Effect of ATM/CHK2/CDC25A Signal Pathway on the Resistance of Colorectal Cancer Cells to L-OHP
Xiaoli YAN, Peng YAN, Jing XUE, Yanfang FAN, Jining ZHENG
Chengde Medical University, Chengde 067000, China
Abstract [Objectives] To compare the expression of key factors ATM, CHK2, CDC25A and CDK2 in ATM/CHK2/CDC25A signal pathway in colorectal cancer cell line HCT116 and L-OHP-resistant cell line HCT116/L-OHP, and to explore the relationship between ATM/CHK2/CDC25A signal pathway and resistance of colorectal cancer cells to L-OHP. [Methods] RT-qPCR was used to detect the expression of ATM, CHK2, CDC25A and CDK2 mRNA in colorectal cancer cell line HCT116 and L-OHP-resistant cell line HCT116/L-OHP, and Western blot was used to detect the expression of corresponding proteins in these two cell lines. [Results] The expression of ATM, CHK2, CDC25A, CDK2 mRNA and protein in HCT116/L-OHP was significantly higher than that in HCT116. [Conclusions] ATM/CHK2/CDC25A signal pathway was associated with resistance of colorectal cancer cell line HCT116 to L-OHP. Blocking this signal pathway may partially reverse the chemotherapy resistance of colorectal cancer patients with L-OHP resistance.
Key words Colorectal cancer, ATM, CHK2, CDC25A
1 Introduction
Colorectal cancer is one of the main cancers that threaten the health of residents at home and abroad, and it is also one of the common malignant tumors in China. In recent years, the morbidity and mortality of colorectal cancer in China have maintained an upward trend. The 2019 China Cancer Statistical Report shows that new cases of colorectal cancer in China account for 9.87% of all malignant tumors, and deaths caused by colorectal cancer account for 8.01% of all malignant tumors[1]. China has become the country with the largest number of new cases and deaths of colorectal cancer every year. Clinically, Oxaliplatin (L-OHP) is a first-line chemotherapeutic drug for patients with advanced colorectal cancer, and has been included in the recommended chemotherapy regimen for stage II, III and recurrent or metastatic colorectal cancer[2]. However, the resistance of colorectal cancer patients to L-OHP is one of the main reasons for the significant decrease or even failure of chemotherapy. Therefore, looking for new biomarkers to reverse L-OHP resistance will help to improve the efficacy and prognosis of patients with L-OHP resistance in colorectal cancer. L-OHP is the third generation of platinum chemotherapeutic drug, which mainly prevents cell division and proliferation by destroying the structure and function of DNA, while the structural damage of DNA is related to multiple signal pathways. At present, there are few studies on the relationship between ATM/CHK2/CDC25A signal pathway and L-OHP resistance in colorectal cancer cells. Therefore, by comparing the expression of ATM, CHK2, CDC25A and CDK2, the key factors of ATM/CHK2/CDC25A signal pathway in colorectal cancer parent cell line HCT116 and L-OHP-resistant cell line HCT116/L-OHP, this experiment explored the relationship between ATM/CHK2/CDC25A signal pathway and L-OHP chemotherapy resistance of colorectal cancer cells.
2 Materials and methods
2.1 MaterialsHuman colorectal cancer cells (HCT-116) were purchased from Guangzhou Jennio Biotechnology Co., Ltd., and drug-resistant human colorectal cancer cells (HCT-116/L-OHP) were purchased from Shanghai Bogoo Biotechnology Co., Ltd. Fetal bovine serum (FBS) was purchased from BI Company, and RPMI-1640 medium and Trypsin-EDTA (0.05%) were purchased from Gibco Company. TRIZOL reagents were purchased from Invitrogen Technology Co., Ltd., RT-qPCR kits were purchased from Tiangen Biochemical Technology Co., Ltd., Hairpin-itTM microRNA and U6 snRNA standardized RT-PCR quantitative kits were purchased from Suzhou GenePharma Co., Ltd. MiRNA-21, miRNA-21mimics, miRNA-21inhibitors and the corresponding negative control sequences were all designed and synthesized by GenePharma, and the other primer sequences were designed and synthesized by Shanghai Sangon Bioengineering Co., Ltd. RIPA lysate was purchased from Solarbio Technology Co., Ltd., ATM rabbit anti-human IgM monoclonal antibody was purchased from ABclonal Company, CHK2, CDK2 rabbit anti-human IgM monoclonal antibody, CDC25A rabbit anti-human IgM polyclonal antibody and goat anti-rabbit IgG secondary antibody were purchased from Beyotime Biotechnology Co., Ltd., and ECL luminous solution was purchased from Beijing Applygen Technology Co., Ltd.
