Intravenous haloperidol for the treatment of intractable vomiting, cyclical vomiting, and gastroparesis
Brad E.Schwartz, Karen Keller Baker, Andrew J.Bleinberger, Amina Lleshi, Raul Cruz-Cano
1 Department of Emergency Medicine, University of Maryland Capital Region Health, UM Prince George’s Hospital Center,Cheverly 20785, USA
2 Ross University School of Medicine Bridgetown, Bridgetown 11093, Barbados
3 Department of Epidemiology and Biostatistics, School of Public Health of the University of Maryland, Baltimore 21201,USA
Dear editor,
The incidence of emergency department (ED) visits from gastroparesis and cannabinoid hyperemesis syndrome has increased by two folds over the past decade.[1-3]Roldan et al[4]reported that patients with these conditions often have prolonged ED stays and are likely to be hospitalized because of the danger of dehydration and challenges inherent in relieving symptoms.Opioid analgesia is a particularly troublesome treatment modality; these conditions are often chronic with frequent recurrences, and repeated opiate therapy can cause opioid dependency and narcotic bowel syndrome.[5]Haloperidol (HP) and droperidol, members of the butyrophenone class, have potent antidopaminergic activity in the chemoreceptor trigger zone.[6,7]Intramuscular administration of HP is being used increasingly in the ED to treat nausea, vomiting secondary to gastroparesis,[6]intractable vomiting,[8]and cyclical vomiting.[9,10]
Patients with intractable vomiting come to EDs seeking symptom relief.Unless they have an established pre-existing diagnosis, the emergency physician has the difficult task of treating symptoms and identifying ailment causes.Many physicians have turned to intravenous (IV) HP over its intramuscular counterpart to manage intractable vomiting[11]as IV HP has a superior onset of action between 3 and 20 minutes.Interestingly,the use of IV HP persists even despite current United States Food and Drug Administration (FDA)recommendations cautioning the use of IV HP.The HP injection is not approved for IV administration.If HP is administered intravenously, the electrocardiogram(ECG) should be monitored for QT prolongation and arrhythmias.[12,13]HP’s most effective dose and administration route have not been well established in patients with undiff erentiated intractable vomiting.
This study is designed to determine the efficacy of IV HP in reducing hospitalization rates for patients with undiff erentiated intractable vomiting, cyclical vomiting,as well as gastroparesis.
METHODS
This retrospective case-control cross-over study used the electronic medical record to analyze visits between October 2013 and March 2018 at an urban community ED with approximately 42,000 visits per year.All patients with a f inal ED diagnosis of intractable vomiting,cyclical vomiting, or gastroparesis were selected initially.Patients were diagnosed with intractable vomiting and cyclical vomiting as a final diagnosis by a treating ED physician.Patients with gastroparesis either had verbally conf irmed to the physician their pre-existing diagnosis or had gastroparesis conf irmed on a prior inpatient stay with a gastric emptying study.
Patients met inclusion criteria if they had two or more ED visits for the treatment of intractable vomiting,cyclical vomiting, or gastroparesis and did not receive HP (non-IV HP) during one visit but did receive it during a previous or subsequent visit (Figure 1).For patients who made multiple visits, the earliest visit fulf illing the inclusion criteria was selected.We planned to exclude visits occurring within seven days of each other to ensure discrete illness events, but there were no instances requiring this exclusion.
