Preliminary study of an open-air watercontacting discharge for direct nitrogen fixation
Zhan SHU (舒展), Chuanqi WANG (汪傳奇), Insaf HOSSAIN,Qiang CHEN (陳強), Wanlian LI (李婉蓮), Jinqi WANG (王晉琪),Pengfei LIU (劉鵬飛) and Qing XIONG (熊青)
1 State Key Laboratory of Power Transmission Equipment and System Security and New Technology,Chongqing University, Chongqing 400044, People’s Republic of China
2 Shenzhen Research Institute of Xiamen University, Institute of Electromagnetics and Acoustics, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance,Key Laboratory of Electromagnetic Wave Science and Detection Technology, Xiamen University, Xiamen 361005, People’s Republic of China
Abstract Efficient nitrogen fixation through a reactive plasma process attracts intense interest due to the environmental issues induced by the conventional Haber-Bosch method.In this work, we present a direct and simple fixation routine without any catalysts for nitrogen in open air using an atmospheric-pressure pin-to-solution plasma electrolytic system.Nitrate,nitrite,and ammonia as the nitrogen-derived chemicals in solution were analyzed as indicators under various discharge conditions to estimate the energy efficiency of this process.The results show that the nitrogen fixation process was much more efficient by the pin-positive discharge compared to the negative one.N chemicals preferred to be formed when the solution was of negative polarity.It was also found that,with the help of solution circulation,the energy efficiency was enhanced compared to that of static liquid.However, an inverse trend was observed with the increase of the discharge current.Further study by optical emission spectroscopy indicates the important roles of activeN2*and water vapour and their derived species near the plasma-water interface in the fixation process.
Keywords:nitrogen fixation,air-water discharge,energy efficiency,active species,plasma-water interface
1.Introduction
Synthetic fertilizers produced via artificial fixation of nitrogen, namely the Haber-Bosch (HB) process, have greatly supported agricultural production since the 20th century [1].However, due to its intrinsic reaction scheme requiring high pressure(150-300 atm)and high temperature(400°C-500°C),the HB process is energy consuming with a considerable environmental impact because of huge greenhouse gas emission.Normally, HB plants are large and centralized to be economical[2].However,this makes it difficult for them to utilize distributed small-scale renewable energy sources.It is reported that about 1%-2% of the total amount of energy worldwide is consumed by HB manufacturing [3, 4], and the growth in demand for nitrogen fertilizers in the future constitutes a significant potential challenge to world energy supplies.
On-demand nitrogen fixation (NF) through distributed(renewable) sources has attracted intense interest in recent years [4, 5].This distributed plant can be of relatively small scale and low energy consumption, thus complementing the HB technology.Electrically powered non-thermal plasma is an emerging technology as a distributed fixer of nitrogen[6, 7].Employing plasma reactors operating at atmospheric pressure and low temperature, and with raw air materials directly providing the final products(nitric oxide/nitric acid),will change the structure of the conventional NF process considerably [8].Various non-thermal plasma sources with different configurations,including dielectric barrier discharge(DBD),spark,glow,and arc discharges,have been developed for NOxproduction with air as raw materials[8].To facilitate the non-thermal plasma NF reaction and enhance its efficiency, various efforts have also been made with the catalyst development for this technology,such as Al2O3and MgO[9].Compared to the hot thermal plasma-based process, which requires high temperature, low energy efficiency of N2activation, and rapid thermal quenching to suppress NO decomposition, non-thermal plasmas generated at atmospheric pressure offer unique perspectives capable of stimulating chemical reactions of N2and other molecules, such as O2or H2, to make NOxor NH3, under limited energy costs and low gas temperatures due to their non-equilibrium properties (electron temperature may be several orders of magnitude higher than the temperature of neutral molecules)[2, 4, 10].Although from a theoretical point the energy efficiency by non-thermal plasma was estimated to be able to reach a comparable level to HB technology[11],the reported energy costs by various non-thermal plasma sources are no lower than 100 GJ/tN (gigajoules expended per metric ton nitrogen produced) [12].Particularly, NF by ‘cold’ room temperature, like plasmas such as DBDs, tends to dissipate more electric energy compared to ‘warm’ discharges like glow or arc-type plasmas [13].Pei et al [12] investigated the energy costs of NOxvia different types of air discharge sources and found the importance of the thermal field T and electric field strength E of discharges in controlling the energy efficiency.A dimensionless parameter defined by ·E Twas proposed as a guiding principle in the design of plasma sources for energy-efficient NF.Modelling work by Wang et al indicated that vibrationally excited N2molecules contribute significantly in N2activation, which effectively enables chemical reactions producing NOx[5].This explains why ‘warm’ discharges are able to deliver energy to the NF process with higher efficiency compared to ‘cold’ plasmas.
