI) to determine the injury site and severity of spine and spinal cord injuries.
Eligibility criteria
Inclusion criteria: 1) Traumatic spinal cord injury (SCI) levels included cervical, thoracic and lumbar spinal levels; 2)neurological examination was AIS-A; 3) diagnosis of SCI was confirmed by MRI.
Exclusion criteria: 1) Penetrating injuries that caused complete transection of the spinal cord; 2) patients with severe brain injuries or other neurological disorders; 3) patients with lower motoneuron diseases; and 4) other conditions including pregnancy, and significant medical, infectious, and psychiatric conditions.
Surgical intervention
Posterior pedicle screw fixation was first implemented to restore stability of the injured vertebrae. Then bilateral laminectomy was performed to expose the spinal cord at the site of injury. A vertebral canal exploration was applied to remove any epidural hematoma or bone fragments. In all cases, a longitudinal posterior midline incision of the dura(durotomy) at a length of 3–5 cm (1.5–2 vertebral body length) was performed to reduce the pressure. The location of spinal durotomy was determined by preoperative MRI,and onsite intraoperative observations. After durotomy,we examined the gross appearance of the spinal cord and the status of the CSF flow of each subject under a surgical microscope to determine corresponding surgical procedures(summarized in Table 1). In general, swelling, pallescence,blockade of CSF flow, loss of rhythmic pulsation of the spinal cord, and injury-induced arachnoid adhesion could be found.Surgical procedures include the removal of spinal arachnoid adhesions to restore pulsations of the injured spinal cord and CSF flow. If the cord at the lesion site was found to contain a softened necrotic region, a longitudinal incision(myelotomy) with a length of 0.3–0.5 cm over the softening region was performed to release the necrotic spinal tissues.Table 1 summarizes the major intraoperative observations,corresponding surgical interventions, and objectives. The released necrotic spinal tissues on the surface of the spinal cord were collected for pathological examination. We rinse the lesion cavity, if opened, with sterile physiological saline to wash out the remaining necrotic tissue. Every effort was made to preserve the residual spinal cord tissue to prevent it fromfurther damage. The dura was then closed with sterile suture.The connective tissue, muscle and skin were closed in layers.After surgery, the subjects were kept in the intensive care unit until they were medically stable.
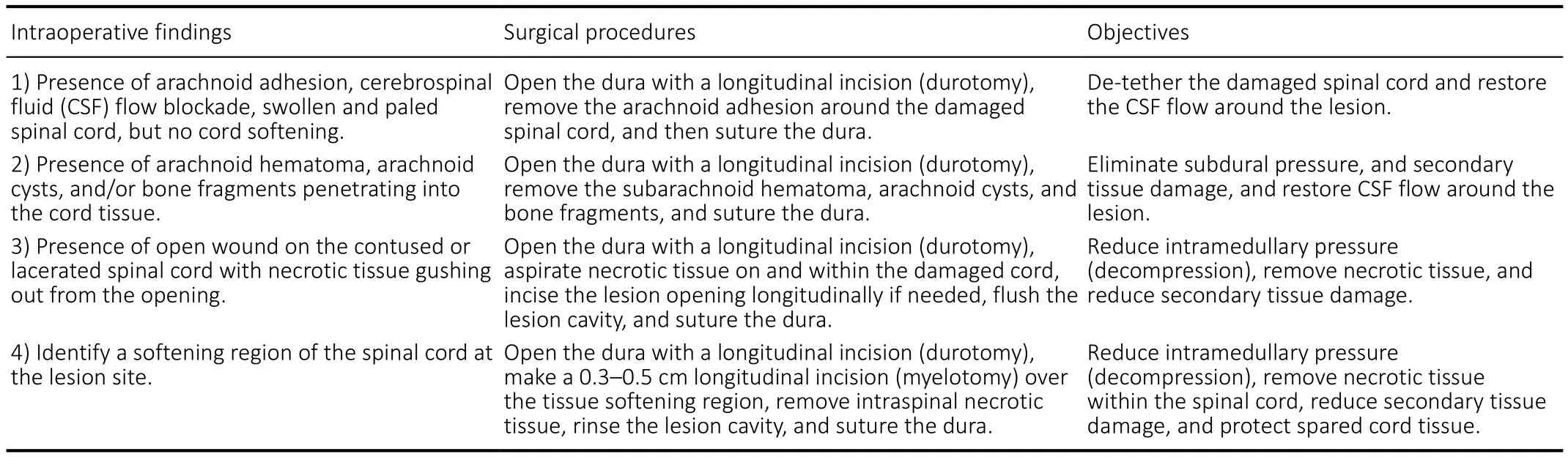
Table 1 |Summary of intraoperative findings, surgical procedures, and objectives
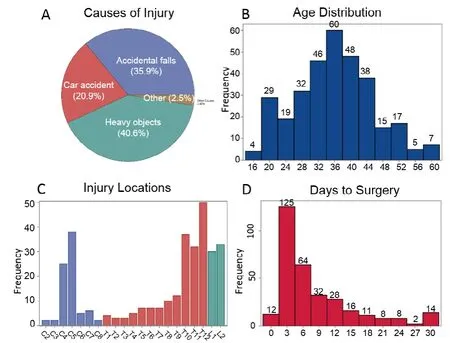
Figure 1 | Demographics of 320 patients with severe traumatic spinal cord injury classified as American Spinal Injury Association Impair Scale-Class A(AIS-A).
Weight-bearing walking training
At 15 days after surgery, with protection of a tailored chestwaist cast made of polyurethane foam (Nanjing Chuniu Technology Co., Ltd., Nanjing, Jiangsu Province, China) for thoracic/lumbar injuries or a neck support for cervical injuries(Figure 2), the subjects were encouraged to start weightbearing ambulation training under careful protection by training therapists of the Center. The training program was named “Kunming Locomotor Training Program (KLTP)” (Figure 3 and Table 2) formulated by our research team (Zhu et al.,2008). The program was made up of 8 progressive steps including: 1) training the subject to stand with weight support when a trainer fixes the knees, 2) training the subject to stand with his/her own weight support, 3) training the subject to walk with wheeled weight support when a trainer holds the knees during walking or holds bandages attached to the patient’s knees during walking, 4) training the subject to make his/her own stride with wheeled weight support, 5) training the subject to walk with the help of a non-wheeled 4-leg support, 6) training the subject to walk with a pair of crutches,7) training the subject to walk with a cane, and 8) training the subject to walk without any support.