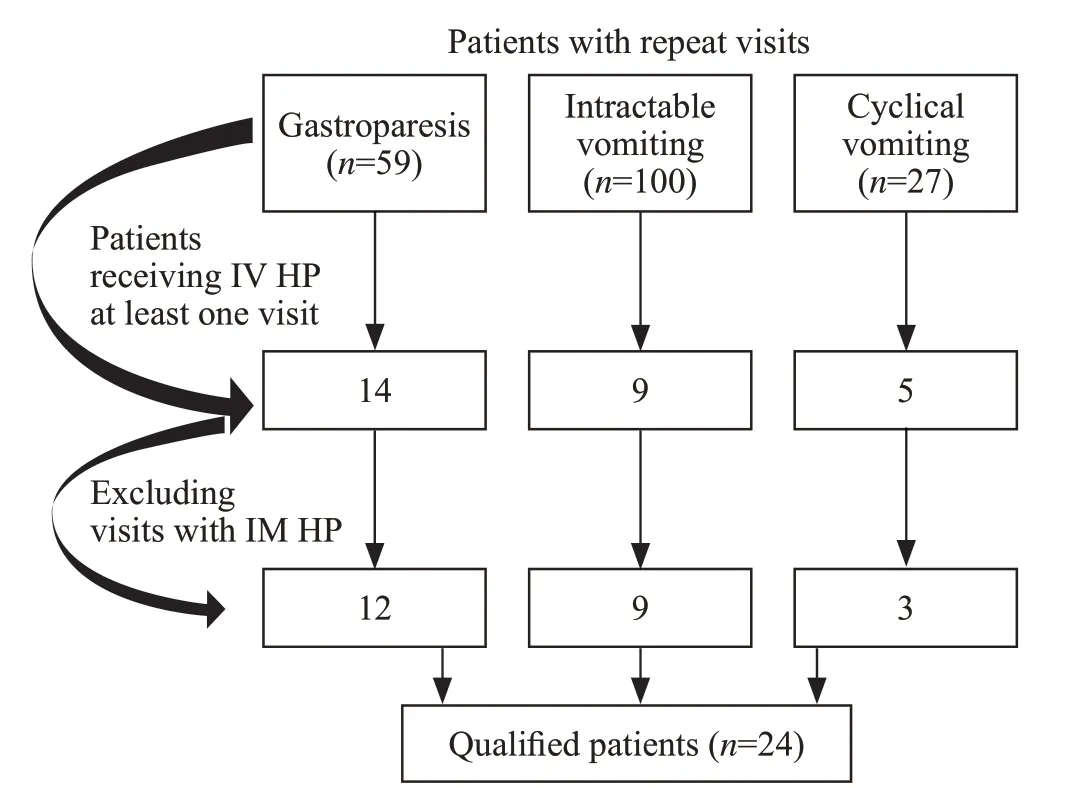
Figure 1.Cohort and inclusion criteria.HP: haloperidol; IV: intravenous;IM: intramuscular.
Our search criteria yielded 24 eligible patients (48 total patient encounters) over the 4.5-year study period.Two independent trained reviewers (medical students) collected the data.No discrepancies were noted by the principal investigator after independently reviewing their work.
The primary outcome of this study was hospitalization, as defined on chart review by the transition of care of the patient from the ED care to the inpatient team care, under observation or inpatient status.Secondary outcomes of interest were opioid analgesic administration calculated using morphine equivalent doses of analgesia (http://www.medcalc.com/narcotics.html), the intensity of abdominal pain recorded on a scale of 1 to 10 at triage and at time of disposition, and ED length of stay.Dosing of IV HP and any adverse events were recorded.We noted if additional medications were administered as well as the sequence in which they were administered.
An incomplete pain score charting was noted in three out of 24 patients.The length of stay could not be determined accurately in one out of 24 patients.
Descriptive statistics, Fisher’s exact test, and McNemar test were used for comparison of categorical variables, and Wilcoxon signed-rank test for continuous variable comparison was performed.Statistical significance was defined as two-tailP-values less than 0.05.Statistical analysis was performed using SAS and R statistical packages.
RESULTS
The majority of patients in this study were between the ages of 31 and 40 years.Of the 24 patients, 19(79.2%) required hospitalization during the visit in which they did not receive HP, whereas eight (33.3%) required hospitalization during the visit in which they were given IV HP (Table 1).The outcome of reduced hospitalization rate in the IV HP group was prominent (odds ratio[OR] 0.083, 95% confidence interval [CI] 0.002-0.563,P=0.004).Further subgroup evaluation demonstrated a trend amongst the gastroparesis group receiving IV HP for decreased hospitalization rate.
