Potential therapeutic effects of polyphenols in Parkinson’s disease: in vivo and in vitro pre-clinical studies
Abstract Parkinson’s disease is a neurodegenerative disorder characterized by a combination of severe motor and non-motor symptoms. Over the years, several factors have been discovered to play a role in the pathogenesis of this disease, in particular,neuroinflammation and oxidative stress. To date, the pharmacological treatments used in Parkinson’s disease are exclusively symptomatic. For this reason, in recent years, the research has been directed towards the discovery and study of new natural molecules to develop potential neuroprotective therapies against Parkinson’s disease. In this context,natural polyphenols have raised much attention for their important anti-inflammatory and antioxidant properties, but also for their ability to modulate protein misfolding. In this review, we propose to summarize the relevant in vivo and in vitro studies concerning the potential therapeutic role of natural polyphenols in Parkinson’s disease.
Key Words: alpha-synuclein; anti-in flammatory; antioxidants; natural molecules;neuroprotection; Parkinson’s disease; polyphenols; syntomatic effect
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease, after Alzheimer’s disease. PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) with consequent dopaminergic denervation of the striatum, which receives the SNc neuron projections (Blandini et al., 2007). The resulting dysfunction of the nigrostriatal pathway is responsible for the classic motor symptoms, which include resting tremor,bradykinesia, rigidity and postural instability. Scientific evidence suggests that different biological mechanism -including oxidative stress, neuroin flammation and α-synuclein(αSyn) accumulation and aggregation - play an important role in the pathogenesis of PD (Mcgeer et al., 1988; Liu et al.,2003; Blandini, 2013; Liddelow et al., 2017; Lee et al., 2019).
The term “Oxidative Stress”, coined by Denham Harman (1956)refers to a pathological process that occurs in the presence of an imbalance between production and detoxification of reactive oxygen (ROS) and nitrogen species by endogenous antioxidant systems, such as superoxide dismutase (SOD),catalase (CAT) and glutathione (GSH) (Blesa et al., 2015). This process leads to mitochondrial dysfunction and irreversible oxidation of macromolecules such as lipids, proteins and nucleic acids, compromising integrity and function of neuronal cells (Lee et al., 2009; Qin et al., 2013; Smeyne et al., 2013).
In the central nervous system, after an injury, a sequence of events take place including the production of inflammatory mediators, the recruitment of immune cells into the parenchyma and the activation of glial cells (microglia and astrocytes): all these phenomena are referred to as neuroinflammation (Lucas et al., 2006; Yong et al., 2019).At the beginning, neuroinflammation plays a defensive role against the pathological insult. However, a persistent and uncontrollable activation of this process can become detrimental (Wyss-Coray and Mucke, 2002). An abnormal intensification of glial cell activation in the brain tissue -especially in nigrostriatal area - was reported both in postmortem brain of PD patients and PD animal models (Mcgeer et al., 1988; Liu et al., 2003; Liddelow et al., 2017). Persistent activation of both microglia and astroglia contributes to exacerbate the neurodegenerative process, mainly through the increased release of pro-inflammatory factors in the extracellular space, including tumor necrosis factor-α (TNF-α),interleukin-1β (IL-1β), IL-2, IL-6, interferon-γ and inducible oxide synthase (Colton et al., 2010; Blandini et al., 2013;Liddelow et al., 2017).
Alpha-Syn is a 140-amino acid protein, encoded by theSNCAgene, that accumulates in Lewy bodies, the pathologic hallmark of PD (Chen et al., 1995). Although the precise physiological function of this protein is not clear, accumulating evidence suggests that αSyn may play an important role in presynaptic dopamine recruitment, vesicle trafficking,synaptic transmission and lipid metabolism (Abeliovich et al., 2000; Murphy et al., 2000; Cabin et al., 2002; Willingham et al., 2003; Liu et al., 2004; Yavich et al., 2004). Missense mutations in the SNCA gene (A30P, E46K and A53T) as well as the overexpression of wild type αSyn causes a decrease in the release of dopamine from presynaptic terminals and increase the propensity of αSyn to form toxic intermediates and to selfaggregate (Polymeropoulos et al., 1997; Kruger et al., 1998;Singleton et al., 2003, 2004; Farrer et al., 2004; Zarranz et al., 2004). Under physiological conditions, the clearance of αSyn is mediated by both the ubiquitin/proteasome system and chaperone-mediated autophagy. However, pathological αSyn may inhibit ubiquitin/proteasome system and impair chaperone-mediated autophagy, consequently affecting autophagy (Cuervo et al., 2004; Engelender et al., 2012).Systematic database search of PubMed was performed to find articles used in this review from 2014. Main keywords related to polyphenols and PD were used.