Since none of the patients was able to stand or walk before surgery, each patient was primarily trained from step 1. Once the patient could complete one step consistently, the training was moved on to the next step. The patients received 3 hours weight-bearing training in 3 sessions per day (each session took 1 hour), 5 days per week, for a duration of 6 months by experienced training therapists. We call the training a 3-5-6 Kunming Locomotor Training Program (KLTP). In addition to the walking training, the subjects received physical (PT) and occupation (OT) therapies on a daily basis.
Kunming Locomotor Scale
We also developed a 10-point locomotor assessment scale called the Kunming Locomotor Scale (KLS) (Zhu et al., 2008)to assess locomotor recovery of the subjects (Figure 3 and Table 2). The KLS is indicated by roman numerals I to X and is based on KLTP. KLS I refers to the patient who can only sit in a wheelchair and cannot stand. Scale II to VIII refer to the patient who can accomplish the KLTP steps 1 to 7,consecutively. Scale IX represents a patient who can walk unstably without support and is equivalent to KLTP step 8.Scale X represents a patient who can walk stably without support. A scale of VI or above indicates that the patient does not need wheeled weight support for walking. For each subject in the study, the KLS was also evaluated and recorded before surgical treatment, 15 days after surgery (before rehabilitation), and 3 and 6 months after rehabilitation.KLS scores were highly positively correlated with AIS motor scores across four time points (Spearman’s partial correlation coefficient = 0.66,P< 0.0001).
At 15 days after surgery, followed by 3 and 6 months after rehabilitation, all patients were carefully tested for motor and sensory scores, AIS Impairment Scale, and KLS. None of the patients’ postoperative conditions were worsened (e.g., moving up neurological levels or development of complications) after surgery and weight-bearing walking training, compared to preoperative conditions, indicating that the surgical procedures and weight-bearing locomotor training were safe.
Neurological evaluation and locomotion scale
The neurological deficit was assessed according to the AIS standards (Maynard et al., 1997). For each patient the AIS motor, pinprick, and touch scores, as well as the AIS Impairment Scales and the neurological levels were evaluated by experienced neurologists and/or neurosurgeons and were recorded carefully before surgical treatment, 15 days after surgery (before rehabilitation), and 3 and 6 months after rehabilitation. The evaluators well trained for spinal cord injury outcome assessments at the Training Course on Neurological Assessment of Spinal Cord Dysfunction (offered by the Department of Orthopedics and Traumatology at University of Hong Kong Faculty of Medicine) and Spinal Cord Outcome Assessment Workshop (offered by the China SCI Net, and HKU Spinal Cord Injury Fund).
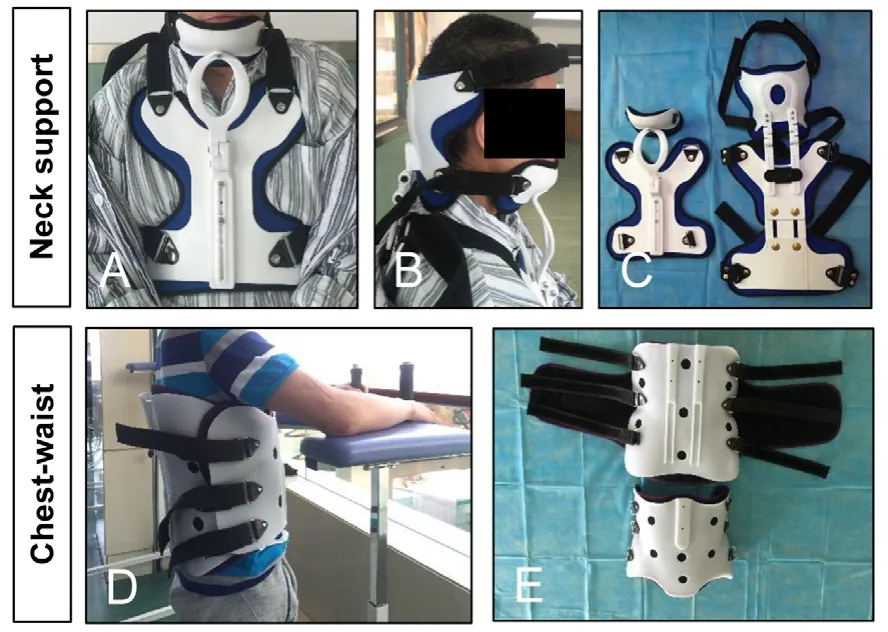
Figure 2|Tailored chest-waist cast for thoracic/lumbar injuries or a neck support for cervical injuries in a patient with severe traumatic spinal cord injury.
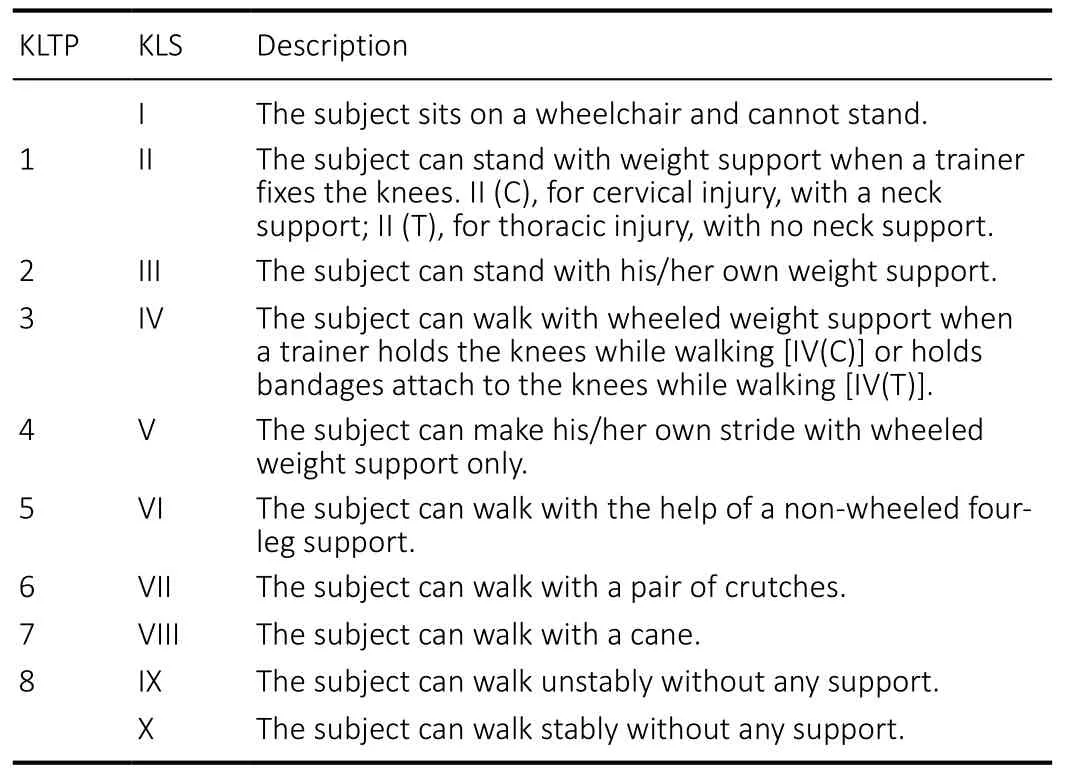
Table 2 |Summary of the Kunming Locomotion Training Program (KLTP)and Kunming Locomotor Scale (KLS)
Magnetic resonance imaging
Magnetic resonance imaging (MRI) represents an excellent method to detect the pathological changes following acute SCI. The spinal MRI scans were performed prior to the surgery and provided important measures determining the injury level, severity, and indication of surgical intervention. With the use of a 1.5T MRI scanner (GE Signa HD, Milwaukee, WI,USA) and a surface spine coil, all patients underwent T1-weighted imaging (T1WI) with a Spin-echo sequence and repetition time/echo time (TR/TE) = 400/20 ms as well as T2-weighted imaging (T2WI) with a fast-spin-echo (FSE) sequence and repetition time/echo time = 3000/120 ms. Imaging parameters for sagittal view were field of view (FOV) = 175× 280 mm, matrix = 240 × 512, and slice thickness = 3 mm.Imaging parameter for axial view were FOV = 156 × 250 mm,matrix = 156 × 256, and slice thickness = 6 mm.
