Hydrogel-based local drug delivery strategies for spinal cord repair
Abstract Spinal cord injury results in significant loss of motor, sensory, and autonomic functions.Although a wide range of therapeutic agents have been shown to attenuate secondary injury or promote regeneration/repair in animal models of spinal cord injury, clinical translation of these strategies has been limited, in part due to difficulty in safely and effectively achieving therapeutic concentrations in the injured spinal cord tissue. Hydrogelbased drug delivery systems offer unique opportunities to locally deliver drugs to the injured spinal cord with sufficient dose and duration, while avoiding deleterious side effects associated with systemic drug administration. Such local drug delivery systems can be readily fabricated from biocompatible and biodegradable materials. In this review,hydrogel-based strategies for local drug delivery to the injured spinal cord are extensively reviewed, and recommendations are made for implementation.
Key Words: drug carriers; drug delivery; hydrogels; microparticles; nanoparticles;neurotrophic factors; scaffolds; spinal cord injury
Introduction
Spinal cord injury (SCI) results in significant loss of sensory,motor function, and autonomous functions below the level of injury. Treatment options for SCI patients are limited, with no cures being available. A number of therapeutic agents have been shown to target various pathological mechanisms following SCI (Schweigreiter and Bandtlow, 2006; Novrup et al., 2014; Shultz and Zhong, 2017). However, safely achieving therapeutic levels in the injured spinal cord tissue can represent a major challenge in clinical settings. Many drugs exhibit negligible accumulation in the central nervous system (CNS) tissue following systemic administration due to the inability to cross the tightly regulated blood-spinal cord barrier (Pardridge, 2005). Even drugs that do exhibit barrier permeability often require high systemic doses to achieve therapeutic level at the injury site, resulting in deleterious off-target effects (Casha et al., 2012; Bowers et al., 2016).For example, minocycline is a highly lipophilic molecule that can readily cross the blood-brain barrier (Garrido-Mesa et al., 2013). However, at the highest tolerable human dose,its local concentration in the cerebrospinal fluid (CSF) is only 2.3 μg/mL (Casha et al., 2012), which is far below the fully neuroprotective levels of 35-75 μg/mL (Wang et al., 2003,2017; Kraus et al., 2005; Garcia-Martinez et al., 2010; Xue et al., 2010). These issues are more severe when continuous administration is required over long periods of time (Ziemba and Gilbert, 2017). To address these obstacles, local drug delivery strategies can be employed for local and sustained drug administration.
Hydrogels have received signi ficant attention for local delivery of cells and drugs. They can also serve as a scaffold to provide structural support and guidance cues to promote regeneration following SCI (Pakulska et al., 2012; Elliott Donaghue et al.,2014; Pradeep et al., 2015; Führmann et al., 2017), because they have high water content and CNS tissue-like mechanical properties (Gustafson et al., 2015). In this review, we will focus on the use of hydrogel to locally deliver drugs to the injured spinal cord. Hydrogels are crosslinked hydrophilic or amphiphilic polymer-based materials that readily absorb water, and thus are predominantly comprised of water by weight when fully hydrated (Ahmed, 2015). Gelation occurs when the polymer chains become crosslinked. Both covalent and noncovalent bonds (ionic, hydrogen, and van der waals,etc.) can participate in crosslinking of polymers, resulting in a massive network of entangled polymer chains. According to crosslinking chemistry, polymer solutions can also be designed to undergo gelation in response to external stimuli such as temperature, pH, and local ion concentration.
Hydrogels can be fabricated from natural or synthetic materials; for an extensive review of CNS-compatible hydrogel materials, readers are directed to Pakulska et al.(2012). Natural polymer hydrogels can be fabricated from polysaccharides (e.g., agarose, alginate, dextran, chitosan,methylcellulose, and hyaluronic acid) and proteins (e.g., fibrin and collagen). These natural materials are excellent candidates for SCI treatment, as they are typically biocompatible (nontoxic and nonimmunogenic) and degrade into safe byproducts(Aurand et al., 2012). Synthetic polymer-based hydrogels offer the advantage of increased tunability, as they allow more control over material properties. Hydrogels built from synthetic polymers, including those derived from polyvinyl alcohol and polyesters such as polyglycolic acid, polyethylene glycol (PEG) acid, polylactic-co-glycolic acid (PLGA) and polycaprolactone can also exhibit desired degradation and biocompatibility pro files (Balu et al., 2018).