Polyphenols
Despite extensive research efforts made in the PD field, no effective therapies able to arrest or slow down the disease are available to date. In the last decade, scientific evidence has shown that natural molecules, including polyphenols(PPH), are able to mitigate pathobiological processes involved in cancer, diabetic, cardiovascular and neurodegenerative diseases (Rodriguez-Mateos et al., 2013; Gunn et al., 2015).In particular, PPH protect neurons from ROS by increasing the activity of NAD-dependent deacetylase sirtuin-1 (SIRT1)(Anekonda et al., 2006; Albani et al., 2010) and dampen the neuroinflammatory process by modulating the signaling of nuclear factor kappa-light-chain-enhancer of activated B cells (Aquilano et al., 2008; Bhullar et al., 2013). In addition,PPH can induce neurite outgrowth, increase cellular antioxidant defense and regulate pro-survival transcription factors and gene expression by targeting different molecular pathways, such as extracellular signal-regulated kinase 1/2,phosphatidylinositol 3-kinase/Akt and mitogen activated protein kinase pathways (Lin et al., 2010). It is noteworthy that PPH also reduce toxicity induced by αSyn misfolded aggregates and amyloid β protein oligomers (Feng et al.,2009; Singh et al., 2013). All these properties strongly point to PPH compounds as potential therapeutic tools for PD.Thus, in the last decade many studies have been performed in order to investigate PPH efficacy in PD and to overcome their limitations, such as bioavailability. However, different factors potentially influencing their biotransformation are still often overlooked (Koutsos et al., 2015). Of note, gut microbiota composition significantly affects PPH biovailability, thereby modulating their effects (Visioli et al., 2011; Frolinger et al.,2019). Analogously, dietary fat can mask the health promoting effects of natural supplements (Maulik et al., 2019).
PPH may be classi fied into two major groups, flavonoids and non-flavonoids, based on the number of benzene rings that they contain and the structural elements that bind these rings together. Non-flavonoids contain up to two benzene rings,whereas flavonoids are compounds with more than two rings(Manach et al., 2004). This review critically appraises the main results obtained byin vivoandin vitrostudies investigating the neuroprotective properties and symptomatic effects of different classes of PPH in PD.
Flavonoids
Flavonoids are widelydistributed within plant kingdom. They are generally found in cereals, fruits, vegetables, legumes as well as in the most common beverages such as tea, wine and beer. Flavonoids include flavonols, flavones, flavanones,flavanols, anthocyanidins and isoflavones (Ferrazzano et al.,2011;Table 1).
Flavonols
The flavonol quercetin is widely distributed in fruits and vegetables, particularly in capers (Capparis spinosa). Ghaffari et al. (2018) demonstrated the antioxidant property of quercetin and its nanocrystals in 6-hydroxydopamine (6-OHDA) rat model of PD. These molecules restored the activity of SOD and CAT enzymes and GSH levels, as well as decreased lipid peroxidation in the hippocampus. Similarly, quercetin treatment (50 mg/kg, 4 weeks) attenuated oxidative stress in the rotenone rat model by restoring thioredoxin activity with consequent decrease of malondialdehyde (MDA) levels (El-Horany et al., 2016). The antioxidant and anti-inflammatory properties of quercetin have been recently confirmed by Sharma et al. (2020) who demonstrated that this compound restores mitochondrial complex I and IV activities and reduces pro-in flammatory marker levels in the striatum of rotenonetreated rats fed with a daily iron supplement. Quercetin has proven also effective on PD-related motor and non-motor symptoms. The administration of quercetin for 28 days after 6-OHDA injection reduced the apomorphine-induced rotational behavior and prevented the memory impairment.These findings are in line with the study of Singh and Kumar(2018), who reported antioxidant, anti-inflammatory and neuroprotective effects of pre-treatment with quercetin -alone or in combination with piperine - in association with an improvement of motor performance. El-Horany et al. (2016)also revealed that quercetin increases autophagy and reduces apoptosis.
Quercetin 3-D-galactoside, also known as hyperoside,is a natural derivative of quercetin isolated fromAcer tegmentosum. This compound significantly promoted the survival of 6-OHDA-treated SH-SY5Y by activating nuclear factor erythroid 2-related factor 2, reducing lactate dehydrogenase release and dampening excessive ROS accumulation (Kwon et al., 2019).
Flavanols
It is well known that metal ions such as Zn(II), Cu(II), Mn and Fe(III) are involved in the pathogenesis of neurodegenerative diseases, as they can enhance oxidative stress by generating highly toxic hydroxyl radicals through the Fenton reaction(Xu et al., 2017; Zhao et al., 2017; Maulik et al., 2019).Furthermore, metal ions can promote and accelerate the fibrillation of αSyn. Zhao et al. (2017) reported that the treatment with epigallocatechin gallate, the most abundant flavonoid in green tea (Camellia sinensis), inhibits αSyn fibrillation and reduces intracellular ROS levels, by chelating Fe(III) in wild-type αSyn-transduced PC12 cells. Xu et al. (2017)confirmed the free-radical scavenging and iron-chelating properties of epigallocatechin gallate in MPTP mice model.In this model, MPTP induces iron accumulation in the SNc,which is associated with altered expression of iron exporter ferroportin, a key regulator of cellular iron metabolism.Chronic treatment with epigallocatechin gallate counteracted MPTP-induced motor impairment and neurochemical de ficits and enhanced ferroportin expression levels in the SNc (Xu et al., 2017). Interestingly, Chen et al. (2015) demonstrated neuroprotective effects of epigallocatechin gallate in MPTP-intoxicated monkeys. Epigallocatechin gallate reduced dopaminergic cell loss in the SNc and improved motor performance while reducing accumulation of neurotoxic αSyn oligomers in the striatum and hippocampus.