Surgical intervention
Surgeries were performed between 12 hours to 30 days after trauma (Figure 1D). Internal fixation was implemented once instability of the injured spinal column was confirmed by radiology and exploration during operation. Circulatory dysfunction of the CSF at the injury site was found in all cases.During the operation the contused spinal cord usually appeared edematous. Patches of surface pial hemorrhage were often seen. At the center of the contusion, pial blood vessels were sparse and the spinal cord was typically pale. In more severely injured spinal cords, gentle probing usually revealed a liquefied or softened region below the surface, covered by a thin layer oftissue. In such cases, we incised the spinal cord longitudinally(0.3–0.5 cm) over the softening region to allow the underlying necrotic tissue to exit (Figure 4A), collected the necrotic tissue exited to the surface of the cord for histological staining,and washed the cavity with a gentle stream of saline. Table 1 summarizes 4 classes of intraoperative findings, correlated surgical procedures and objectives.
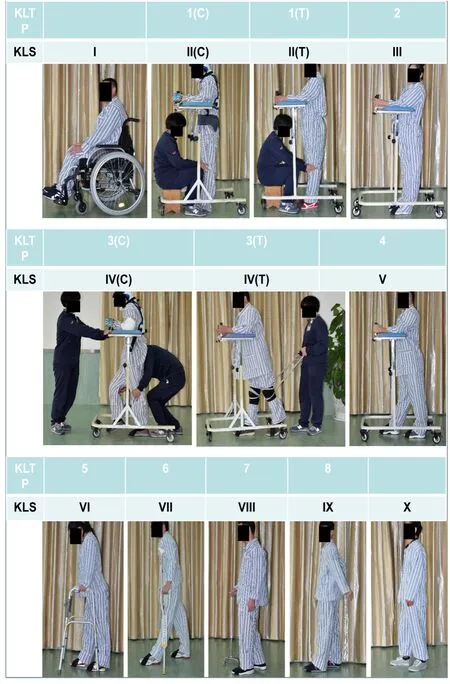
Figure 3|Kunming locomotor training program (KLTP) and Kunming locomotor scale (KLS) in patients with severe traumatic spinal cord injury.
Histology and immunohistochemistry
Tissue fixation and paraffin embedding
The necrotic spinal tissue samples released from the injury center following myelotomy were fixed in 4%paraformaldehyde (PFA) for 2–3 days. Subsequently, the tissue was gradient dehydrated in 75% alcohol overnight,85% alcohol for 2 hours, 95% alcohol overnight, 100% alcohol for 0.5 hours twice, followed by xylene transparent for 0.5 hours twice and paraffin saturation for 4 hours. The tissue was embedded in paraffin and sectioned serially with a rotary microtome (Leica RM2235; Leica, Wetzlar, Germany) at a thickness of 3–4 μm. Paraffin sections were flattened in 40°C water bath (Leica HI1210; Leica) and mounted on the coated microscope slides.
Dewaxing and rehydration
Paraffin sections were warmed in 65°C incubator overnight for softening paraffin and soaked in xylene 3 times, each time for 20 minutes. Sections were subsequently gradient,rehydrated in 100%, 95%, 85%, and 75% alcohol for 10 minutes, respectively, followed by running water 10 minutes for hydration.
Hematoxylin and eosin staining
Sections were stained with hematoxylin and eosin (H&E;Sigma, St. Louis, MO, USA) as previously described and were viewed using an Olympus IX71 (Olympus, Center Valley, PA,USA) (Iannotti et al., 2004). Briefly, sections were stained in hematoxylin for 7–8 minutes, followed by 0.5% HCl/95%alcohol for color separation removing plasma stain, and running water flushing for restoring nucleus back to blue. After 75% and 85% alcohol transition, sections were stained with eosin for 25 seconds, and dipped and rinsed in 95% alcohol five times. And sections were dehydrated in 100% alcohol for 5 minutes twice, transparent in xylene and coverslipped with neutral balsam.
Luxol fast blue staining
Luxol fast blue (LFB) staining demonstrates the state of white matter myelination following SCI (Iannotti et al., 2004). Briefly,slides were warmed at 37°C overnight. After dehydrating with 70% and 95% alcohol for 2 minutes, each section was immersed in a 0.1% LFB solution at 37°C overnight. After cooling to 4°C for 20 minutes, slides were dipped in 95%alcohol 5 times then placed in distilled water. The sections were then differentiated in 0.05% lithium carbonate at room temperature. After washing with 70% alcohol for 5 minutes and distilled H2O for 1 minute, the sections were dehydrated,cleaned, and cover-slipped. The sections were viewed using an Olympus IX71.
