Quality Standard of Quercetin in the Leaves of Heritiera littoralis
Yu LIN, Jianning TAN, Ping ZHANG
1. College of Pharmacy, Guangxi University of Chinese Medicine, Nanning 530001, China; 2. Teaching Experiment and Training Center of Guangxi University of Chinese Medicine, Nanning 530001, China
Abstract [Objectives] To establish a quality standard method for the determination of quercetin in leaves of Heritiera littoralis. [Methods] Quercetin was extracted from the leaves of H. littoralis by alcohol extraction, and the quercetin was quantitatively analyzed by HPLC. [Results] There was a good linear relationship between the peak area (A) and the content (M) of quercetin in the range of 0.048-0.168 μg, A=2 615.5M+1.528 6, r=0.999 9. The method was applied to the determination of ethanol extract of H. littoralis leaves, the average recovery rate was 98.77% and RSD was 1.57%. [Conclusions] The method is simple, convenient, reproducible and accurate, and provides an idea for the determination of quercetin in leaves of H. littoralis.
Key words Heritiera littoralis, Quercetin, Content determination, High performance liquid chromatography
1 Introduction
Heritieralittoralisis one of the most important semi-mangroves in the family Sterculiaceae, and has typical adaptability to land and sea habitats. It is mainly distributed in Thailand, India, Vietnam and other Southeast Asia countries, as well as eastern Africa, Oceania and other tropical coasts. It is mainly distributed in Guangxi, Guangdong, Hainan and Taiwan in China[1-2]. It has buttress-like roots, tall and large stems, silvery white abaxial surfaces of leaves, and special fruit shape[3-4]. It has the characteristics of salt tolerance and wind resistance, so it is a good landscape tree species and coastal shelterbelt tree species[5].H.littoralishas a long history of folk medicine in Southeast Asia, and its seeds can treat diseases such as diarrhea, indigestion and dysentery. Its bark can be used to make juice to treat hematuria, diarrhea and dysentery. Its twigs contain tannins, which are good for the gums. Its roots can be decocted to treat oral infections and toothache and so on. The flavonoids extracted from its leaves have anticancer activity.
So far, it has been found that the main chemical constituents of leaves are flavonoids, triterpenoids, steroids and sesquiterpenes[6-11]. However, for a long time, there have been many researches on the extraction methods of total flavonoids, the determination of total flavonoids and the chemical composition of flavonoids in the leaves ofH.littoralis, but few researches focus on the determination of a single component. Li Yuejuan[12]studied the chemical constituents of flavonoids in the leaves ofH.littoralis, and isolated and identified protocatechuic acid, epicatechin, quercetin-3-O-α-L-rhamnoside and other monomers. Tian Yanetal.[13]isolated and identified flavonoids such as quercetin, kaempferol, kaempferitrin, eriodictyol and catechin in the leaves ofH.littoralis.
On the basis of these research results, the method for content determination of a single component in the flavonoid extract of leaves ofH.littoraliswas established in this experiment. The main active components of flavonoids in the leaves ofH.littoralisare quercetin, epicatechin, kaempferol and so on, among which quercetin has many pharmacological effects such as anti-inflammation, anti-oxidation and anti-cancer[14]. In this experiment, the content of quercetin was determined by high performance liquid chromatography[15-16]. This method is simple, convenient, fast, feasible, accurate, with good separation effect and high repeatability. It provides a reference for the subsequent overall quality evaluation and quality control of the leaves ofH.littoralis.
2 Materials and methods
2.1 Experimental medicinal materials and reagentsQuercetin reference substance (Shanghai Ronghe Pharmaceutical Science and Technology Development Co., Ltd., No.161120, purity: 99.73%), the leaves ofH.littoralis, were collected in Guangxi Beilun Estuary Mangrove Reserve on July 10, 2016. It was identified by Xu Mingben of Beibu Gulf Marine Research Center of Guangxi Academy of Sciences as the leaf ofH.littoralisDryand, a semi-mangrove plant in the family Sterculiaceae. Chromatographic methanol [Thermo Fisher Scientific (China) Co., Ltd.)], phosphoric acid (AR, Sinopharm Group Chemical Reagent Co., Ltd.), methanol (AR, Chengdu Kelong Chemicals Co., Ltd.), experimental water (ultra-pure water).