Hydrogels can encapsulate drug or drug-loaded carriers to provide sustained localized drug release. For local delivery,hydrogels can be implanted into parenchymal, intrathecal(subdural), or epidural spaces, via injection of preformed hydrogels (often shear-thinning) or polymer solutions that can gel in situ. Among the three implantation locations, intrathecal implantation is typically the most ideal for drug delivery applications as it bypasses diffusional barriers presented by the dura mater and fatty tissue in the epidural space, yet does not cause additional spinal cord tissue damage (Shoichet et al., 2007). Some drug loss to CSF circulation is expected, but the direct contact of hydrogel and spinal cord tissue allows for improved spatial control of drug delivery, resulting in improved targeting of the injured tissue over conventional intrathecal catheterization methods (Figure 1AandC). In addition,hydrogels exhibit tunable mechanical properties compatible with native spinal cord tissue, minimizing the risk of shearinduced tissue damage associated with mechanical mismatch.First proposed by Shoichet et al. (2007), feasibility and safety of intrathecal hydrogel injection was first shown with both collagen and hyaluronic acid/methylcellulose hydrogels. In this study, Shoichet et al. (2007) proved that intrathecally injected collagen hydrogels could safely provide local delivery of epidermal growth factor to the spinal cord. The safety and efficacy of this route of local delivery was further con firmed by Wang et al. (2017) and others (Thomas et al., 2015; Ghosh et al., 2018).
Finally, hydrogels can also be implanted directly into lesion cavities and serve as both drug delivery systems and tissue engineering scaffolds to support tissue regeneration across the lesion. In many cases, drug delivery and scaffold functionalities can be achieved with a single hydrogel construct (Jain et al.,2006, 2011; Han et al., 2009; Johnson et al., 2010; Lee et al., 2010). Notably, the same material properties than make hydrogels excellent drug delivery systems also make them excellent tissue engineering scaffolds. High water content allows for rapid mass transport of nutrients and waste products, while mechanical similarities to native CNS tissue limit mechanical stresses experienced by healthy spinal cord tissue surrounding the lesion cavity. In addition, many hydrogels can gell or shapein situto accommodate irregular lesion geometries. Because lesion cavities do not form for at least 1 week after SCI (Hong et al., 2017), however, delivery of drugs targeting early mechanisms of injury progression is limited to intrathecal or epidural routes of administration.
Search Strategy and Selection Criteria
In order to identify relevant scientific reports, PubMed database was searched with the keywords “spinal cord injury”and “hydrogel”. The search was first performed in November 2018, and literature was continuously monitored until January 2020 to identify any additional articles.
Hydrogels As Drug Carriers
Implanted hydrogels can either directly act as drug carriers or immobilize other drug delivery carriers at the injury site.A summary of studies investigating the use of hydrogels as drug carriers for treatment of SCI is provided inAdditionalTable 1. When hydrogels act as direct drug carriers, several methods can be employed to encapsulate drugs. The simplest form involves suspension of a drug within the hydrogel matrix, allowing the drug to diffuse out of the gel and into the surrounding space. By modulating various composition parameters such as polymer concentration and crosslinking density, hydrogel pore size can be varied to control drug release, with larger pore sizes leading to faster drug release(Varghese et al., 2014). For example, Burdick et al. (2006)studied the release of various neurotrophic factors from PEG-based hydrogels of different crosslinking densities, and found that drug release was slower from gels with higher crosslinking densities, while Lindsey et al. (2015) showed that the release rates of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) from beta hairpin peptide hydrogels were inversely related to the hydrogel concentration. Similarly, varying hydrogel degradation rate can be used to modalate drug release rate, as effective pore size increases during degradation (Caccavo et al., 2015).Drug release rate is also highly dependent on drug properties such as molecular weight, as larger molecules will be more sterically hindered from escaping the hydrogel network (Perale et al., 2012).