Flavanones
Naringenin is a flavanone found in a variety of fruits and herbs, particularly in grapefruit. naringenin possesses immunomodulatory and antioxidant effects that may be beneficial to a variety of pathological conditions, including neurodegenerative diseases (Maatouk et al., 2016; Oguido et al., 2017; Yang et al., 2019). Mani et al. (2018) showed that the naringenin treatment counteracts MPTP-induced dopaminergic degeneration and significantly upregulates DA-transporter and tyrosine hydroxylase protein expression.Oral pre-treatment with naringenin for 5 days significantly counteracted the MPTP-induced lipid peroxidation and increased GSH, SOD and CAT levels, improving the behavioral performance (Sugumar et al., 2019). Naringenin also downregulated the mRNA expression levels of proinflammatory mediators (i.e., TNF-α, IL-1β, and inducible oxide synthase; Sugumar et al., 2019) and inhibited αSyn fibrillation in MPTP-intoxicated animals (Mani et al., 2018).Although these results are encouraging, naringenin treatment shows several limitations due to its poor bioavailability and water insolubility, which prompted the development of new formulations and delivery routes. Recently, intranasal delivery of a naringenin-nanoemulsion proved able to enhance the uptake and bioavailability of naringenin in the brain (Gaba et al., 2019). Intranasally-administered naringenin improved the motor performance in a 6-OHDA rat model and modulated the oxidative stress response, as demonstrated by the signi ficant increase of GSH and SOD levels, and the decrease of MDA and lipid peroxidation (Shakeel et al., 2017; Gaba et al., 2019).
Treatment with naringin, the major flavonoid glycoside in grapefruit and pummelo, protected dopaminergic neurons by reducing microglial activation and enhancing themechanistic target of rapamycincomplex-1 prosurvival pathway (Kim et al., 2016).

Table 1 |Summary of the effects of flavonoids in PD models
In vivo6-OHDA ↓Neurodegeneration
28 d Quercetin (10 and 25 mg/kg, p.o.) Ghaffari et al., 2018
↑Motor performance
↑Cognitive performance↓Oxidative stress
In vivo6-OHDA ↓Oxidative stress↓Neuroinflammation↑Motor performance
14 d Quercetin (25 mg/kg, p.o.) with piperine
(2.5 mg/kg, p.o.) Singh and Kumar, 2018
In vitro6-OHDA ↓Oxidative stress↑Cell viability 4 h Hyperoside (0, 0.25, 0.5, 1, 2.5, 5, and 10 μM) Kwon et al., 2019In vivoRotenone ↓Oxidative stress
↓Endoplasmic reticulum stress↓Apoptosis
↑Motor performance
↑Autophagy efficiency 28 d Quercetin (50 mg/kg, i.p.) El-Horany et al.,2016
In vivoRotenone/Iron ↑Motor performance
↓Oxidative stress
↓Neuroinflammation↓Neurodegeneration
28 d Quercetin (25 and 50 mg/kg, p.o.) Sharma et al., 2020
In vitroαSyn ↓Oxidative stress↓αSyn fibrillation 24 h Epigallocatechin gallate (0, 10, 20, 50, 100 and 200 μM) Zhao et al., 2017In vivoMPTP ↑Motor performance↓Oxidative stress↓ Iron levels
7 d Epigallocatechin gallate (25 mg/kg, p.o.)Xu et al., 2017
In vivoMPTP ↑Motor performance↓Neurodegeneration↓αSyn
80 d Epigallocatechin gallate (40 mg/kg, p.o.) Chen et al., 2015a
In vivoMPTP ↓Oxidative stress↓Neuroinflammation↓Neurodegeneration↓αSyn
5 d Naringenin (25, 50 and 100 mg/kg, p.o.)Mani et al., 2018
In vivoMPTP ↓Oxidative stress↓Neuroinflammation↑Motor performance
5 d Naringenin (25, 50 and 100 mg/kg, p.o.)Sugumar et al., 2019
In vivo6-OHDA ↓Oxidative stress↑Motor performance 28 d Vitamin E Loaded Naringenin Nanoemulsion (0.72 mg/kg, intranasal)Gaba et al., 2019In vivo6-OHDA ↓Oxidative stress↓Neuronal damage 15 d Naringenin (20 and 40 mg/kg, p.o.) Shakeel et al., 2017In vivo6-OHDA ↓Neurodegeneration↓Neuroinflammation 7 d Naringin (80 mg/kg, i.p.) Kim et al. 2016In vivo6-OHDA ↑Motor performance
7 d Hesperetin (50 mg/kg, p.o.) Kiasalari et al. 2015
↓Oxidative stress
↓Neuroinflammation↓DNA fragmentation↓Apoptosis
In vivoMPTP ↑Motor performance
↓Presynaptic glutamate release↓Glutamatergic transmission
↑Synaptic GluR1subunit
↓Neuroinflammation
14 d Baicalein (10 mg/kg, i.p.) Xue et al., 2014