2.2 Experimental instrumentsAgilent high performance liquid chromatograph (American Agilent 1260 quaternary pump DEADO15981, American Agilent 1260 automatic sampler DEAAC29697, American Agilent 1260 column incubator DEACN41839, American Agilent 1260 detector DEABB16815, American Agilent 1260 chromatographic workstation); ultraviolet-visible spectrophotometer (Shimadzu Co., Ltd., UV-1780); electronic analytical balance (Sartorius Scientific Instruments Co., Ltd., specification: PRACTUM224-1CN); pure water filter (Sichuan Youpu Ultra Pure Technology Co., Ltd., model: UPC-II-10T); KQ5200B ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., power: 200W, working frequency: 40KHZ); centrifuge (Shanghai Anting Scientific Instrument Factory, TGL-16G); vacuum pump with circulated water system (Zhengzhou Greatwall Scientific Industrial and Trade Co., Ltd., SHB-III).
2.3 Methods
2.3.1Preparation of reference substance solution. The reference substance of quercetin (1.2 mg) was weighed precisely and placed in a 100 mL volumetric flask, dissolved with methanol to a constant volume, shaken well, and the quercetin reference solution was obtained. According to the purity of the reference substance of quercetin, the concentration of quercetin was 12 μg/mL.
2.3.2Extraction and preparation of sample solution. The collected leaves ofH.littoraliswere washed and dried in the sun, and beaten into coarse powder by a pulverizer for later use. A proper amount of coarse powder was placed in a 1 L beaker, a proper amount of 95% ethanol was added, cold soaked for 7 d, then the residue was filtered out. The extract was concentrated under reduced pressure, and dried to obtain ethanol extract.
About 0.5 g of the extract was precisely weighed and placed in a 50 mL conical flask with a stopper. The pipette was used to precisely take 10 mL of methanol and put it into a conical flask with a stopper, and the total weight was measured. After ultrasonic treatment at 50 ℃ for 1.5 h, cooling and weighing were carried out, and then methanol solvent was used to make up for the lost weight. A proper amount of sample solution was put in a 2 mL centrifuge tube and placed in a centrifuge for 10 min centrifugation at 13 000 r/min. Then the supernatant was absorbed with a disposable 1 mL syringe, the tiny particles were filtered with 0.22 μm microporous membrane, and then placed in an automatic injection bottle and measured.
2.3.3Chromatographic conditions. Chromatographic column: Phenomenex Gemini 5u C18110R (250 mm×4.6 mm, 5 μm); mobile phase: methanol (A)-0.1% phosphoric acid solution (B) (V/V); the volume ratio of methanol in the organic phase ranged from 20 to 80, and the gradient elution was carried out in a certain proportion (Table 1); flow rate: 1 mL/min; detection wavelength: 375 nm; column temperature: 40 ℃; injection volume: 10 μL. Under these conditions, the chromatographic peaks of quercetin and other adjacent components could achieve baseline separation, and the degree of separation was higher than 1.5.
2.3.4Methodological investigation. (i) Linear relationship investigation. An appropriate amount of prepared quercetin reference substance was taken, and the reference substance solution (4, 6, 8, 10, 12, 14 μL) was injected into high performance liquid chromatograph (HPLC), respectively. The samples were de-termined three times in parallel according to Section2.3.3chromatographic conditions. Taking the peak areaAof the quercetin reference substance as the ordinate and the content of the reference substance absorbed (injection volume)M(μg) as the abscissa, the standard curve was drawn (Fig.1). The linear regression equation was obtained:A=2 615.5M+1.528 6, and the correlation coefficientr=0.999 9. The results showed that the linear relationship was good when the injection volume of quercetin reference substance was in the range of 0.048-0.168 g.