In the above NF process via gaseous discharges, the created NOxstill needs to be converted to a stable form, like aqueous NO?3.The latter may be used to fix unstable NH3to NH4NO3as well, an in-volatile fertilizer for crops [7].Novel attempts were made to directly produce nitrogen-based fertilizer in an aqueous phase by water-contacting or under-water plasma discharge processes with N2or air as the working gas[14-18].The utilization of a water solution provides another advantage of H2circumvention in the NF system, which is more economical from the perspective of industrial application.Hawtof et al reported a hybrid electrolytic system consisting of a negative-polarity metal pin and sulfuric acid solution, which enabled highly selective synthesis of NH3from nitrogen gas and water at atmospheric pressure and temperature [15].High faradaic efficiency up to 100% was achievable without any help from a catalyst material.They demonstrated that this high selectivity was dominantly attributed to the solvated electron chemistry at the plasma-water interface.Peng et al investigated a bubble-like under-water discharge for the NF process by applying a concentrated high-intensity electric field [16].A fixation rate of 11.2 μmol min?1was achieved by this system with pure N2as the working gas.Kubota et al investigated the effect of the ratio of ethanol in pure water on the ammonia production by an atmospheric N2plasma jet directly treating a water solution[17].These studies hint that an increase in the H content in solution, such as by adding ethanol or the use of an acid solution like H2SO4, will enhance the synthesis of NH3[15].An increase in the number of protons also creates a solution with specific conductivity to stabilize the discharge.Alternatives like phosphate or tap water lead to a drop in the production of N chemicals due to competing processes forming other chemicals, for example, chlorides if using tap water.
Compared to under-water discharge, direct generation of plasma at the water surface is easier to implement, avoiding the effect of solution conductivity[19].The former is difficult in conductive solution and a high-intensity electric field,which means more energy consumption is required normally[20].Meanwhile,plasma at the water surface monopolizes the superiority of free ambient air as the raw material for the NF process.
In this work, a preliminary study was performed on a typical warm air/water discharge electrolytic system as a potential direct fixation means for nitrogen.The effects of operation parameters,including solution polarity,status of air gas or solution(static or flowing),and discharge current,were investigated in the NF process by this air/water discharge system.Energy efficiency was estimated and analyzed to clarify the critical roles of the interface process at the solution surface in the NF process based on the production of total fixed aqueous nitrogen species.It is shown that the solution dynamics exhibit an enhanced improvement in nitrogen synthesis and energy efficiency.
2.Experimental setup and analysis approaches
2.1.The pin-to-water discharge
The configuration of the pin-to-water device is shown in figure 1.A tungsten needle(diameter 0.8 mm)is inserted in a quartz tube (length 72 mm, inner and outer diameters are 3.8 and 6.4 mm, respectively).A DC power supply (HAPS06-6000, DPAI Ltd, China), with both positive and negative polarities, was used for comparison of NF energy efficiency.A 50 kΩ resistor is connected between the tungsten electrode and the DC power supply to limit the flowing current in the circuit to avoid the arc discharge transition.A 100 ml volume Petri dish full of diluted H2SO4solution was placed below the pin-electrode, wherein a grounded graphite rod was immersed.The purpose of the H2SO4solution is to supply the proton source for NH3formation at the plasma-water interface [17].The pH value of the solution was kept at 3.0(concentration of 0.5 mM l?1), and the gap distance between the needle tip and solution surface was set at 1 mm for all discharge processes here.The DC power source was set to constant-current mode, with discharge currents ranging from 10 to 40 mA.