In vivoMPTP ↑Motor performance↓Neurodegeneration 7 d Baicalein (1 and 10 mg/kg, i.p.) Lee et al., 2014In vivoMPTP ↑Motor performance
7 d Baicalein (140 and 280 mg/kg, p.o.) Gao et al., 2015
↓Neurodegeneration↑Neurogenesis
↑Proliferation
In vivoMPTP ↓Neurodegeneration↑Motor performance 9 d Baicalein (10 mg/kg, i.p.) Zheng et al., 2019In vivoTransgenic ↑Motor performance
↓Oxidative stress
↓Neurodegeneration
24 h Tangeritin (5, 10, and 20 μM, p.o.) Fatima et al., 2017
In vivoTransgenic/6-OHDA ↓αSyn
↓Neurodegeneration
↑Food-sensing behavior↑Life span72 h Irisflorentin (0, 0.1, 0.5, 2.5, and 12.5mM, p.o.) Chen et al., 2015c
In vitroαSyn ↓Oxidative stress
↓Apoptosis 24 h Genistein (20 μM) Wu et al., 2018
6-OHDA: 6-Hydroxydopamine; αSyn: α-synuclein; i.p.: intraperitoneal; MPTP: methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD: Parkinson’s disease; p.o.: oral administration or per os.Hesperetin, a flavanone derived from hesperidin and found in citrus fruit, reduced MDA levels, enhanced CAT activity and GSH content in the striatum of 6-OHDA rat model; these effects were accompanied by reduced astrocyte activation and increased B-cell lymphoma 2 expression levels. Hesperetin exerted neuroprotective effects also in the SNc by preventing the loss of dopaminergic neurons and restoring motor performance (Kiasalari et al., 2016).
Flavones
Baicalein is a flavone extracted from the roots ofScutellaria baicalensisand used in traditional Chinese herbal medicine for its antibacterial, antiviral, and anti-in flammatory effects (Lee et al., 2014; Gao et al., 2015). Baicalein attenuates presynaptic glutamate release and reduce cytokines levels in the nigrostriatal pathway in mice injected with MPTP (Xue et al.,2014). Moreover, baicalein treatment - alone or in combination with levodopa - increased motor performance and prevented MPTP-induced dopaminergic cell loss (Lee et al., 2014; Gao et al., 2015; Zheng et al., 2019). This neuroprotective effect was accompanied by glial cells deactivation. In addition, baicalein suppressed the MPP+-induced nuclear translocation of nuclear factor-κB, consequently decreasing c-Jun N-terminal kinase and extracellular signal-regulated kinase activation (Lee et al., 2014). Interestingly, Gao et al. (2015) demonstrated that baicalein promotes neurogenesis, neuroblast proliferation and neurotrophin signaling pathway.
Oral treatment with Tangeritin, a flavone isolated from peels of citrus fruits, for 24 days improved motor climbing performance in a transgenic Drosophila model of PD that expresses human wild type αSyn (Fatima et al., 2017). This symptomatic effect was accompanied by an increase of dopamine and a modulation of oxidative stress markers(Fatima et al., 2017).
Iso flavones
Chen et al. (2015) demonstrated the therapeutic properties of irisflorentin, an isoflavone derived fromBelamcandae Rhizoma, in two differentCaenorhabditis elegansPD models.In particular, the treatment with iris florentin prevented αSyn accumulation in the transgenicC. elegansmodel, whereas reduced dopaminergic neuron degeneration, and improves food-sensing behavior and life-span in 6-OHDA-induced model. According to the authors, iris florentin may exert their effects by modulating the expression of both 26S-proteasome non-ATPase regulatory subunit 3 and Egg-laying defective protein 1, consequently enhancing the activity of the proteasome and blocking apoptosis (Chen et al., 2015c).
It was reported that genistein, a molecule present in lupin,fava beans, soybeans and kudzu, reduces rotenone-induced oxidative stress and cell apoptosis in neuroblastoma SH-SY5Y cells overexpressing A53T mutant αSyn (Wu et al., 2018).Speci fically, genistein induced its cytoprotective properties by activating estrogen receptors, increasing the phosphorylation of pro-apoptotic protein BAD and modulating the levels of nuclear factor-erythroid 2-related factor. This event led to the reduction of rotenone-induced mitochondrial oxidative injury and MDA levels, and an increase of the activity of Heme Oxygenase 1, which plays a central role in neuronal protection(Wu et al., 2018).