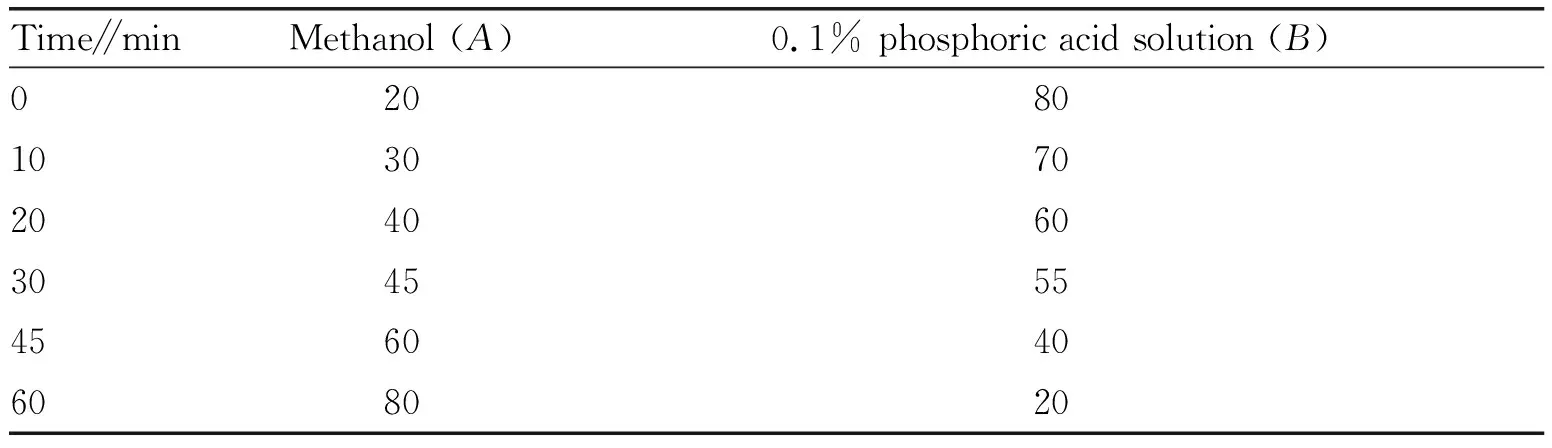
Table 1 Ratio of gradient elution in mobile phase
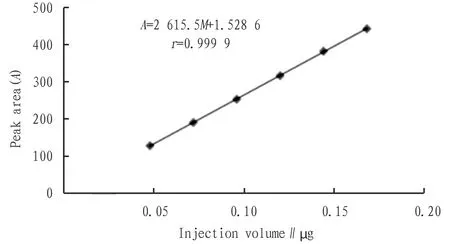
Fig.1 Standard curve
(ii) Precision investigation. 10 μL of prepared quercetin reference substance was precisely absorbed and determined under Section2.3.3chromatographic conditions. Injection was carried out 6 times continuously, and the peak area was recorded. The average peak area of quercetin was 308.8 and theRSDwas 0.33%, which indicated that the precision of quercetin was good.
(iii) Stability investigation. 0.540 5 g of ethanol extract from the leaves ofH.littoraliswas precisely weighed. After preparation according to the experimental method, let it stand for 0.1, 2, 4, 8, 10 and 24 h, respectively. 10 μL of the sample solution was precisely sucked for injection and determined according to Section2.3.3chromatographic conditions, and 3 samples were treated in parallel at each time. The average peak area was 206.6 and theRSDwas 1.90. The results showed that the sample solution prepared by this method had good stability within 24 h.
(iv) Reproducibility test. 6 parts of ethanol extract from leaves ofH.littoraliswere precisely weighed, each of which was about 0.5 g, and 6 samples were prepared in parallel according to the experimental method. The peak area was recorded under Section2.3.3chromatographic conditions, and the peak area was recorded. The average content of quercetin in the sample was 0.145 8 mg/g, and theRSDwas 1.85%. The results showed that the method had good reproducibility.
(v) Sample recovery test. A total of 9 parts of ethanol extract from leaves ofH.littoraliswere precisely weighed. 2.50 mL of quercetin reference solution was precisely added to 1-3 parts, 3.30 mL of quercetin reference solution was precisely added to 4-6 parts, and 4.00 mL of quercetin reference solution was precisely added to 7-9 parts. Then the solution was prepared according to the test method for sample injection and determination (Table 2). Through the calculation, the average recovery rate was 98.77% and theRSDwas 1.57% (n=9), which indicated that the accuracy of the method was good.