Immunohistochemistry
The procedures of immunohistochemistry staining were performed according to previously reported protocol in rodents(Liu et al., 2006) and monkey (Wu et al., 2013; Ma et al., 2016).Briefly, sections were warmed at 37°C overnight, and then rinsed with phosphate-buffered saline (PBS, Sigma, St. Louis,MO, USA) (0.1 M, pH 7.4) and treated with 3% H2O2for 1 hour to quench endogenous peroxidase. After rinsing with PBS, the sections then were incubated in 10% goat serum - 0.3% Triton X-100 for 1 hour. Primary antibodies, including mouse anti-SMI-31 (1:1000, Sternberger Monoclonals Inc., Baltimore,MD, USA) to recognize the phosphorylated neurofilament epitope of axons, mouse anti-glial fibrillary acidic protein (GFAP;1:1000, Sigma) to identify astrocytes, and mouse anti-KP1/CD68 (1:200, Dako, Carpinteria, CA, USA) to identify neutrophils and macrophages. All antibodies were diluted in 1% bovine serum albumin (BSA, Sigma)/PBS (pH 7.2) and the sections were incubated with the individual antibodies overnight at 4°C. On the second day, sections were incubated at room temperature with biotinylated goat-anti-mouse IgG antibody(1:300, Vector Laboratories, Inc., Burlingame, CA, USA) for 1 hour. After incubation with ABC reagent (Vectastain ABC kit,Vector Laboratories), the reaction complex was visualized with 3,3-diaminobenzidine (Dako). Then all sections were dehydrated with graded alcohols, cleared with xylene, and coverslipped. Sections were viewed using an Olympus IX71. The specificity of labeling was confirmed by using isotype control of mouse primary antibody (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
Mixed effect models were used for repeated AIS scores over the four time points (0, 15 days, 3 and 6 months) with separate scores as the dependent variable. Demographic variables, time, injury location and time and injury location were included as independent variables while controlling for potential covariates including age, sex and injury cause. Since KLS scores are ordinal in nature, we approximated KLS scores to a continuous outcome using mixed effect models to model changes in KLS scores over time. We used the generalized estimating equation (GEE) method to model KLS scores as Poisson data and obtained similar mode results to those obtained from mixed effects models. We prefer to present the mixed effects model results for ease of result interpretation and comparability with ASI scores. An unstructured variancecovariance matrix was used in all models to allow for correlations from repeated scores from the same individuals over time.Post hoccomparisons among scores within an injury location from the four time points were adjusted using the Bonferroni method to control for multiple comparison errors. Analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Significance level was set to 0.05 for all hypothesis tests.
Results
Demographics
In this study, all the 320 patients were classified as AIS-A at the time of admission to the clinical center, showing no evidence of perianal sensation or voluntary anal sphincter contraction. Since all of the subjects arrived at the hospital later than 8 hours after injury, none of them received highdose methylprednisolone. Figure 1A displays a pie chart for injury causes. Of all causes, 130 subjects (40.6%) were injured by heavy objects, 115 (35.9%) suffered accidental falls, 67 (20.9%) were injured in car accidents, and 8 (2.5%)had other causes of injury. The study population comprised 271 (84.7%) male subjects and 49 female subjects (15.3%).The mean age of subjects in this analysis was 35.4 years (SD= 9.6) and the mean time from injury to surgery was 8.3 days(SD = 7.4). Figure 1B and D display histograms of age and days between injury and surgery, respectively. In Figure 1C, we present the frequency of injury location grouped into cervical,thoracic and lumbar regions. The most frequent injuries were in C4-5 of the cervical region, T10–12 of the thoracic region,and L1-2 of the lumbar region. Subjects with early surgical interventions (≤ 7 days after SCI) are not different in age (P=0.2424), sex (P= 0.6947) or any of the scores at baseline (P>0.05) from those who received delayed interventions (≥ 8 days after SCI). In mixed effects models with longitudinal scores as the dependent variables, whether subjects received early or delayed surgeries were not significantly related to any of the scores or change of scores (P> 0.05 in all models).
MRI results
High-quality MRI was recommended for determining the injury and its severity (Hadley and Walters, 2013). The MRI was performed in all cases prior to and after the surgery.For example, in one subject of the study, a T2-weighted MR image shows a sagittal image of a C3 spinal cord injury at 5 days post injury (Figure 5A). Hyper-intensive CSF image was found surrounding the spinal cord. At the injury site, the CSF signal became narrowed, indicating the obstruction of the CSF flow at this region (Figure 5A). Within the injury epicenter,MR images demonstrated an increased T2 signal, indicative of tissue damage (Figure 5A). Eleven years after the surgery,T2- and T1-weighted MR images in the same case showed restored CSF signal, particularly in the ventral subarachnoid space (Figure 5B and C). A defined cystic cavitation was found with hyper-intensive T2 and T1 signals at the injury epicenter(Figure 5B and C). Note that, after surgery, spared cord tissue was found surrounding the lesion cavity (Figure 5B and C).
Histology and immunohistochemistry results
In a subset of subjects, necrotic cord tissues exiting to the surface of the spinal cord during the surgery were collected and examined histologically and immunohistochemically.Hematoxylin and eosin staining showed profound neutrophil infiltration (Figure 4B and C), hemorrhage (Figure 4B and C)and degenerated segments of blood vessels (Figure 4B and C) in the intraspinal necrotic tissue. Adjacent sections in the same region showed fragments of degenerated axons (Figure 4D, SMI-31 immunoreactivity) and myelin debris (Figure 4E,LFB-staining), along with degenerating astrocytes (Figure 4F, GFAP-IR). KP1/CD68 antibody staining further confirmed that the necrotic tissue contained both infiltrated neutrophils(Figure 4G) and reactive macrophages (Figure 4G).