2.2 Methods
2.2.1Cell culture. Human colorectal cancer cells and drug-resistant cells were cultured by a RPMI-1640 complete medium containing 10% fetal bovine serum (FBS) in a cell incubator containing 5% CO2at 37 ℃. When the cell growth coverage rate reached 85% to 90% of the bottom of the culture dish, it was digested with trypsin and subcultured at the proportion of 1∶3.
2.2.2Real-time quantitative PCR (RT-qPCR) detection of the expression level of related genes. Cell RNA extraction: when the coverage rate of the cells to be cultured reached 80% to 90%, the cells were washed with PBS for 3 times, and the total RNA of the cells was extracted with TRIZOL. The concentration of RNA was detected by Nanodrop 2000 spectrophotometer. If the A260/A280ratio was in the range of 1.8 to 2.1, it was the RNA that conformed to the standard. Three independent repeated experiments were carried out. In each experiment, there were two double holes for each sample, and the difference ofCtvalue between the holes should be less than 1.2-ΔΔCtrepresents the ratio between the target genes of the experimental group and the target genes of the control group, and ΔCt= (Cttarget gene-Ctreference gene)experimental group-(Cttarget gene-Ctreference gene)control group.

Table 1 Primers of each gene by RT-qPCR
2.2.3Western blot experiment. The cells in logarithmic growth phase were taken, and RIPA lysate was used to lyse each group of cells to extract total protein, and the protein concentration was measured by ELIASA. 30 μg of protein samples were taken for SDS-PAGE electrophoresis; 200 mA constant current was used for PVDF membrane transfer; it was closed for 2 h with 5% skimmed milk powder; ATM, CHK2, CDC25A, CDK2 primary antibodies (diluted at 1∶1 000) and β-actin primary antibody (diluted at 1∶100 000) were incubated overnight at 4 ℃ and PVDF membrane was washed by TBST for 3 times (15 min each time); the secondary antibody (diluted at 1∶1 000) was incubated for 2 h at room temperature and PVDF membrane was washed by TBST for 3 times (10 min each time); ECL was used for development and then collect images. The gray value of the image was analyzed by Quantity One, and the ratio of the gray value of the target protein band to the reference band was used as the relative expression of the target protein. The experiment was repeated 3 times.

3 Results and analysis
3.1 Expression level of ATM, CHK2, CDC25A and CDK2 mRNA in HCT116 cell and HCT116/L-OHP cellThe expression level of ATM, CHK2, CDC25A and CDK2 mRNA in drug-resistant cell HCT116/L-OHP was significantly higher than that in parent cell HCT116, and the difference was statistically significant (P<0.05). The results are shown in Fig.1.
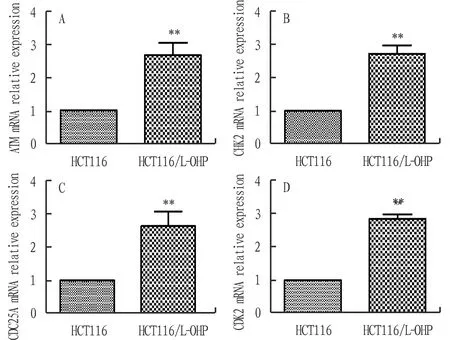
Note: *P<0.05, **P<0.01 compared with HCT116.
3.2 Expression level of ATM, CHK2, CDC25A and CDK2 proteins in two groups of cellsThe expression level of ATM, CHK2, CDC25A and CDK2 proteins in drug-resistant cell HCT116/L-OHP was significantly higher than that in parent cell HCT116, and the difference was statistically significant (P<0.05). The experimental results of protein level were consistent with the gene expression results of various factors, and the results are shown in Fig.2.
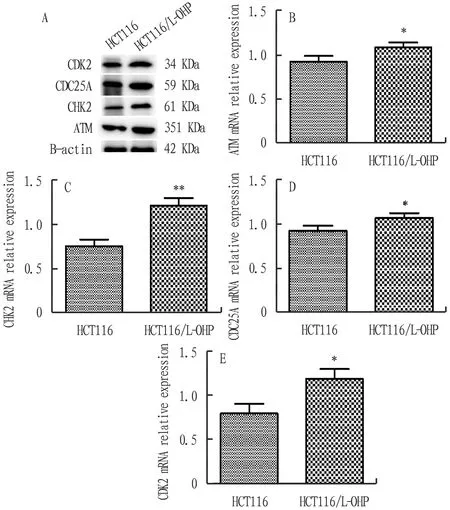
Note: A. Protein expression of associated factors in the two groups. B—E. The statistical result of relative expression of associated factors in the two groups. *P<0.05, **P<0.01 compared with HCT116.