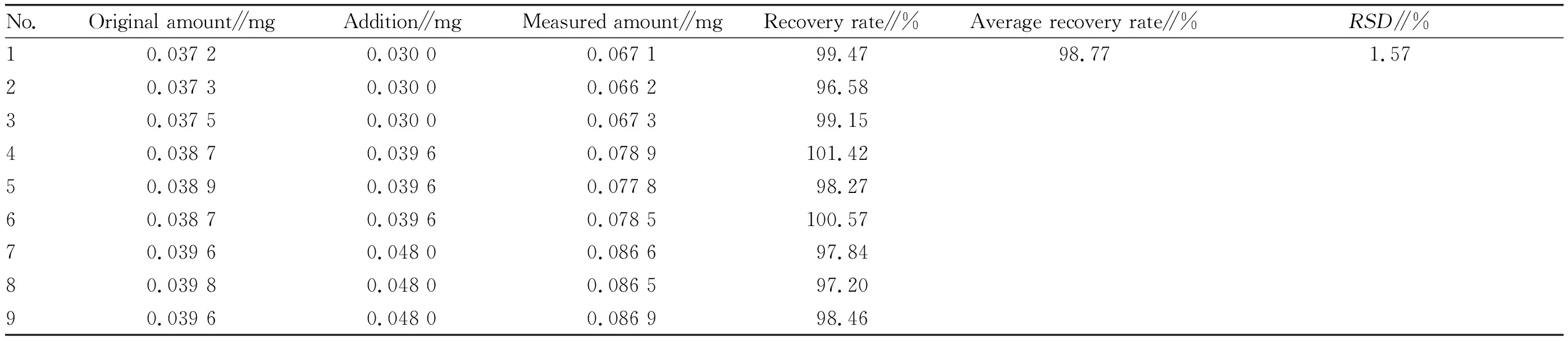
Table 2 Recovery test of ethanol parts from the leaves of Heritiera littoralis
3 Results and analysis
3.1 Selection of solventsAbout 0.5 g of ethanol extract from the leaves ofH.littoraliswas precisely weighed, and put into a 50 mL conical bottle with stopper. 10 mL of methanol, ethanol, 70% methanol, 70% ethanol, 50% methanol, 50% ethanol, were precisely pipetted, respectively, and placed in a corked conical flask, and the total weight was weighed. After ultrasonic treatment for 30 min at room temperature, it was cooled, weighed, and then the corresponding solvent was used to make up for the lost weight. A proper amount of sample solution was taken and placed in a 2 mL centrifuge tube and put in a centrifuge for 10 min centrifugation at 13 000 r/min. Then the supernatant was absorbed with a disposable 1 mL syringe, and the tiny particles were filtered with 0.22 μm microporous membrane, and then placed in an automatic injection bottle and measured. The results showed that the dissolution rate in methanol was the highest, followed by ethanol, 70% methanol and 70% ethanol, and the dissolution efficiency was the lowest in 50% methanol and 50% ethanol, so methanol was chosen as the solvent.
3.2 Investigation of different ultrasonic timeAbout 0.5 g of ethanol extract from the leaves ofH.littoraliswas precisely weighed, and put into a 50 mL conical bottle with stopper. 10 mL of methanol was precisely pipetted and placed in a corked conical flask, and the total weight was weighed. After ultrasonic treatment for 0.5, 1, 1.5, 2 and 2.5 h at room temperature, respectively, it was cooled, weighed, and then the methanol solvent was used to make up for the lost weight. A proper amount of sample solution was taken and placed in a 2 mL centrifuge tube and put in a centrifuge for 10 min centrifugation at 13 000 r/min. Then the supernatant was absorbed with a disposable 1 mL syringe, and the tiny particles were filtered with 0.22 μm microporous membrane, and then placed in an automatic injection bottle and measured. The results showed that the ultrasonic time was too short and the effective components were not completely dissolved, resulting in low dissolution efficiency; if the ultrasonic time was too long, the activity of quercetin might be destroyed and the dissolution efficiency would become lower; when the ultrasonic time was 1.5 h, the dissolution efficiency was the highest, so 1.5 h was chosen as the ultrasonic time.
3.3 Investigation of different ultrasonic temperaturesAbout 0.5 g of ethanol extract from the leaves ofH.littoraliswas precisely weighed, and put into a 50 mL conical bottle with stopper. 10 mL of methanol was precisely pipetted and placed in a corked conical flask, and the total weight was weighed. After ultrasonic treatment at room temperature, 40, 50, 60 and 70 ℃, respectively, it was cooled, weighed, and then the methanol solvent was used to make up for the lost weight. A proper amount of sample solution was taken and placed in a 2 mL centrifuge tube and put in a centrifuge for 10 min centrifugation at 13 000 r/min. Then the supernatant was absorbed with a disposable 1 mL syringe, and the tiny particles were filtered with 0.22 μm microporous membrane, and then placed in an automatic injection bottle and measured. The results showed that the dissolution efficiency was the highest at 50 ℃; if the ultrasonic temperature was too low, the sample dissolved slowly, resulting in low dissolution efficiency; increasing the ultrasonic temperature could help to accelerate the dissolution of the sample, but too high temperature would destroy the activity of quercetin and reduce the dissolution efficiency. Therefore, 50 ℃ was chosen as the ultrasonic temperature.