Non- flavonoids molecules
Non-flavonoid PPH include phenolic acids, phenolic alcohol,stilbenes and lignans (Ferrazzano et al., 2011). The first two sub-classes of molecules are characterized by the presence of a single benzene ring and a carboxylic (phenolic acids)or hydroxyl (phenolic alcohols) terminal functional group.Conversely, stilbenes and lignans have both two benzene rings, but a different polymer structure: linear for stilbenes and branched for lignans (Manach et al., 2004;Table 2).
Stilbenes
Resveratrol is the most important compound of this subclasse and it is found mainly in grape skin. Resveratrol modulates mitochondrial function and redox biology, more likely by its acting as gene regulator (Xia et al., 2017; Jardim et al., 2018). In a study on MPTP-treated Drosophila, resveratrol increased CAT, GSH and acetylcholinesterase activities(Abolaji et al., 2018). Resveratrol significantly alleviated MPP+-induced cytotoxicity and mitochondrial dysfunction by modulating Akt/Glycogen synthase kinase-3 pathway.Indeed, the inhibition of glycogen synthase kinase-3 activity clearly abolished the protective effects of resveratrol (Zeng et al., 2017). Analogously, resveratrol administration has been demonstrated to suppress rotenone induced-neurotoxicity in PC12 cells, whereas this compound increases heat-shock 8 and 90 protein levels and protein degradation systems in parkin-mutant skin primary fibroblasts (Vergara et al.,2017). Recent evidence has indicated that also SIRT1/Akt1 pathway is involved in the neuroprotective effect performed by resveratrol (Zhang et al., 2015; Diaz-Gerevini et., 2016).Accordingly, blocking SIRT1 or Akt1 leads to a decrease of the protective effect exerted by this compound (Wang et al.,2018). Guo et al. (2016) reported that resveratrol-induced activation of SIRT1 and subsequent LC3 deacetylation in MPTP model contribute to autophagic degradation of αSyn.Furthermore, an improvement of behavioral deficits has been reported in this model. In line with this, Zhang et al.(2018) showed that a 5-week oral treatment with resveratrol improves motor performance and alleviates cognitive de ficits in the A53T αSyn mice. This effect on behavior was associated with an increase of tyrosine hydroxylase levels and a reduction of neuroinflammatory process and αSyn levels in the brain,especially phospho-Ser129-αSyn form. The anti-in flammatory effects of resveratrol have been confirmed in a rat model of rotenone-induced PD (Gaballah et al., 2016). MPTP treatment in mice also affects olfactory discrimination and social recognition memory. Administration of resveratrol for 15 days restored these behavioral and neurochemical de ficits and reduced the severity of neurodegenerative process in the striatum (da Rocha Lindner et al., 2015).
Oral treatment with piceid, a non-glycosylated derivative of resveratrol, prevented oxidative damage in the rotenone rat model. In particular, Chen et al. (2015b) demonstrated that piceid restores the levels of SOD, thioredoxin and GSH and decreases MDA in the striatum. At the same time, this PPH contributed to restore proper mitochondrial function by increasing ATP levels and rescued rotenone-induced dopaminergic cell loss in the SNc.
Other non- flavonoids molecules
The lignans are a large group of PPH found in plants. In a recent study, treatment with 7-hydroxymatairesinol, a lignan extracted from the heartwood of the Norway spruce (Udani et al., 2013), induced a significant reduction of the striatal damage and improvement of motor performance in rats bearing a 6-OHDA induced nigrostriatal lesion. These effects may be ascribable to a reduction of neuroinflammatory response to 6-OHDA, induced by this compound. Indeed, fewer activated glial cells (microglia and astrocytes) and modulation of microglia phenotypes, with an important reduction of the cytotoxic phenotype, were observed in animals orally treated with this PPH. In addition, a moderate reduction of proin flammatory mediator mRNA levels and an increase of anti-in flammatory mediators were detected (Giuliano et al., 2020).Similarly, the PPH ellagic acid, a phenolic acid found in blackberry, improved the motor impairment via reducing the levels of neuroin flammatory biomarkers TNF-α and IL-1β and protected the brain against free radicals-induced neural damage (Farbood et al., 2015).