Drug-loaded hydrogels have been shown to exhibit efficacy in animal models of SCI. When a degradable polylactic acid/PEG-based hydrogel capable of releasing neurotrophin-3(NT3) for 2 weeks was implanted directly into the transected rat spinal cord parenchyma, animals exhibited increased axon density and improved behavioral outcomes (Piantino et al., 2006). Kang et al. (2009) utilized a hydrogel comprised of hyaluronic acid (HA) and methylcellulose (MC) to deliver a neuroprotective molecule erythropoietin. The drug was rapidly released from HA/MC hydrogels rapidly within 16 hoursin vitro, and implantation in the intrathecal space reduced cavitation and improved neuron survival after SCI. Liu et al. (2016) delivered a neuroprotective agent monosialoganglioside GM-1 from a thermo-responsive poloxamer-based hydrogel. Room temperature polymer solution was pipetted directly on top of the hemisected spinal cord where it was warmed to body temperature and subsequently gelledin situ(combined epidural and intraparenchymal implantation, as the solution flowed throughout the implantation site down into the injured cord prior to gelation). Hydrogel treatment improved neuroprotection and locomotor recovery, and inhibited apoptosis and glial scar formation. Conova et al. (2011)delivered BDNF to the injured spinal cord from poly(N-isopropylacrylamide)-g-polyethylene glycol or poly(N-isopropylacrylamide)-g-MC hydrogels designed to support transplanted cells and fill gaps in tissue following an aspiration lesion resulting in a 2-3 mm long cavity. Feasibility of intraparenchymal implantation was established, and axonal outgrowth into the lesion was observed following implantation of BDNF-loaded poly(N-isopropylacrylamide)-g-polyethylene glycol, although this was not quantified and robustly compared with other groups. Thomas et al. (2015) delivered lentivirus encoding for sonic hedgehog (Shh) from intrathecally implanted gelatin and PEG-based hydrogels, resulting in enhanced localization of virus to the implantation site and increased Shh activity for up to 45 days post-implantation.Myelination was observed in Shh-hydrogel treated groups, but again quanti fication and statistical comparisons with relevant controls were not reported.
For SCI applications, releasing free drugs from a hydrogel matrix may not always provide sufficient temporal control to achieve sustained drug release. Because hydrogel pore size and stiffness are inversely proportional (Chiu et al., 2013), the small pore sizes required for sustained release may yield gels too stiff to be safely implanted on top of or inside the soft and fragile spinal cord tissue. An alternative strategy to control drug release from hydrogels is to attach drugs to hydrogels via degradable covalent bonding. In these systems, reversibly bound drugs detach from the polymer matrix, yielding free drug that diffuses into the surrounding environment (Cheng et al., 2011). For example, Tian et al. (2005) employed covalent coupling to attach and release Nogo-66 receptor antibody from a HA hydrogel to promote axon regrowth. In this study,the antibody was covalently conjugated to the polymer backbone via a condensation reaction between aldehyde groups added to in the antibody and hydrazide groups added to HA, yielding a pH-sensitive, hydrolytically unstable hydrazone linkage. Under physiologic pHin vitro, an approximately linear release pro file was obtained, with ~80% of loaded drug releasing over 400 hours (16-17 days). The released antibody was found to retain bioactivity, as evidenced by being able to bind to the Nogo receptor in rat brain tissue.
Reversible physical interactions between drugs and drug carriers can also be utilized to achieve sustained drug release.This drug delivery mechanism is de fined as affinity-based drug delivery. Drug release from affinity-based delivery systems can be controlled by modulating the affinity between the drug and its carrier as well as the ratio of drug to binding sites (Mohtaram et al., 2013). Negatively charged sulfated polysaccharides including glycosaminoglycans, for example,exhibit known affinities for a variety of positively charged growth factors (Xu et al., 2016), and thus have proven useful for applications in growth factor delivery. Butterfield et al.(2011) utilized such a strategy, harnessing a biochemical affinity between NGF and chondroitin sulfate. To incorporate NGF, multi-armed PEG molecules were modified with chondroitin-sulfate binding peptides, which immobilized NGF-chondroitin sulfate complexes within the hydrogel network.Neurite outgrowth was significantly longer on NGF-loaded hydrogels than on control hydrogelsin vitro. Similarly, a hydrogel containing homogenized and lyophilized acellular spinal cord matrix was found to readily bind to basic fibroblast growth factor, presumably via binding to glycosaminoglycans retained in the matrix during decellularization (Xu et al.,2016). Heparin-binding affinity has also been exploited to deliver a number of growth factors to the injured spinal cord.Zhao et al. (2016) incorporated NGF into a heparin-poloxamer