Neurological recovery
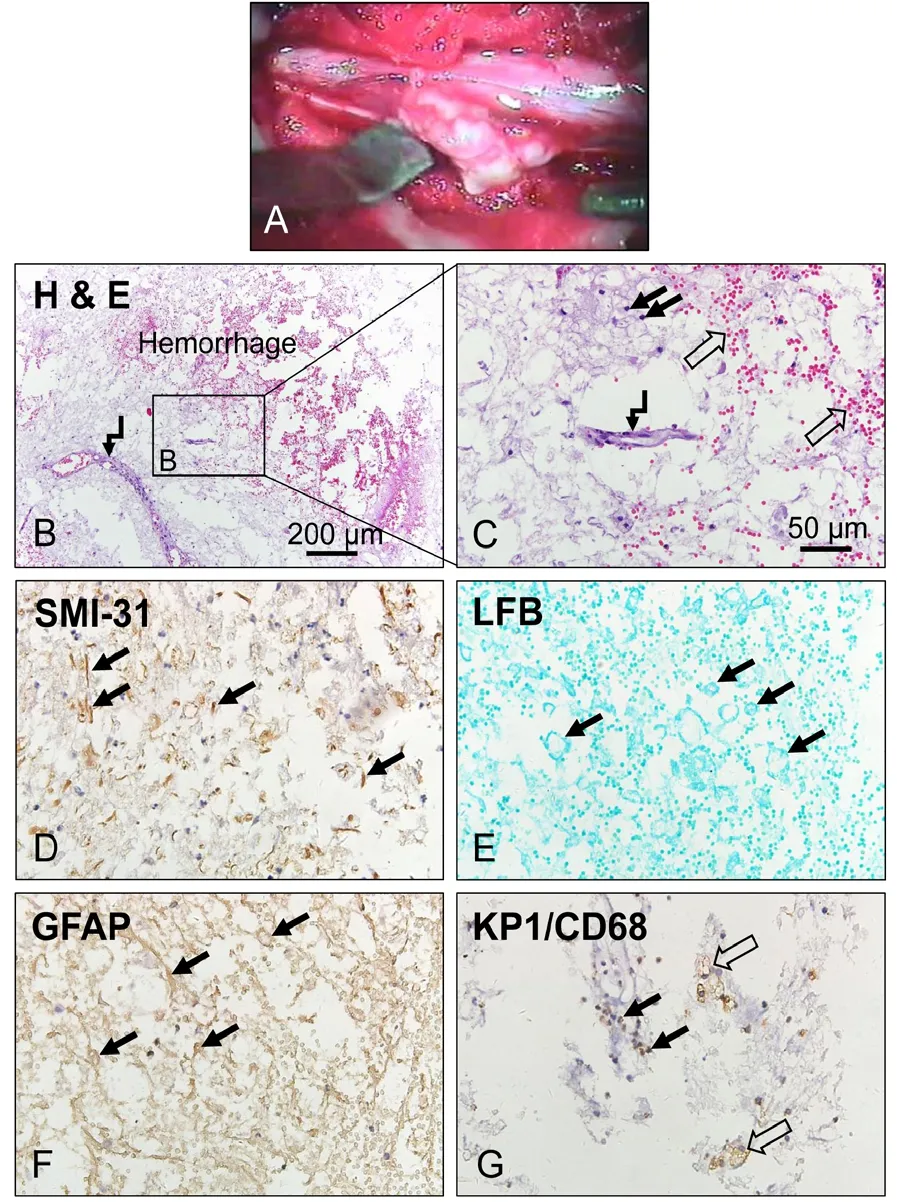
Figure 4| Histology and immunohistochemistry of the necrotic spinaltissue from the injury center after severe traumatic spinal cord injury.
First, we compared sensory and motor scores over the four time points from the three injury locations, i.e. the cervical,thoracic, and lumbar (Figure 6). The mean AIS pinprick scores and 95% confidence intervals (Figure 6A) showed significant difference at baseline (P< 0.0001), post-surgery scores at 15 days, 3 months or 6 months were significantly higher than pre-intervention baseline scores for each injury type (15 daysvs.before sugeryP< 0.05, all other comparisonsP< 0.0001).Scores at 3 and 6 months were also significantly higher than scores at 15 days for each injury type (P< 0.001). However,scores at 3 months were not significantly different from those at 6 months (P> 0.05), suggesting that no significant improvement in AIS pinprick scores was observed beyond 3 months after surgery. No other covariates were significant in the model for AIS pinprick scores. Mean AIS touch scores and 95% confidence intervals over the 4 time points (Figure 6B) are stratified by injury location. Results for the touch scores were similar to those of the pinprick scores. Scores at 15 days, 3 months or 6 months were significantly higher than baseline scores (15 daysvs. before sugeryP< 0.05, all other comparisonsP< 0.0001) for each injury location. Scores at 3 and 6 months are also significantly higher than scores at 15 days. No significant further improvement from 3 month to 6 months was found (P> 0.05). Of the set of covariates considered, injury cause was significantly associated with touch score (P= 0.0256) indicating that subjects injured by heavy objects had higher touch scores than those with other causes. Mean AIS motor scores (Figure 6C) over the 4 time points stratified by injury location. Scores at 15 days, 3 or 6 months were significantly different from baseline scores(P< 0.0001 for all comparisons) for each injury location. In addition, significant difference was also detected between the 15 day and 3 and 6 month scores (P< 0.0001), but no significant difference between the 3 and 6 month scores (P> 0.8862). No other covariates were significantly associated with motor scores in this model. Mean Kunming locomotor scores (KLS) over the 4 time points by injury location was presented in Figure 6D. Scores at 15 days, 3 or 6 months were significantly different from baseline scores for each injury location (P< 0.0001). In addition, there were significant differences between all pair-wise comparisons of time points of 15 days, 3 months or 6 months (P< 0.001) for lumbar and thoracic injuries indicating continued significant improvement in walk scores during the 6 months follow-up period. For cervical injuries, significant increase in scores was seen from 15 days to 3 months, but the improvement from 3 months to 6 months was not significant (P= 0.1545). KLS scores were highly correlated with AIS motor scores across four time points (Spearman’s partial correlation coefficient=0.66,P<0.0001). No other covariates were significant in the KLS score model.
Since injuries at neurological level below T10 may have partial or complete lower motoneuron damage and may show a completely different recovery profile, we compared sensory and motor scores by two injury levels: upper (C2–T10) and lower (T11–L2) levels over the 4 time points (Figure 7). Results were similar to those obtained in the three regions (cervical,thoracic and lumbar) as we discussed above. Within each level, subjects exhibited significant improvement in all scores at 0.5 and 3 months. However, for ASIA pinprick (Figure 7A)and touch (Figure 7B) scores, no further improvement was seen from 3 to 6 months. For the motor score, significant difference between 3 and 6 months was only seen in the lower level (Figure 7C). For the KLS, significant differences were seen between 3 and 6 months for both upper and lower injury levels (Figure 7D). In addition, there were significant differences between all pair-wise comparisons of KLS at time points of 15 days, 3 months or 6 months (P< 0.001) for both upper and lower level injuries, indicating continued significant improvement during the 6 month follow-up period.

Figure 5 | Magnetic resonance (MR) images in a patient with severe traumatic spinal cord injury.