García-Moreno et al. (2019) demonstrated that treatment
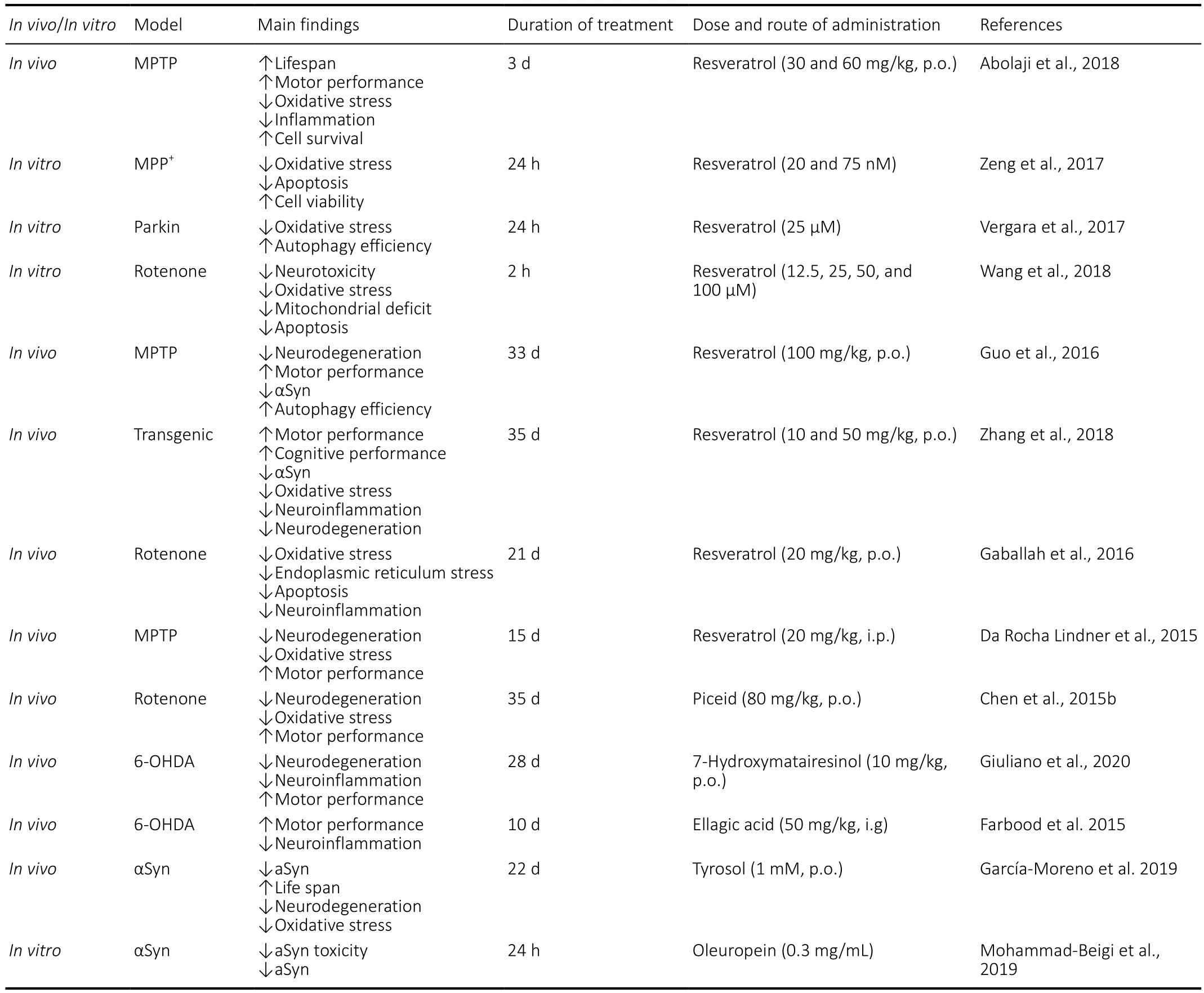
Table 2 |Summary of the effects of non-flavonoids molecules in PD models
with tyrosol, one of the main PPH in olive leaf and fruit,reduced αSyn inclusions in transgenicC. elegansthat expressing human αSyn. This effect resulted in a reduced dopaminergic cell loss, lower αSyn toxicity and extended life span. Tyrosol also reduced ROS levels and promoted the expression of speci fic chaperones and antioxidant enzymes.
Analogously, oleuropein, a phenolic compound from olive fruit extracts belonging to the sub-classe of tyrosols, reduced αSyn toxic aggregation and, consequently, counteracted αSyn cytotoxicity in SH-SY5Y cells treated with αSyn aggregates(Mohammad-Beigi et al., 2019).
Complex Mixture of Polyphenols
Fruits and vegetables contain high levels of complex mixtures of PPH (both flavonoids and non-flavonoids) that can act synergistically to provide neuroprotective effects (Table 3).
Zhang et al. (2015) demonstratedin vitrosynergistic neuroprotective effects of chrysin ( flavones) and protocatechuic acid (phenolic acid), two PPH identi fied in the fruits ofAlpinia oxyphylla. In particular, the co-treatment resulted in greater cell viability with decreased lactate dehydrogenase release in 6-OHDA-treated PC12 cells. These cytoprotective effects could be related to the decrease of kappa-light-chain-enhancer of activated B expression and an increased transcriptional activity of nuclear factor erythroid 2-related factor 2, with consequent upregulated expression of antioxidant enzymes (SOD, CAT and GSH) and reduction of MDA levels (Zhang et al., 2015).
Mangrulkar and Chaple (2019) have recently demonstrated the potential of the PPH-extract ofcymbopogon citratus(composed by flavonoids, tannin and phenolic acid-rich fractions) in two different animal models of PD (haloperidol and reserpine). The treatment with this extract reduced motor de ficits by restoring DA levels and improving the effectiveness of antioxidant system.