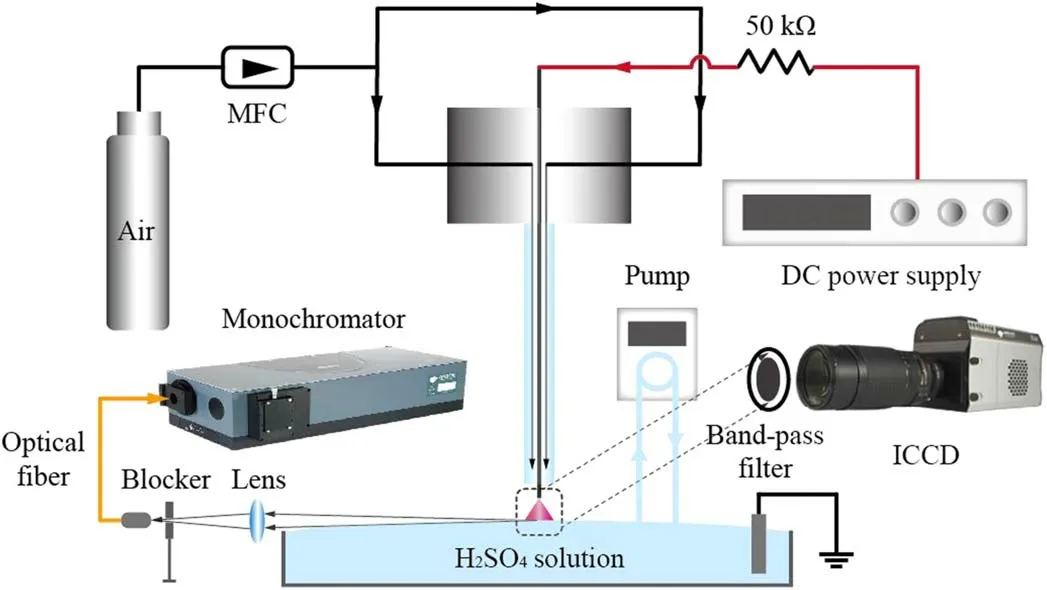
Figure 1.A schematic diagram of the atmospheric-pressure pin-to-water air plasma electrolytic system for in situ NF.
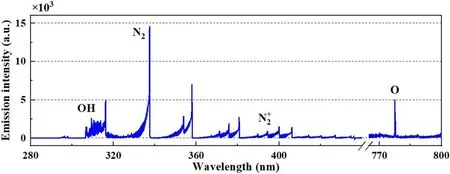
Figure 2.An emission spectrum from the discharge area close to the cathode-solution surface, under a discharge current of 20 mA.
Static ambient air, or flowing air controlled by a mass flow controller(MKS Instruments Ltd,USA),was used as the discharge gas.Therefore, the dynamic effect of flowing gas on this water-contacting discharge and the accompanying nitrogen fixation process was studied.Meanwhile, the dynamic status of the solution was controlled by a peristaltic pump through a circulation unit connecting the Petri dish.Pumping rates ranging from 0 to 170 ml min?1(maximum level here by the pump) were applied and investigated for their impact on the NF process by this plasma electrolytic system.