thermo-sensitive hydrogel. When PC12 cells were cultured with freshly prepared drug-loaded hydrogel, released NGF could rescue PC12 cells from oxidative insult. Further studies showed improved histological and functional outcomes in animals treated with heparin-poloxamer hydrogel loaded with GDNF following compressive SCI (Zhao et al., 2017) and purified acellular matrix and basic fibroblast growth factor following hemisection (Xu et al., 2018). Heparin has also been conjugated into fibrin gels via a bifunctional peptide linker to provide sustained delivery of growth factors. Sustained release of NT3 for up to 2 weeks was achieved by increasing the ratio of heparin: NT3, with released NT3 exhibiting enhanced stability and preserved capacity to induce dorsal root ganglion neurite outgrowthin vitro(Taylor et al., 2004). When NT3 loaded heparin/fibrin hydrogels were implanted into a 2-mm ablation lesion, glial scarring was markedly reduced and neuronal fiber was enhanced (Taylor et al., 2006). NT3 exhibited similar effects on neuronal fiber growth in a delayed repair model where hydrogel was implanted at the injury site 2 weeks after hemisection (Johnson et al., 2009). Heparin has also been used to modulate growth factor release from fibrin-based hydrogels designed to support implanted stem cells and direct differentiation. Heparin/ fibrin hydrogels have been used to release platelet-derived growth factor (PDGF)and Shh to direct differentiation of encapsulated mouse stem cellsin vitro(Willerth et al., 2008). Similarly, fibrin scaffolds containing heparin-binding delivery systems have been used to simultaneously deliver embryonic stem cell-derived neural progenitor cells and growth factors PDGF and NT3 to direct progenitor cell differentiation. Following hemisection and implantation directly into the lesion site, fibrin scaffolds in combination with PDGF and NT3 supported embryonic stem cell-derived neural progenitor cells survival and neuronal differentiation (Johnson et al., 2010).
Peptide or protein-based drugs can also be modified to incorporate moieties with binding affinity for hydrogel constituents, although care must be taken such that any irreversible chemical conjugation does not affect the bioactivity of drug molecules. Han et al. (2009) utilized a recombinant BDNF, collagen-binding domain polypeptide, and then released BDNF-/collagen-binding domain fused with a from collagen gels. Implantation in a rat hemisection model resulted in improved histological and behavioral outcomes.Pakulska et al. (2013) delivered a recombinant chondroitinase ABC (ChABC)/Src homology domain 3 (SH3) fusion protein from a crosslinked MC hydrogel that was conjugated with an SH3 binding peptide. Drug release could be modulated by varying SH3-protein/SH3 binding peptide ratio and binding strength. A later study found that local intrathecal implantation of the hydrogel following a moderate impactcompression injury in rats signi ficantly decreased chondroitin sulfate proteoglycan levels and improved behavioral outcomes(Pakulska et al., 2017).
Hydrogels Encapsulating Drug Carriers
In addition to acting as direct drug carriers, hydrogels have been utilized to localize drug-releasing vehicles such as fibers,particles, and microtubes at the injury site. A summary of studies investigating the use of hydrogels encapsulating drug carriers for treatment of SCI is provided inAdditional Table 2. When hydrogel pore size is sufficiently smaller than the delivery vehicles encapsulated in the hydrogel, the vehicles are effectively immobilized within the hydrogel matrix (Rossi et al., 2013). Because drugs are much smaller than the pore size of a hydrogel, however, therapeutics can still diffuse out of the hydrogel matrix following liberation from biomaterial carriers. These composite hydrogel systems are attractive for intrathecal drug delivery to the spinal cord because they combine temporal control over release provided by drug carriers with spatial control over release provided by hydrogels. If nano- and micro-sized drug carriers are implanted intrathecally without being embedded in a hydrogel, the smaller carriers can diffuse away from the injury site via CSF.As a result, these drug carriers injected intrathecally into CSF can nonspecifically diffuse throughout the CNS, dramatically reducing localization at the injury site (Figure 1B). In addition,high CSF turnover drives drug expulsion from the CSF to the lymphatic and circulatory systems (Pardridge, 2011).This expulsion may outpace diffusion into tissue, resulting in inefficient drug delivery from circulating carriers to targettissue. Composite hydrogel systems, on the other hand, allow small nano- and micro-sized drug carriers to be immobilized at the injury site, increasing the percentage of released drugs delivered to the injury site (Figure 1C). In addition, composite systems can allow for multi-drug release with independently tunable release profiles, as various drugs encapsulated in different carriers can all be suspended within a single hydrogel matrix (Burdick et al., 2006; Baumann et al., 2009).