Wu et al. (2018) demonstrated the benefits of daily treatment with grape skin extract, which contains PPH such as resveratrol (stilbenes) proanthocyanidine (tannins) and quercetin ( flavonols), in Drosophila melanogaster with PTEN-induced kinase 1 loss-of-function. In this latter, the loss of PTEN-induced kinase 1 function causes important motor deficit, associated with energy depletion, degeneration of dopaminergic neurons and shortened lifespan (Yang et al., 2006). Notably, consumption of this extract protected mitochondrial structure and extended lifespan with an important effect on motor function (Wu et al., 2018).
PPH-digested metabolites fromarbutus unedo(including flavanols, flavonols, ellagitannins and phenolic acids) have been shown to reduce oxidative and endoplasmic reticulum stress and mitochondrial impairment in PD cell models by reducing aSyn aggregation (Macedo et al., 2018).
Similarly, Maulik et al. (2018) demonstrated the ability of the crude extract of Alaskan bog blueberry (phenolic,anthocyanin and flavonoids), fromvaccinum uliginosum, to attenuate human αSyn aggregation and improve motility in a transgenic C. elegans model that expressed αSyn. Maulik et al. (2018)indicated that the reduction of gene expression of NAD-dependent protein deacetylase sir-2.1 (ortholog of mammalian Sirtuin 1) could be a potential mechanism through which blueberry exerts its bene ficial effects (Maulik et al., 2018). In a more recentin vivostudy, Maulik et al.(2019) evaluated the interplay between dietary fat and extract of Alaskan bog blueberry supplementation on manganeseinduced neurotoxicity in mice. They demonstrated that low-fat or normal-fat diets, in combination with extract supplementation, improved motor performance and attenuated the molecular hallmarks of neurotoxicity. However,when animal are fed with a high-fat diet, the therapeutic effects of the extract were suppressed, demonstrating the importance of including PPH in low-fat diet to counteract agerelated neurodegenerative disorders.
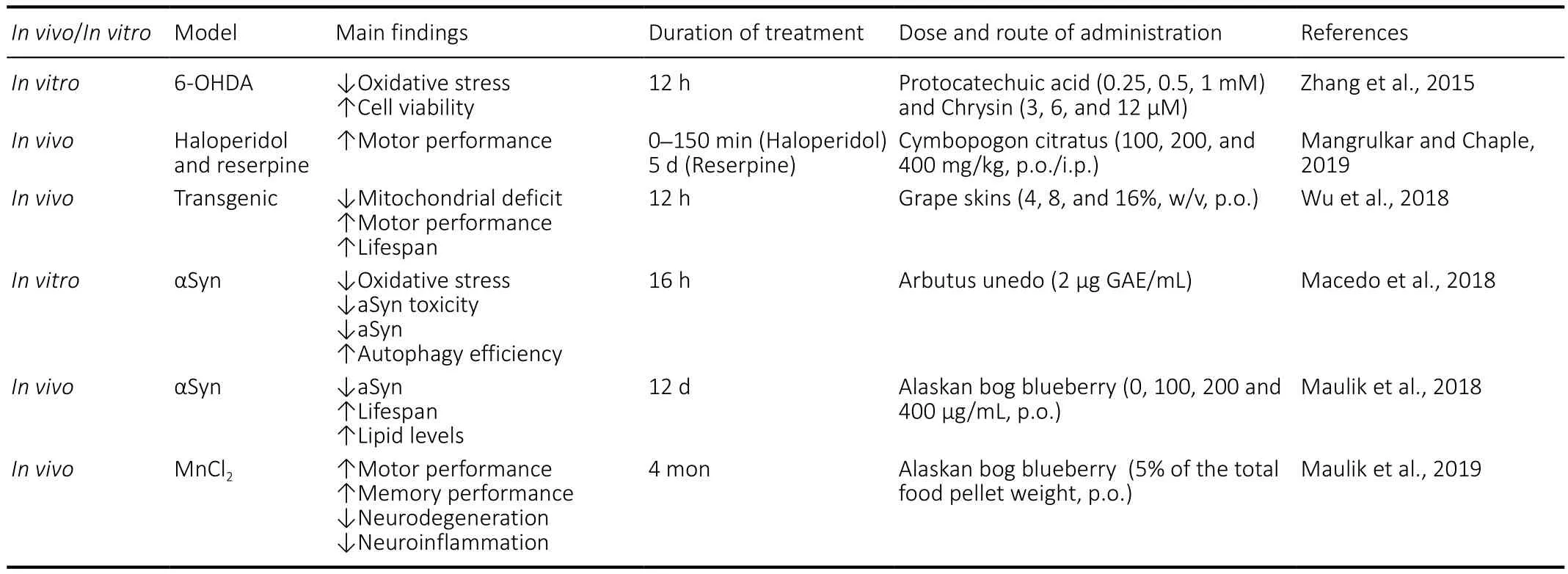
Table 3 |Summary of the effects of complex mixture of polyphenols in PD models
Conclusion
PD is a neurodegenerative disorder with a remarkable impact on the patient’s quality of life and on the economic and welfare burden that weighs on family caregivers. Current therapeutic strategies for PD are mainly symptomatic and hampered by important side effects. It is therefore essential to promote the development of new therapeutic strategies aimed to interrupt or, at least, slow down the neurodegenerative process and thus the onset or progression of symptoms. Compounds able to positively modulate glial cells activity, reduce ROS levels and enhance autophagy may represent a valuable innovation in the treatment of PD.Thein vivoandin vitroexperimental results reported in the current review suggest that several PPH molecules could be protective against the pathogenic processes implicated in PD.A distinctive trait of these compounds deserving attention is their multi-target activity. Indeed, each PPH molecule may act simultaneously on different pathomechanisms, promoting the restoration of oxidative homeostasis, the reduction of the neuroinflammatory process and the enhancement of αSyn clearance. All together, these effects bring to a reduction of the neurodegenerative process in the nigrostriatal pathway with a consequent improvement of motor performance and,apparently, without side effects (Figure 1).