4 Discussion
L-OHP is a first-line chemotherapeutic drug commonly used in patients with advanced colorectal cancer. Drug resistance to L-OHP is the main reason for poor chemotherapy effect and poor prognosis. L-OHP is the third generation of platinum chemotherapeutic drug. After entering the cells, platinum drugs interact with intracellular nucleophilic molecules such as DNA, RNA and proteins through changes in molecular structure[3]. And DNA is the preferred target of platinum drugs. When it binds to DNA, it leads to cross-linking between DNA single strands or double strands, which affects the structure and function of DNA, and then inhibits the replication and transcription of DNA, resulting in cell death[4].
ATM/CHK2/CDC25A signal pathway is related to DNA damage checkpoint. Under normal circumstances, cells will cause DNA damage after being subjected to various adverse stimuli. For example, cells will have DNA structural changes and dysfunction after being treated by L-OHP. Serine-threonine kinases (ATM) is one of the key points involved in cell DNA damage identification and repair[5]. After DNA injury, ATM is activated to be phosphorylated. Phosphorylation of ATM can induce phosphorylation of many substrates, including cell cycle checkpoint kinase (CHK2), further amplify DNA damage signals and inactivate cyclin 25A (CDC25A). CDC25A is a member of the CDC25 protein phosphatase family. The CDC25 protein phosphatase family plays a key role in activating cyclin-dependent kinase (CDKs) by removing phosphate from the conservative Thr14/Tyr15 inhibitory phosphorylation site. The main substrate of CDC25A is CDK2, and CDK2 is allowed to enter the internal checkpoints of G1/S and S when activated. DNA damage causes CDC25A to be phosphorylated by CHK1 and CHK2 at multiple inhibitory sites (Ser123, Ser177, Ser278, Ser292 and Thr506), which leads to binding to inhibitory 14-3-3 protein and ubiquitin-mediated degradation, resulting in cell cycle arrest. At present, studies have confirmed that cyclin 25A (CDC25A) is an oncogene, which is highly expressed in a variety of tissues, including breast cancer, non-small cell lung cancer, colorectal cancer and so on. Overexpression of CDC25A promotes tumorigenesis and is one of the main reasons for unlimited proliferation of tumor cells[6-8].
The purpose of this study was to compare the expression of ATM, CHK2, CDC25A and CDK2, the key factors of ATM/CHK2/CDC25A signal pathway in colorectal cancer cell line HCT116 and L-OHP-resistant cell line HCT116/L-OHP. The results showed that the expression level of ATM, CHK2, CDC25A, CDK2 mRNA and protein in HCT116/L-OHP was higher than that in HCT116. This shows that in HCT116/L-OHP cell line, the ATM/CHK2/CDC25A signal pathway is inhibited, and the corresponding kinase in the pathway can not be phosphorylated, resulting in the increased expression of CDC25A without specific degradation, which makes the sensitivity of HCT116/L-OHP to L-OHP decrease or even disappear, resulting in drug resistance, and finally the cells are in a state of unlimited proliferation. Therefore, the activation of ATM/CHK2/CDC25A signal pathway in L-OHP-resistant cell line HCT116/L-OHP may enhance the sensitivity of drug-resistant cells to L-OHP, which may provide a theoretical basis for reversing the resistance to L-OHP in patients with colorectal cancer. Further experimental studies are needed to find the upstream gene that can effectively activate the expression of key factors in the ATM/CHK2/CDC25A signal pathway of drug-resistant cell lines, and this upstream gene may also become a new therapeutic target for patients with colorectal cancer resistant to L-OHP chemotherapy.
- Medicinal Plant的其它文章
- Difference Analysis of Secondary Metabolites of Herba Polygoni Chinensis from Guangxi
- Effects of Temperature on Seed Germination and Metabolism of Scutellaria baicalensis Georgi
- Quality Standard of Quercetin in the Leaves of Heritiera littoralis
- Microbial Limit Test of Zhideke Granules
- Meta-analysis of Therapeutic Efficacy of Purgation and Catharsis Method for Treating Severe Pneumonia
- Based on the Theory of Five Yun Six Qi to Study the Clinical Effect of Chaigui Decoction on Jueyin Type Hypertension