The simplest type of composite hydrogels involves dispersion of drug aggregates or particles into hydrogel matrices. This approach can be utilized to provide sustained release of sparingly soluble drugs, as release is controlled by solvation rate of drug precipitates. Wang et al. (2009) blended neuroprotectant nimodipine particles into a HA/MC hydrogel and found that drug release was dependent upon drug particle size. Similarly, our lab found that by precipitating thyroid hormone T3 inside an agarose hydrogel, release could be sustained for months, with released T3 retaining the ability to stimulate oligodendrocyte differentiation and promote oligogenesis in the injured spinal cord when the hydrogel was implanted subdurally on top of contused rat spinal cords(Shultz et al., 2017).
For delivery of drugs with solubility well beyond the effective doses, more sophisticated carriers are required. Reservoirbased nano and microspheres such PLGA particles embedded in hydrogels have been extensively studied for this purpose.Ansorena et al. (2013) loaded alginate hydrogel with either free glial derived neurotrophic factor (GDNF) or GDNF-loaded PLGA microspheres. Microsphere encapsulation significantly reduced drug release rate; after 30 days of releasein vitro,more than 70% of GDNF remained in microsphere-loaded hydrogels, as compared to less than 30% of GDNF left in free drug-loaded hydrogels. Similarly, des Rieux et al. (2014)incorporated vascular endothelial growth factor into a fibrinogen/alginate hydrogel in the form of free, nanoparticle,or microsphere-encapsulated drug, and found that drug release was slower from nanoparticle and microsphere formulations with release rates being inversely proportional to particle size. Chvatal et al. (2008) loaded PLGA nanoparticles encapsulating methylprednisolone (MP) into agarose hydrogel to provide sustained local delivery of the small molecule drug without eliciting deleterious side effects associated with systemic corticosteroid administration. Epidural implantation of MP-loaded nanoparticles following contusion SCI resulted in significantly reduced acute inflammatory response and diminished lesion volumes. Similarly, MP delivery from polycaprolactone nanoparticles embedded in fibrin gel that was implanted epidurally exhibited equivalent anti-inflammatory activity as systemic MP administration following contusion SCI (Karabey-Akyurek et al., 2017).Rooney et al. (2011) encapsulated dibutyryl cyclic adenosine monophosphate into PLGA microparticles, and suspended the particles into oligo [(PEG) fumarate] hydrogels (Rooney et al., 2011). Steady drug release lasted for up to 42 days, and intraparenchymal