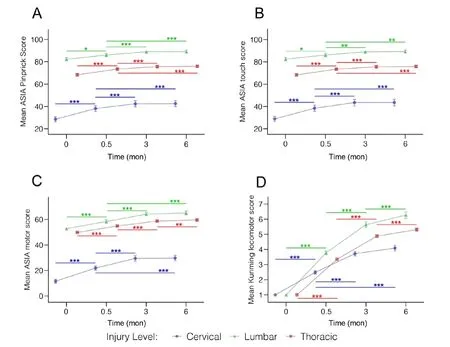
Figure 6 | Functional assessments at cervical, thoracic, and lumbar injury levels over time after severe traumatic spinal cord.
The distribution of admitting and follow-up AIS Impairment Scale is illustrated in Figure 8 and Table 3. By 15 days after surgery, 181 patients (56.6%) remained AIS-A. However, 100(31.3%) became ASIA-B, 34 (10.6%) became AIS-C, and 5 (1.6%)become AIS-D. By the end of the 6th month rehabilitation,151 patients (47.2%) improved their neurological scales from AIS-A to AIS-B (19.1%), AIS-C (18.1%), AIS-D (8.8%) or AIS-E(1.3%), gaining significant neurological recovery. Remarkably, 3 patients (1.3%) regained normal motor and sensory functions.The recovery patterns are similar in cervical, lumbar and thoracic injuries.
A diagram summarizes the advantages of our surgical intervention that involves intradural/intramedullary decompression (Figure 9). We reason that a laminectomy decompression only removes extradural pressure on the spinal cord and, therefore, the decompression is incomplete(Figure 9A). Such epidural decompression does not remove pressures built up beneath the dura or within the spinal cord proper caused by subdural hemorrhage or intramedullary edema generated by the secondary injury. A durotomy alone or combined with a myelotomy may effectively release the intradural and intramedullary pressure (Figure 9B). In addition,the durotomy procedure allows neurosurgeons to remove the intradural hematoma, bone fragments, arachnoid adhesion,and arachnoid cysts, which cannot be accomplished without a durotomy. Furthermore, a myelotomy was performed in some cases allowing the release of degenerated cord tissue.Together, these procedures helped to reduce the cord swelling and restore the CSF flow (Figure 9C). Thus, our surgical intervention procedure may minimize the edema, restore vascular perfusion, and reduce secondary degeneration, which could contribute to the observed functional improvement at 15 days post-surgery.
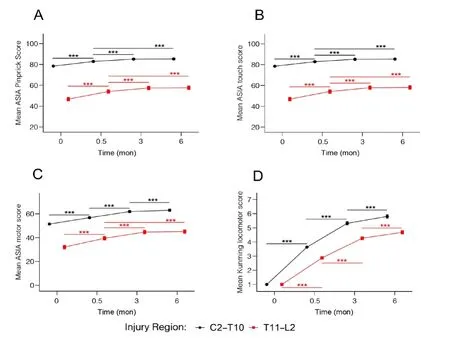
Figure 7 | Functional assessments at upper (C2–T10) and lower (T11–L2)spinal injury levels over time after severe traumatic spinal cord.
Discussion
In the present study, we demonstrated that surgical intervention with durotomy/myelotomy, combined with weight-bearing locomotor training promoted statistically significant neurological recoveries in subjects with clinically complete SCI or AIS-A (absence of deep anal pressure). The injury severity and level were confirmed with MRI. In contrast to the conventional extradural decompression, our surgical decompression was intradural (durotomy) and, in some cases, intramedullary (myelotomy), which, we believe, had provided a more complete spinal cord decompression than a laminectomy decompression. The surgery procedure was proven to be safe since all subjects survived the surgery and their neurological conditions were not worsened. In addition,the 3-5-6 KLTP provided an active, weight-bearing training for the subjects. We chose only AIS-A subjects (n= 320) for the current study to minimize the possibility of spontaneous recoveries that might occur in patients with incomplete SCI making data interpretation difficult. In clinically complete SCI,defined as AIS-A, the percent of spontaneous recovery after an acute SCI is fairly limited (Fawcett et al., 2007). For example,the International Campaign for Cures of Spinal Cord Injury Paralysis (ICCP) supported panel reported that about 80% of the initial AIS-A patients remained as AIS-A, 10% converted to AIS-B (i.e. some sensory function), and 10% to AIS-C (regain some motor function) (Fawcett et al., 2007). In our study,however, 151 subjects (47.2%) improved their neurological scales from AIS-A to AIS-B (19.1%), AIS-C (18.1%), AIS-D(8.8%) or AIS-E (1.3%), assessed at the end of the 6th month of rehabilitation, gaining significant neurological recovery.Since the surgical intervention showed initial improvement and the locomotor training showed future improvement on locomotor recovery, as compared to pre-surgery baseline conditions, we conclude that the combination of the two is beneficial. This study is consistent with our early report on the neurological improvement in 30 AIS-A cases after early surgical intervention and rehabilitation training for up to 3 months (Zhu et al., 2008). One limitation of the study is that we used presurgical neurological measures as baseline controls. Another limitation is that we did not have injury only and rehabilitation only control groups. These limitations would be addressed in future prospective, controlled, and randomized clinical trials.
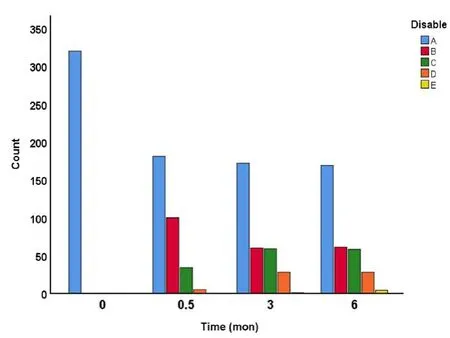
Figure 8| Distribution of American Spinal Injury Association Impairment Scale (AIS) disability over time after severe traumatic spinal cord.