2.2.Electrical and optical diagnostics
The discharge voltage was monitored by a Tektronix P6015A probe at the pin-electrode end, and the discharge current was kept stable by setting the DC power to the constant-current mode.The optical emissions from the air plasma,particularly the part in the vicinity of the solution surface, were detected.As illustrated in figure 1, a block was set behind a two-inch biconvex lens(f = 20 cm)to allow only the radiation close to the gas-solution interface to be collected by a collimating lens.The captured radiation was then transferred by an optical fibre to a monochromator(Andor Shamrock 750,UK)with a CCD camera (Andor DU416A) as the detector.A typical emission spectrum collected from the interface area close to the cathode-solution surface, which is presented in figure 2,obviously shows the peak wavelengths of excited OH radicals, N2molecules, and O atoms.The strong OH(A-X)emission band indicates abundant production of excited OH radicals, which indirectly implies plentiful H2O molecules produced from evaporation of the solution surface due to the heating effect of the discharge.Besides,the emission bands of excited N2and atomic O indicate the occurrence of N2vibrational excitation and O2dissociation therein, which are important for the formation of NOx.
The emission band of OH(A-X, 0-0) was detected with high spectral resolution by a 3600 l mm?1holographic reflection grating, to estimate the discharge gas temperature Tgthrough the OH (A ,v′ =0) rotational temperature Tr[21, 22].A Boltzmann plotting approach was applied to rotational transitions P1, Q1, and Q2of OH(A-X, 0-0) with rotational numbers J<7, assuming an equilibrium between Trof OH (A,v′=0,J≤7) and the translational (gas)temperature.This assumption is commonly valid and helpful for avoiding Tgoverestimation due to overpopulation of higher rotational levels, which typically occurs in atomic gas discharges mixed with a high content of water vapour due to the fast quenching process of OH(A) [23].
Meanwhile, two-dimensional distributions of typical radiant species in the discharge region were captured by an ICCD camera with the help of various band-pass filters.Here,emissions from the excited species OH(A), NO(A), N2(C),N+2(B), and O(3p5p) in the air plasma were imaged with filters with peak transmission at 308 nm, 248 nm, 337 nm,391 nm, and 777 nm, respectively.All these filters have the same 10 nm full-width at half-maximum of their transmission ranges.The variations in emission intensities of these excited species are compared to the trends of NF efficiency by this air/water plasma electrolytic system.
2.3.Long-lifetime species detection
Standard chemical analysis was applied to determine the concentrations of fixed aqueous nitrogen species.In this work, the production of three important aqueous nitrogen products, namely nitrate), nitrite (), and ammonium(),were quantified and,based on their amounts,the NF energy efficiency was evaluated by considering the dissipated electrical energy.Ion chromatography(Thermo Fisher ICS-600)was used for the detection of nitrate and nitrite,and their amounts were determined through calibration of NaNO3and NaNO2solutions of known concentrations.Theconcentration was measured using the Nessler’s reagent method.The chemical reaction below, betweenand[H gI4]2?,forms a yellow or brown colour sediment, with maximum absorption at 420 nm
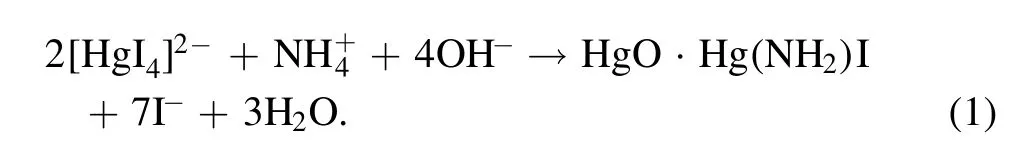
A photometric method was then applied, and the absorption intensity was calibrated by a NH4NO3solution of known concentration.The energy efficiency of aqueous N-product yield in the studied plasma electrolytic system is then evaluated by
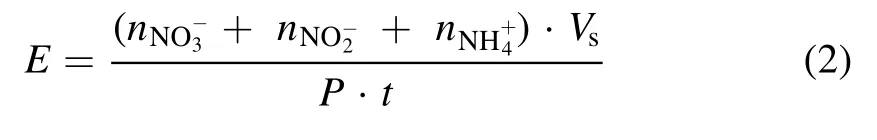
where ·P tis the dissipated electrical energy of the discharge,andVsis the H2SO4solution volume.For all synthesis results here, a constantVsof 100 ml was applied.