hydrogel implantation following transection resulted in significant functional recovery. Cox et al. (2015)delivered estrogen to contused rat spinal cords from PLGA nanoparticles embedded in agarose hydrogel. When the hydrogel was implanted epidurally, drug concentrations in spinal cord tissue were twice as much as that in plasma, and rapid anti-inflammatory effects were observed. Pakulska et al. (2017) used a combined strategy: the crosslinked MC hydrogel served as a drug carrier for ChABC as discussed in the previous section; it also encasulated PLGA nanoparticles to deliver stromal cell-derived factor 1α. Stromal cell-derived factor 1α was released via its electrostatic affinity with PLGA nanoparticles to facilitate recruitement of endogenous neural precursors (NPCs). However, in this study, local delivery of stromal cell-derived factor 1α alone did not increase NPC number and distribution.
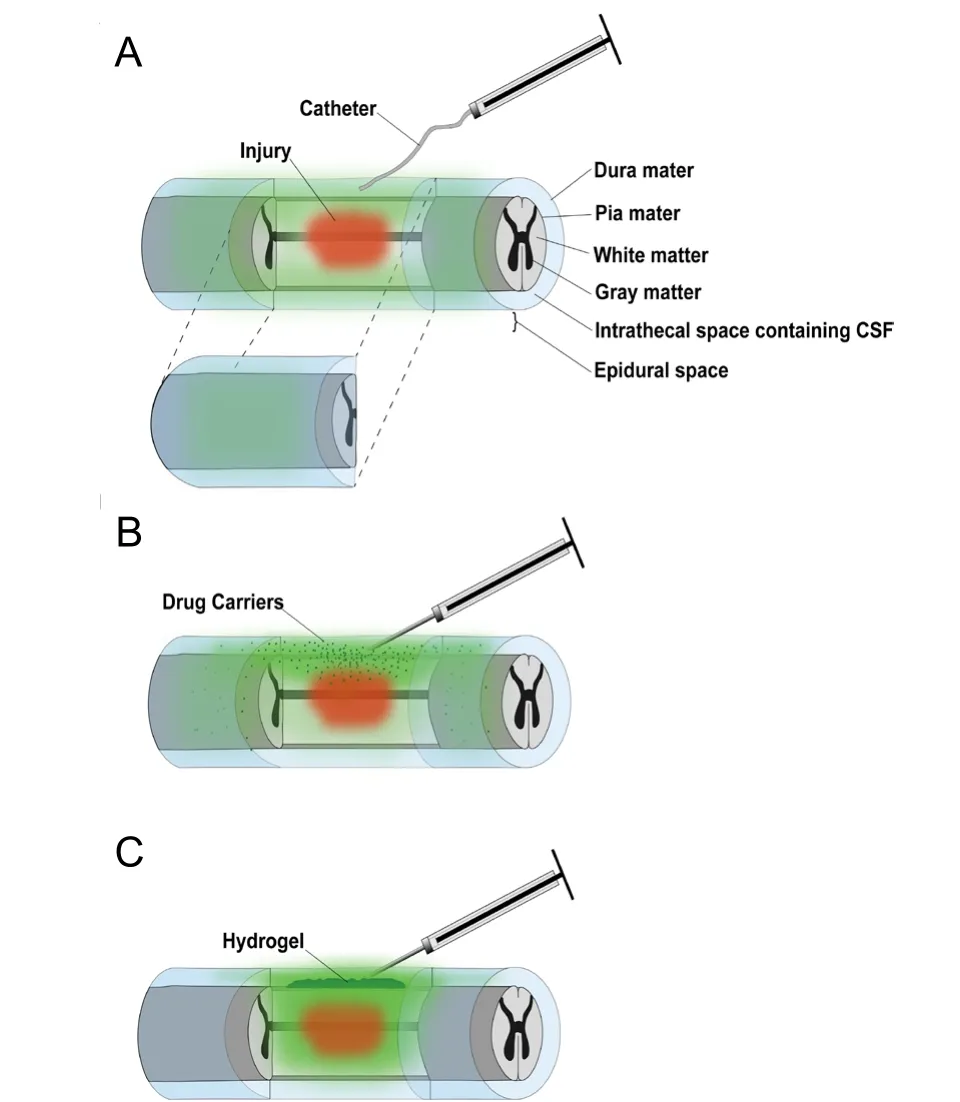
Figure 1|Illustration of contused spinal cord receiving local drug delivery.
HA and HA/MC hydrogels have also been used to encapsulate PLGA nanoparticles for local drug delivery to the injured spinal cord. BDNF was released for up to 30 days from PLGA nanoparticles embedded in a click-crosslinked HA hydrogel(Führmann et al., 2015). Sustained release of PDGF-AA for 21 days was achieved from PLGA nanoparticles encapsulated in an HA/MC hydrogel (Elliott Donaghue and Shoichet, 2015).This drug delivery system was also used to release NT3 (at least 28 days) and anti-NogoA (at least 55 days) (Stanwick et al., 2012a, b). Interestingly, encapsulation of nanoparticles in HAMC hydrogel resulted in more stable release pro files as compared to nano particles alone, potentially because MC acted as a diffusion-limiting layer on the particle surface.In vivoefficacy of this drug delivery system was demonstrated by Kang et al. (2013), who delivered basic fibroblast growth factor from intrathecally implanted HA/MC/PLGA to compressed rat spinal cords, resulting in increased blood vessel density at 28 days post-injury. Likewise, NT3 released from PLGA nanoparticles dispersed in an HA/MC hydrogel that was implanted intrathecally stimulated axon growth in the injured rat spinal cord and promoted functional recovery(Elliott Donaghue et al., 2015). Co-delivery of anti-NogoA and NT3 was achieved by suspending free anti-NogoA and NT3-loaded PLGA nano particles in the HA/MC hydrogel to combine a shorter-term 10 day release of anti-NogoA with a longer-term 58-day release of NT3 (Elliott Donaghue et al.,2016). Intrathecal implantation following compression SCI yielded significant functional improvements as compared to control.