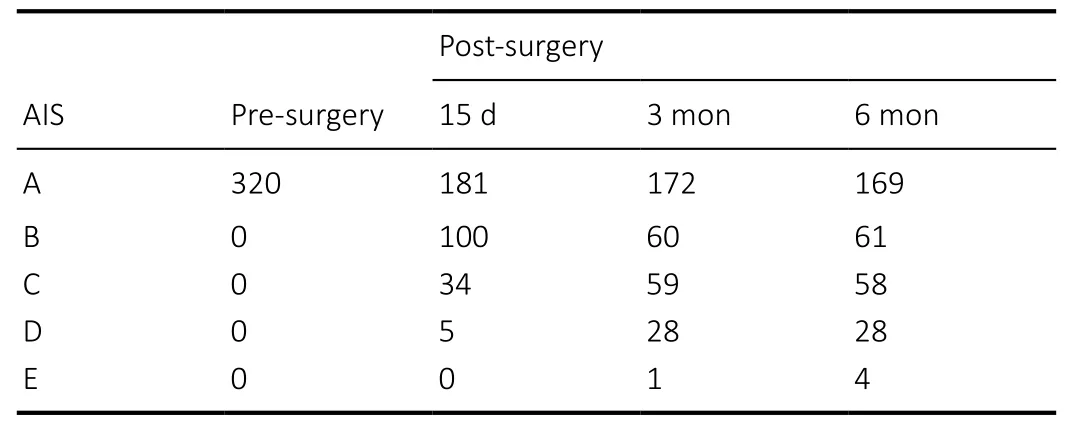
Table 3|American Spinal Injury Association Impairment Scale (AIS)distributions pre- and post-surgery
Demographically, the primary cause of the injury in our subjects was by heavy objects (40.6%), followed by accidental falls (35.9%), car accidents (20.9%), and others (2.5%), which is different from what was reported in the United States with the car accident and falls as the primary and secondary causes of the injury (National Spinal Cord Injury Statistical, 2016). In our subjects, the average age at injury was 35.4 years and,among them, 84.7% were males and 15.3% were females,similar to what was reported in the United States (National Spinal Cord Injury Statistical, 2016). The injuries occurred at cervical to lumbar levels with the most frequent injuries occurring at C4–5, T10–12, and L1–2. The time from injury to surgery ranged from 1 day to 30 days with a mean time of 8.3 days. In this study, we define surgeries performed ≤ 7 days after injury as “early surgical intervention” and ≥ 8 days after injury as “delayed surgical intervention”. Surgeries performed between the two time windows were not significantly related to any of the functional scores or change of scores that we measured (P> 0.05 in all models).
Surgical intervention could minimize edema, restore vascular perfusion, and reduce secondary degeneration. In contrast to the conventional extradural decompression, our surgical decompression was intradural and, in some cases,intramedullary, which, we believe, had provided a more complete spinal cord decompression than a laminectomy decompression alone. In animal models of SCI, early surgical decompression with durotomy plus a dural allograft promotedtissue repair and functional recovery (Smith et al., 2010). Early surgical intervention has 2 principal goals: decompression and stabilization (Fehlings and Arvin, 2009; Raslan and Nemecek,2012). In most studies, surgical stabilization is considered beneficial whereas laminectomy or extradural decompression remains controversial (Morgan et al., 1971). Some literature suggests that early surgical decompression may improve outcomes and decrease length of stay, morbidities, and potentially mortality, particularly when intervention is within 24 hours (Harrop et al., 2014). Others suggest that early surgical decompression has no significant neurological benefit(Vaccaro et al., 1997). We reasoned that the laminectomy decompression only removes extradural pressure on the spinal cord and, therefore, the decompression is incomplete since it does not remove pressures built up beneath the dura or within the spinal cord proper caused by hemorrhage,intramedullary edema or tissue degeneration. This idea is supported by the human cadaveric study that cervical and thoracic kyphotic deformities resulted in significant increases in intramedullary pressure (IMP) (Winestone et al., 2012).A durotomy can reduce the mean cervical and thoracic IMP by 62.8% and 69.9%, respectively, and a piotomy can reduce the mean cervical and thoracic IMP by 91.3% and 105.9%,respectively, which is much greater than laminectomy which only reduced the mean cervical and thoracic IMP by 22.5% and 18.5%, respectively (Winestone et al., 2012).Furthermore, tethered cord syndrome and secondary retethering can only be removed when durotomy is performed.

Figure 9 | Schematic illustration summarizes beneficial effects of the intradural/intramedullary decompression after severe traumatic spinal cord.
In our study, all subjects received durotomy, a procedure not only allows to reduce the intradural pressure but also to remove the arachnoid adhesion or tethering around the damaged spinal cord, the subarachnoid hematoma, the arachnoid cysts, and bone fragments often occurring after an acute SCI. Moreover, if tissue swelling or necrotic tissue was found at the lesion site, a myelotomy was applied to decompress the swollen spinal cord or to remove the degenerative tissue built up within the spinal cord. Our results showed that such intradural and/or intramedullary decompression significantly improved sensory (pinprick,touch) and locomotor (AIS and KWS) scores at 15 days postsurgery as compared to pre-surgical assessments, indicating that the intradural/intramedullary surgical intervention alone is beneficial for neurological recoveries in AIS-A subjects.
A possible mechanism of recovery after surgical intervention performed within the first month post-injury could be related to the prevention of further neurological deterioration at the lesion site and protection of more spared axons and myelin around it. Animal models of SCI have provided evidence that early surgical decompression can lead to improved neurological recovery after SCI possibly through the prevention of secondary injury (Delamarter et al., 1995;Dimar et al., 1999). The protection of spared axons is of vital importance since axons traversing the injury site are the only remaining connections between the brain and caudal spinal cord. The functional consequences of SCI are determined by the level and extent of damage to the white matter (Bradbury and McMahon, 2006; Anderberg et al., 2007). Human pathological studies indicate that, even in clinically complete SCI patients, their spinal injuries are anatomically incomplete and a certain portion of axons might have been spared (Bunge et al., 1993; Puckett et al., 1997). Our MRI imaging also showed sparing of white matter surrounding the lesion cavity.Thus, surgical intervention aimed at enhancing axonal sparing and reducing secondary degeneration could be an attractive and achievable practice.
Accurate appraisals of a surgical therapeutic window for acute SCI are fundamental to the establishment of optimal treatments (Li et al., 2014). Literature suggests that early surgical decompression may play a significant role in functional recovery (Fehlings and Arvin, 2009; Fehlings et al., 2012).Unfortunately, the timing of “early” surgical decompression has been difficult to establish because it is defined at different times by different authors and there is a lack of evidence supporting the definition. A systematic review on class II evidence concluded that early surgery (< 24 hours) resulted in better neurological outcome, reduced complications,and reduced length of ICU and overall hospital stay when compared to delayed surgery (> 24 hours) (Cengiz et al.,2008; Fehlings and Arvin, 2009). Another study published a prospective randomized trial investigating surgical treatment of SCI and showed no significant neurologic benefit when cervical spinal cord decompression after trauma is performed≤ 72 hours after injury as opposed to ≥ 5 days (Vaccaro et al., 1997). In the present study, the “time window” from injury to surgery ranged from 1 day to 30 days with a meantime of 8.3 days. We defined “early surgical intervention” as intervention performed ≤ 7 days after injury and “delayed surgical intervention” performed ≥ 8 days after injury. Our results showed that days to surgery were not significantly related to the motor score. Even within such a time window(1–30 days), far beyond the 24 hour or 72 hour time points,significant recoveries (47.2% of 320 AIS-A patients) could still be achieved when surgical intervention was combined with long-term weight-bearing walking training.