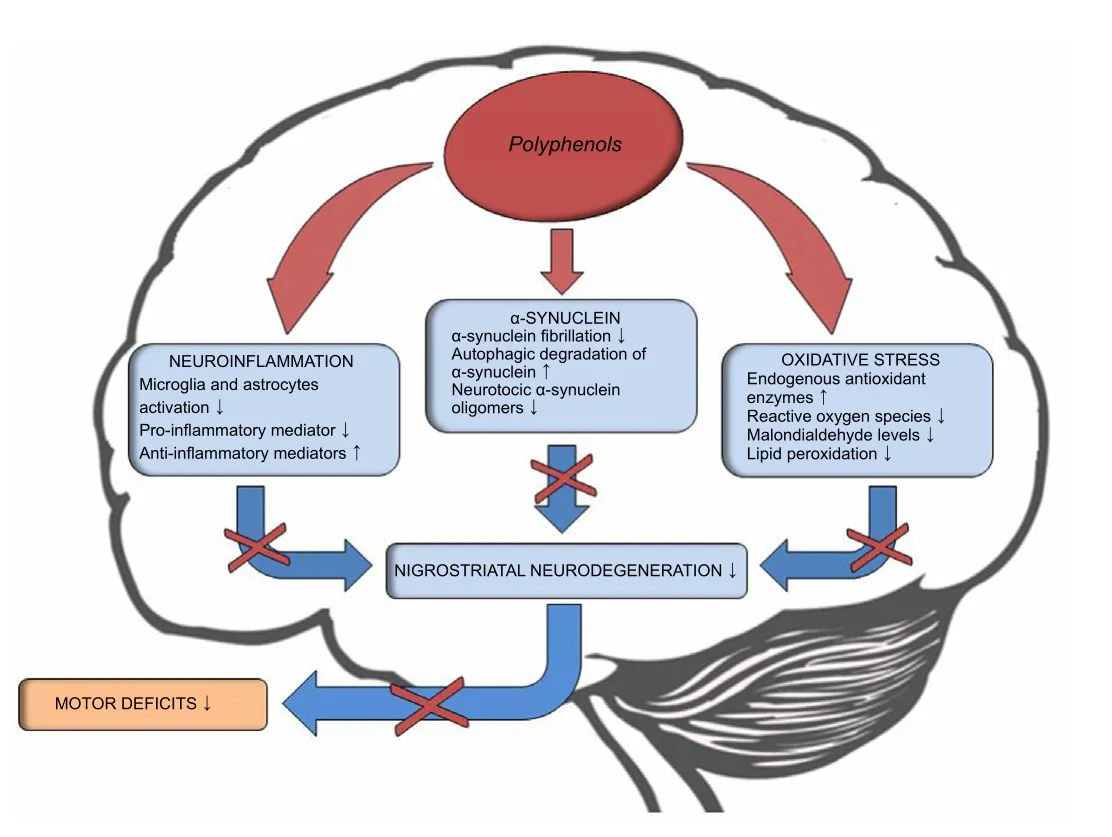
Figure 1 | Potential effects of polyphenols in Parkinson’s disease.
Further studies are needed to investigate new formulations and route of administration for these molecules in order to ameliorate their bioavailability and, consequently, to maximize their biological activity in view of their potential use in PD therapy.
Author contributions:Review conception and design: CG. Manuscript writing: CG and SC. Paper review and review guiding: SC and FB. All authors approved the final manuscript.
Con flicts of interest:The authors declare no con flicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury
- Dysfunctional glia: contributors to neurodegenerative disorders
- In flammation/bioenergetics-associated neurodegenerative pathologies and concomitant diseases: a role of mitochondria targeted catalase and xanthophylls
- Possible implications of dysregulated nicotinic acetylcholine receptor diffusion and nanocluster formation in myasthenia gravis
- Hydrogel-based local drug delivery strategies for spinal cord repair
- Altered physiology of gastrointestinal vagal afferents following neurotrauma