Meanwhile, the H2O2production was determined by a similar photometric method based on the reaction of H2O2with metavanadate ammonium as well.In acid medium, this reaction forms a red-orange colour peroxovanadium cation with maximum absorption at 450 nm.This absorption intensity (at 450 nm) is proportional to the reacted H2O2concentration in the plasma-irradiated solution.
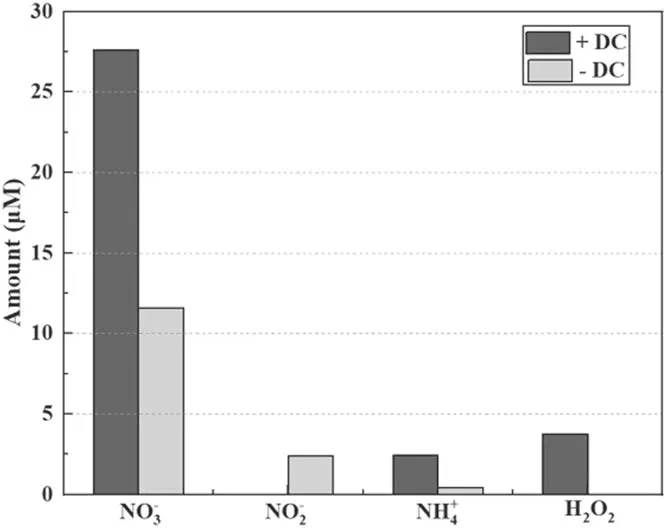
Figure 3.Production of aqueous chemicals by the DC open-air (no flow) plasma electrolytic system, under positive and negative polarities of the tungsten needle: discharge current 10 mA, no solution circulation, and operation time 5 min.
Before the above analysis procedures, the treated H2SO4solution after discharge operation was stirred for 10 min and then left standing for 30 min.This long-time standing was adopted to avoid the necessary detection of another typical aqueous nitrogen species, namely peroxynitrous acid(ONOOH), which is typically formed in N2/air plasma electrolytic systems through the reaction ofand H2O2in the solution with a pH value around 3 [24]

The peroxynitrous acid is not stable and dissociates fast under acidic conditions, dominantly collapsing to a final product of[24].The absence of ONOOH in the solution after storage was proved through fluorescence analysis by 2.7-dichlorodihydrofluorescein diacetate (DCFH-DA), an organic reagent able to react with ONOOH and generate fluorescence at 523 nm [25].Therefore, three samples were taken for detection of the above four aqueous species individually,and the average concentrations were used.Since the three samples were taken from the same well-mixed solution,the deviations of the measured concentrations of interested chemicals are small and less than 10%.Meanwhile, the variations in pH, conductivity, and temperature of the bulk solution were monitored immediately after the discharge operation.