Other drug carriers loaded in hydrogels include polyelectrolyte complexes, lipid microtubes, and liposomes. Lee et al.(2010) delivered thermostabilized ChABC and NT3 from lipid microtubes suspended in agarose hydrogel, resulting in enhanced axonal sprouting and outgrowth in a rat hemisection model after intraparenclymal implantation.Using a similar approach, Jain et al. (2006) promoted neurite outgrowth using an agarose hydrogel containing BDNF-loaded lipid microtubules. Jain et al. (2011) also employed this drug delivery system to locally deliver constitutively active Rho-GTPases and BDNF to the injured spinal cord,increasing the number of corticospinal tract axons traversing the chondroitin sulfate proteoglycan-rich glial scar. In these studies, lipid microtubule-loaded agarose hydrogel also served as a growth-permissive substrate when implanted directly into SCI lesions, allowing for in filtration of host axons into the hydrogel matrix. Wilems encapsulated ChABC-loaded lipid microtubules and partial Nogo receptor blocker NEP1-40-loaded PLGA microspheres in fibrin scaffolds, which achieved 1-week release of ChABC and two-week release of NEP1-40in vitro(Wilems and Sakiyama-Elbert, 2015). Implantation directly into a hemisection lesion reduced chondroitin sulfate proteoglycan deposition and enhanced axon growth into the fibrin hydrogel. Ghosh et al. (2018) improved respiratory function in rats with clinically relevant cervical contusion SCI via local BDNF delivery from intrathecally implanted agarose hydrogels containing BDNF-loaded polyelectrolyte complexes developed in our lab. We also achieved local delivery of minocycline, a tetracycline-derived antibiotic with potent anti-in flammatory, anti-oxidative and neuroprotective properties,via intrathecal implantation of agarose hydrogel containing minocycline/dextran sulfate/metal ion complexes. We found that local delivery was more effective than systemic delivery in achieving high local minocycline concentrations in the injured spinal cord and in promoting neuroprotection and functional recovery in a rat cervical contusion model (Wang et al., 2017).Liposomes modified with a scar-targeted peptide sequence and loaded with docetaxel and BDNF were encapsulated into a heparin-modified poloxamer hydrogel containing acidic fibroblast growth factor, providing sustained release of three different drugs for 21 days (Wang et al., 2018). Upon injection directly into contused rat spinal cords, liposome-loaded hydrogel improved axon regeneration and motor function.
Finally, Nguyen et al. (2017) developed a nanoparticle/ fiber/hydrogel composite system for co-delivery of proteins and nucleic acids. Nucleic acid-loaded micellular nanoparticles were incorporated into aligned electrospun fibers and encapsulated together with free proteins and heparin in a collagen gel. Notably, this composite hydrogel was designed for implantation into the injured spinal cord, with aligned fibers providing guidance cues for regenerating axons.Implanted nanofiber/collagen hydrogels containing NT3 and miR-222 (miRNA with potent regenerative properties)integrated well with the host tissue, directed neurite extensions and supported myelination within the lesion site in a mild hemisection model of SCI.
Considerations for Hydrogel Implantation following Spinal Cord Injury
Hydrogels are a promising option for drug delivery to the injured spinal cord. They can either directly serve as drug carriers or encapsulate smaller drug carriers for further control over drug delivery. In addition, hydrogels can be either implanted into the injured spinal cord, or be applied to the epidural or intrathecal space. The ideal route of hydrogel implantation is dependent on application, as well as drug properties. When the goal of implantation is solely to provide drug delivery, epidural and intrathecal administration allow for avoidance of highly invasive injection procedures and can typically provide sufficient localized drug delivery. If the dura mater is damaged during injury, drugs can diffuse from epidurally placed hydrogels to initially move into and across the intrathecal space and reach spinal cord tissue.Once the dura heals, however, this fibrous matrix offers a dense diffusional barrier to most drugs, especially those that are high in molecular weight such as proteins. Intrathecal administration generally offers the best compromise between invasiveness and localization, and bypasses the dura as a barrier to delivery. Injectable hydrogels can be implanted intrathecally via a small dural puncture. Typically, durotomies from needle punctures heal spontaneously (Turnbull and Shepherd, 2003; Ahmed et al., 2006). This is particularly true if the hole is generated by a small-bore needle. Occasionally(if needed), a durotomy site can easily be repaired by suture,autologous blood patch, or fibrin glue. On the other hand,even though intrathecal injection of hydrogel is less invasive,neurosurgeons may prefer to perform a larger durotomy to expose the spinal cord tissue where the gel will be applied.This allows for better control of gel implantation. The dura can be easily repaired by suturing to a clinically available dura graft, such as DuraGen matrix. Hydrogels can be implanted at the epidural or intrathecal space when a spinal surgery is performed to decompress and stabilize the spine. The earliest possible time to perform a spinal surgery is 8 hours after injury (Ng et al., 1999), and by 24 hours it is possible to perform the surgery on most patients. Therefore, when implanted intrathecally or epidurally, hydrogels can be applied no later than 24 hours after SCI. This is especially useful when the hydrogel is intended to deliver drugs targeting injury mechanisms that emerge rapidly after initial trauma.