3.4 Selection of maximum absorption wavelengthAn appropriate amount of quercetin reference solution was taken and it was scanned by UV-vis chromatographic scanner in the absorption spectrum range of 200-500 nm. Quercetin had two absorption peaks at 255 and 375 nm, respectively, and the absorption intensity was similar at these two wavelengths. However, the absorption peak at 255 nm was sharp and easily affected by non-monochromatic light and solvent, while the absorption peak at 375 nm was smooth and the interference was small, so 375 nm was chosen as the detection wavelength.
3.5 Investigation of different column temperaturesAbout 0.5 g of ethanol extract from the leaves ofH.littoraliswas precisely weighed, and put into a 50 mL conical bottle with stopper. 10 mL of methanol was precisely pipetted and placed in a corked conical flask, and the total weight was weighed. After ultrasonic treatment for 1.5 h at 50 ℃, it was cooled, weighed, and then the methanol solvent was used to make up for the lost weight. A proper amount of sample solution was taken and placed in a 2 mL centrifuge tube and put in a centrifuge for 10 min centrifugation at 13 000 r/min. Then the supernatant was absorbed with a disposable 1 mL syringe, and the tiny particles were filtered with 0.22 μm microporous membrane, and then placed in an automatic injection bottle and determined at the column temperature of 25, 30, 35 and 40 ℃, respectively. The results showed that the higher the column temperature, the larger the peak area (the peak area was in the order of 25 ℃<30 ℃<35 ℃<40 ℃), the peak area was the largest at 40 ℃, and the degree of separation was higher than 1.5. The symmetry factor met the conditions, so 40 ℃ was chosen as the column temperature.
3.6 Investigation of different mobile phasesDifferent mobile phase systems (acetonitrile-0.1% formic acid, acetonitrile-0.1% phosphoric acid, methanol-0.1% phosphoric acid) and elution ratios were investigated. According to the chromatogram comparison, when the mobile phase was acetonitrile-0.1% phosphoric acid, the peak of the sample appeared faster, and the chromatographic peaks of different components were easy to pile together; when the mobile phase was acetonitrile-0.1% formic acid, the chromatographic peaks of different components were difficult to separate, there were more peaks coincident together, and there were more miscellaneous peaks; when the mobile phase was methanol-0.1% phosphoric acid, the peaks were separated, the degree of separation was better, and the symmetry factor was also good. Therefore, methanol-0.1% phosphoric acid solution was selected as the mobile phase.
4 Conclusions
The quantitative analysis of quercetin in the ethanol part of leaves ofH.littoralisby high performance liquid chromatography (HPLC) was carried out. From the test results of linearity, stability, repeatability and recovery, it was seen that the HPLC method could effectively control the content of quercetin in the ethanol part of leaves ofH.littoralis. The method is simple and feasible, the result is accurate, the reproducibility is good, and the recovery rate is high. It can be used for the determination of quercetin in the ethanol part of leaves ofH.littoralis.
Through the research in recent years, people have a certain understanding of the extraction technology and chemical composition of flavonoids from the leaves ofH.littoralis, but there are few studies on the determination of single component or multiple components. Therefore, it is necessary to make an in-depth study on the determination of chemical components and pharmacological activity of leaves ofH.littoralis, which can provide reference for the subsequent comprehensive study of the quality and quality control of medicinal materials.
- Medicinal Plant的其它文章
- Difference Analysis of Secondary Metabolites of Herba Polygoni Chinensis from Guangxi
- Effects of Temperature on Seed Germination and Metabolism of Scutellaria baicalensis Georgi
- Effect of ATM/CHK2/CDC25A Signal Pathway on the Resistance of Colorectal Cancer Cells to L-OHP
- Microbial Limit Test of Zhideke Granules
- Meta-analysis of Therapeutic Efficacy of Purgation and Catharsis Method for Treating Severe Pneumonia
- Based on the Theory of Five Yun Six Qi to Study the Clinical Effect of Chaigui Decoction on Jueyin Type Hypertension