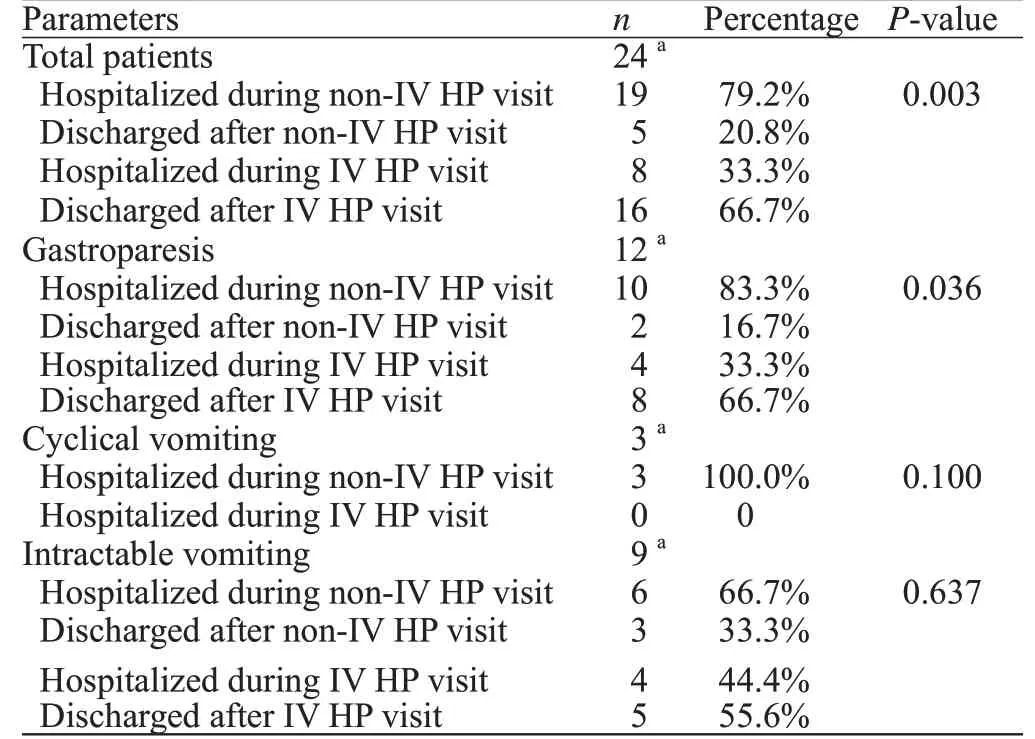
Table 1.Hospitalization rate comparison between the non-IV HP group and the IV HP group

Table 2.Medication classes administered, n (%)
The median morphine equivalent analgesic dose was 7.7 mg in the non-IV HP group and 0 mg in the IV HP group.The median of the differences in analgesic dosing provided was statistically signif icant (-5.2, 95%CI-9.6 to-3.0,P<0.005).
The median value of pain reduction was lower in the non-IV HP group than in the IV HP group (5 vs.7), but the difference was not statistically significant (-2.0, 95%CI-0.5 to 4.5,P=0.108).The length of stay in the ED was not signif icantly diff erent between the two groups (9.8 hours in the non-IV HP group vs.9.3 hours in the IV HP group).
The most frequently administered dose of IV HP was 5 mg, and it was typically given as a secondary agent (Table 2).No adverse side effects (dystonia, akathisia, excessive sedation, dysrhythmia, or intervention for QTc prolongation)were documented in the group that received IV HP.
DISCUSSION
HP is a butyrophenone-type antipsychotic that is available in oral and intramuscular preparations.It emerged from the pharmaceutical landscape in 1958 and was approved for entry into the USA market in 1967.It is commonly used as an antipsychotic due to its potent dopamine D2 receptor antagonist activity, which also causes it to be an eff ective agent in treating nausea.Over the past 40 years, it has been used extensively in psychiatry.[14]In fact, the authors of a meta-analysis comprising 21 randomized controlled trials based on more than 3,000 patient visits calculated that cardiac dysrhythmias occurred in 0.21% of cases in which a dose of 5 mg of IV HP was administered.[14]A precautionary ECG prior to IV HP administration as well as continuous cardiac monitoring is recommended.[15]HP use has been described in the palliative care and anesthesiology literature, being administered for intractable vomiting and postoperative vomiting, respectively, with positive outcomes.[16-18]In addition to its antiemetic eff ects, HP appears to amplify the analgesic eff ect of opiates.This eff ect is suspected for several reasons: HP synergistically augments the dopaminergic pathway in the striata niagara in the brain,[19]it has an isomeric similarity to meperidine, and it plays a role in the N-methyl-D-aspartate (NMDA) pain modulation pathway in animal models.[4]
We found signif icant benef its of IV HP administration in terms of fewer patients requiring hospitalization in our study.The decreased hospitalization rate was consistent with prior studies and case reports using intramuscular and IV HP.[4,6,10]In a study by Ramirez et al,[6]a cohort of 52 patients who received intramuscular HP for management of diabetes-associated gastroparesis were case-matched to a prior ED visit when they had not received HP.Their study noted a reduction of hospitalization rates in their HP group of approximately 17%, while our study noted a nearly 50% reduction in hospitalization.It remains to be determined if this significant reduction in hospitalization rates is due to the IV route of HP used in our cohort, rather than the intramuscular route from Ramirez et al.[6]
Our study also noted fewer requirements for opioid analgesia.Ramirez et al[6]found that HP as an adjunctive therapy was superior to placebo for acute gastroparesis symptoms.[4]The median morphine equivalent analgesic dose was 7.7 mg in the non-IV HP group and 0 mg in the IV HP group.Also, the median reduction in pain (-2 on the pain scale), while not statistically significant, did show a moderate association.IV HP reduced analgesia requirements and appeared to have reduced pain scores in patients.