When the goal of implantation is not only to provide drug delivery, but also to act as a regeneration-promoting tissue engineered scaffold and/or deliver transplanted cells,intraparenchymal implantation is required. While this can be readily achieved follow sharp hemisections/transections,contusion and compression injuries are far more common among human patients than sharp lacerations (Alizadeh et al.,2019). For the more clinically relevant contusion/compression injuries, implantation is usually delayed for 1 week or more to allow for the formation of the cystic cavity in the injured spinal cord (Hong et al., 2017). Therefore, this route of implantation cannot target early injury mechanisms. Because cavities are generally found towards the center of the cord and may not be easily visualized by eye, delayed implantation into the cystic cavity may require the use of imaging modalities such as magnetic resonance imaging to measure cavity volumes and to ensure injections are performed at the correct needle depths and locations, as recently described by Zhang et al.(2015).
Future Directions
Hydrogel-based local drug delivery systems represent an exciting field of biomaterials research with great potential to improve patient outcomes following SCI. Despite promising results from a number of studies using small animal models of SCI, however, no human clinical trials investigating the use of hydrogel-based delivery systems for the treatment of SCI have been initiated to date. In order to facilitate the translation of experimental successes into clinical trials, successful scaleup into large animal models must first be demonstrated. For example, the UBC porcine model of traumatic SCI utilizes a weight drop device and Yucatan miniature pigs, which more accurately recapitulate human spinal cord anatomy,to produce human-like contusion injuries (Kim et al., 2018).Preclinical SCI models utilizing macaque and rhesus monkeys have also been described and used to evaluate the efficacy of experimental treatments (Freund et al., 2007; Rosenzweig et al., 2019). To our knowledge, no studies investigating the efficacy of hydrogel-based SCI treatment strategies in large animals have been reported, although MRI-guided strategies for hydrogel implantation into the intrathecal space from a simple lumbar puncture have been described in uninjured swine and dogs (Malysz-Cymborska et al., 2018). As the field progresses, promising hydrogel-based local drug delivery treatments for SCI should be first tested in small-animal models, then evaluated in larger animal models and ultimately moved to clinical trials.
Conclusion
Hydrogel-based drug delivery strategies present opportunities to dramatically expand the toolkits of researchers and clinicians, allowing evaluation of theraepeutic agents for treating SCI via clinically viable methodologies. Several approaches, including encapsulation of free drugs or drug aggregates in hydrogels, drug/polymer conjugation chemistries, use of biochemical affinities, and/or embedding of smaller drug carriers can be utilized to provide localized drug release. Hydrogels can be implanted either epidurally,intrathecally, or directly into the spinal cord parenchyma,depending on the indication for which drug(s) are being delivered. In some cases, hydrogels can also serve as tissue engineering scaffolds to promote regeneration. Currently,there is an abundance of studies in small animal models; next steps involve evaluating the efficacy of promising strategies in large animal preclinical models and ultimately moving the top performers into clinical trials.
Author contributions:RBS and YZ wrote the manuscript and approved the final manuscript.
Con flicts of interest:Both authors declare no con flicts of interest.
Financial support:RBS was funded by the USA Department of Education’s Graduate Assistance in Areas of National Need (GAANN) Program and the National Institute of Biomedical Imaging and Bioengineering of theNational Institutes of Health under Award Number T32EB005583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the USA Department of Education or the National Institutes of Health.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Summary of studies investigating hydrogels containing free or immobilized drugs for spinal cord repair.
Additional Table 2:Summary of studies investigating hydrogels containing drug carriers for spinal cord repair.
 中國(guó)神經(jīng)再生研究(英文版)2021年2期
中國(guó)神經(jīng)再生研究(英文版)2021年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury
- Dysfunctional glia: contributors to neurodegenerative disorders
- In flammation/bioenergetics-associated neurodegenerative pathologies and concomitant diseases: a role of mitochondria targeted catalase and xanthophylls
- Potential therapeutic effects of polyphenols in Parkinson’s disease: in vivo and in vitro pre-clinical studies
- Possible implications of dysregulated nicotinic acetylcholine receptor diffusion and nanocluster formation in myasthenia gravis
- Altered physiology of gastrointestinal vagal afferents following neurotrauma