Locomotor rehabilitation holds great promise in spinal cord repair (Field-Fote et al., 2017; Magnuson and Dietrich, 2017).A basic mechanism underlying locomotor rehabilitation is thought to “retrain” the residual spinal circuitry responsible for functional locomotion after SCI. The emerging concept is that, after SCI, the capacity of neural circuitry to generate a locomotor pattern is not lost and can recover with a combination of therapies including locomotor training(Magnuson and Dietrich, 2017). A landmark study in the laboratory of Serge Rossignol (Belanger et al., 1996) indicates that an isolated locomotor circuitry exists below a complete lower thoracic transection in cats and that this circuitry is able to initiate near normal hindlimb stepping when treadmill training is initiated. Using shallow water as a body weight support strategy for step training, the Magnuson laboratory (Kuerzi et al., 2010) showed that all tested animals demonstrated plantar hindlimb stepping in the shallow water at as early as 2 weeks after a T10 contusive SCI, even when they had less than 10% spared white matter at the injury epicenter. Other animal studies also support the importance of rehabilitation alone or in combination with other repair strategies in promoting locomotor recovery (Courtine et al., 2009). In human subjects with complete SCI, the spinal circuitry of motor complete paraplegics can generate motor patterns effective for standing in response to task-specific training with optimized epidural stimulation parameters;however, step training can lead to neural adaptations resulting in impaired motor function for standing (Rejc et al., 2017).In human subjects with incomplete SCI, treadmill training improved functional locomotor recovery possibly through increasing the function of the spared corticospinal tract(Thomas and Gorassini, 2005). In individuals with incomplete SCI, high intensive locomotor training increased serum concentrations of brain-derived neurotrophic factor (Leech and Hornby, 2017) which could be one possible explanation for facilitating the plasticity of neural circuitry by brain-derived neurotrophic factor. However, according to a recent survey,the majority of SCI participants indicated that exercise was important to functional recovery, yet more than half either did not have access to exercise or did not have access to a trained therapist to oversee that exercise (Anderson, 2004).
Our locomotor training is unique in several ways. First, we start the training as early as 15 days after surgery. Second, the training is active and weight-bearing under careful protection by the training therapists. Third, we have established KLTP to train subjects from one step to another. Last, the training is intensive (training time 3 hours per day, 5 days per week),and long-term (for a minimum of 6 months). By the end of the 6thmonth of rehabilitation, 47.2% patients improved their neurological scales from AIS-A to B (19%), C (18%), D (9%) or E(1%), gaining significant neurological recovery. Remarkably, 3 patients (~1%) achieved complete recovery, regaining normal motor and sensory function. The percentage of recovery is far beyond what has been reported with spontaneous recovery(Fawcett et al., 2007). One possible mechanism of locomotor training-mediated recovery is that the training enhances neural circuit remodeling within the spinal cord (Frigon and Rossignol, 2006; Dunlop, 2008; Fouad and Tetzlaff, 2012).Another possibility is that the locomotor training enhances proprioceptive sensory feedback which provides a primary source for excitatory drive entering the spinal cord to engage local circuits distal to the lesion. Indeed, partial or complete loss of sensory input impairs recovery of gait control and locomotion (Bouyer and Rossignol, 2003; Rossignol et al.,2006). Thus, locomotor training, as we have shown in the current study, could “wake up” or “retrain” the remaining neural circuitry within the spinal cord to improve neurological functions.
Our results showed that surgical intervention within 1 month post-injury significantly improved sensory (pinprick, touch)and locomotor (AIS and KWS) scores at 15 days post-surgery as compared to pre-surgical assessments, indicating that the surgical intervention alone is beneficial for neurological recoveries in AIS-A patients. We also found continued significant improvement in walk scores during the 6 month locomotor training period, as compared to 15 days postsurgery and pre-surgical assessments, indicating the beneficial effect of walking training. This led us to believe that surgical intervention and locomotor training each plays a role in neurological recovery and that a combination of the two synergistically enhances the recovery. In addition, significant differences between all pair-wise comparisons of time points of 15 days, 3 months or 6 months were only found using KLS,implying that the KLS is more sensitive in detecting changes of locomotor recovery as compared to ASIA motor score assessment.
In conclusion, our study demonstrates that intradural/intramedullary surgical intervention within a month postinjury, combined with long-term weight-bearing locomotor training, promoted significant neurological recoveries in subjects with clinically complete or AIS-A SCI. We propose that both early and delayed surgical interventions (within 1 month)and weight-bearing walking training play a role in mediating locomotor recovery and that combination of the two offers an even greater synergistic effect.
Acknowledgments:We thank Ms. Patti Raley, a Medical Editor, for her critical reading of the manuscript.
Author contributions:YL, HZ, KFS, WW, XMX discussed and designed the study. YL, HZ, jXX, FN, ZX, PT, CS, HG, SL performed the study. ZM performed histology and immunohistochemistry. SG performed statistical analysis. jXX, HZ, CC and XMX wrote the manuscript.
Conflicts of interest:All authors declare that there is no conflict of interest in this paper. No conflicts of interest exist between Re-Stem Biotechnology, Co., Ltd and publication of this paper.
Financial support:This work was supported in part by the Hong Kong Spinal Cord Injury Fund.
Institutional review board statement:All procedures were approved by the Science and Research Committee of Kunming General Hospital of PLA and Kunming Tongren Hospital.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement:This study followed the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statement.
Biostatistics statement:All statistical analyses of this study were performed by the biostatistician, Sujuan Gao, a co-author and Professor at Indiana University School of Medicine.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study
- Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy
- Recognition of moyamoya disease and its hemorrhagic risk using deep learning algorithms: sourced from retrospective studies
- D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure
- An integrative multivariate approach for predicting functional recovery using magnetic resonance imaging parameters in a translational pig ischemic stroke model
- AAV8 transduction capacity is reduced by prior exposure to endosome-like pH conditions