Limitations
This study’s main limitation was its small sample size.Our inclusion criteria encompassed intractable vomiting, cyclical vomiting, and gastroparesis.Generally, at our clinical site, HP administration for nausea was reserved for patients with severe and intractable vomiting, which limited our ability to include patients with undifferentiated nausea and vomiting as their final diagnosis.Our study’s secondary outcome of ED length of stay did not reach statistical significance.Our small sample size may have been underpowered to detect this outcome measure.
Our study design enabled us to capture repeat visits to one ED for the same gastrointestinal condition, but patients could have gone to other hospitals seeking treatment for the same condition.The study site is in a metropolitan area with a variety of secondary and tertiary hospitals, so it was possible that we did not capture all ED visits by the patients who met our inclusion criteria.Our search criteria focused on intractable vomiting and did not include other f inal diagnoses, such as abdominal pain.Additionally, our use of three subcategories of vomiting could have created confounding variables that were not controlled for in our retrospective analysis.
Our patient population was a demographically homogenous group, which made our results less generalizable.Our study period spanned more than four years.Although most visits were clustered over several months, there were some visits with a temporal gap of over six months.
This study was not powered to evaluate the frequency of adverse eff ects related to the IV administration of HP.We found no documentation of adverse events in the patients’ charts, but HP’s action has a half-life of 21 to 24 hours, so dystonic reactions or other adverse effects could have arisen after the typical ED course.No cardiac events occurred in either of our cohort groups.
CONCLUSIONS
In this small retrospective study, IV HP appears to be an effective adjunctive treatment in the ED for patients suffering from intractable vomiting, cyclical vomiting,or gastroparesis.HP is more effective than traditional care in reducing the hospitalization rate.Patients who receive IV HP require less narcotic pain control.Continued research is needed to compare the effi cacy of intramuscular and IV administration of HP and whether the IV administration route has a superior reduction in rates of hospitalization and analgesic dosing.
ACKNOWLEDGMENT
The manuscript was copyedited by Linda J.Kesselring, MS, ELS.
Funding:This study did not receive any funding.
Ethical approval:The study was approved by the institutional review board.
Conf licts of interests:The authors have no conf licts of interest to declare.
Contributors:BES and KKB designed the study.BES obtained approval from the IRB for the study.AJB and AL collected the data and, in conjunction with BES and KKB, wrote the f irst draft.BES,KKB, AJB, AL, and RCC analyzed the results and contributed to the manuscript.All authors have reviewed and approved the f inal draft of the manuscript.
 World journal of emergency medicine2021年3期
World journal of emergency medicine2021年3期
- World journal of emergency medicine的其它文章
- Chemical pneumonitis caused by intravenous injection of insecticide spray
- Myocardial infarction detected by a smartwatch after transcatheter aortic valve replacement during the COVID-19 pandemic
- Mediastinum metastasis in a post-surgical pancreatic cancer patient successfully conf irmed with endoscopic ultrasonography
- Biphasic anaphylaxis manifested as type I Kounis syndrome induced by ingestion of raw f ish gallbladder: A case report
- Tension hydropneumothorax in a Boerhaave syndrome patient: A case report
- Performance of extracorporeal membrane oxygenation in patients with fatal paraquat poisoning:grasp for straws?