3.Synthesis results and discussion
3.1.Fixation efficiency by ±DC discharges
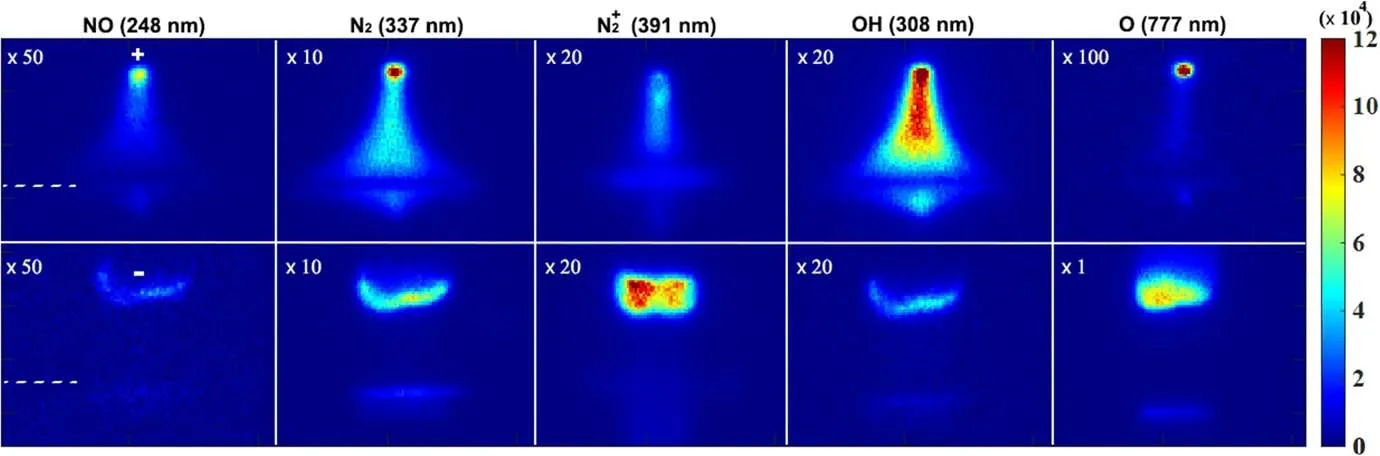
Figure 4.Two-dimensional spatial distributions of emission intensity of excited species above the solution surface.A different constant value was multiplied for a comparable image contrast.The discharge conditions are the same as above.
The synthesis of concerned nitrogen products by the studied open-air plasma electrolytic system with different polarities of solution is presented in figure 3.It is apparent that under positive polarity of the tungsten needle, the yield of N chemicals is much higher than that of the negative case.Except for nitrite,the other formed aqueous species,including H2O2,exhibit a faster synthesis rate when the solution works as the cathode.This enhanced fixation of nitrogen is supposed to be attributed to the important cathode sheath effect close to the solution surface, where an intense electrical field existed[26, 27].Correspondingly, strong ionization discharge is expected therein with abundant accompanying physical and chemical processes.This qualitative supposition is well proved by the detected emission intensity from the discharge,as shown in figure 4.
Similar to the trend of the NF yield presented above,almost all these excited species involved in synthesis reactions of nitrogen are of higher emission intensities under the cathode-solution conditions.Particularly,the excited nitrogen species, including N2(C) and(B), indicate plentiful electron stimulated processes of excitation, ionization of N2molecules in the region of the gas-solution interface.The formation of vibrationally excited N2molecules through electron collision is significantly important in the nitrogen fixation process, by efficiently breaking the N-N chemical bond and enabling synthesis of NO according to the Zeldovich mechanism [12]
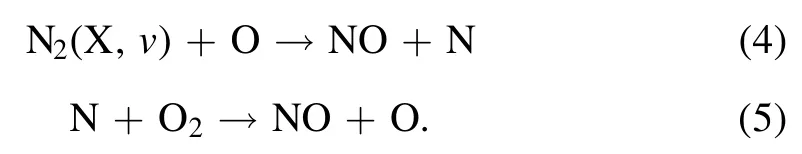
The above reactions will result in enhanced generation of NO species,consistent with the observed stronger radiation of NO(A)in the cathode-solution case.Meanwhile,the brighter luminous zone of OH(A), similar to the emission spectrum presented in figure 2, implies abundant dissociation-related water vapour processes occur above the cathode-solution surface.In other words, higher contents of water vapour are introduced to the gaseous discharge column.This could be the reason for a weak 777 nm radiation of excited O atoms above the surface of the cathode solution, compared to the anode case, since H2O is an effective quencher to excited-state O.Or, the produced O atoms might be depleted significantly by chemical reactions with plenty of species, like vibrationally excited N2(X, v) and water vapour.In water-contacting discharges, much higher H2O pressure typically accompanies,while the water solution acts as a cathode electrode,dominantly due to the effects of plasma heating and ion bombardment (non-existent in the solution-anode case) [28].A larger gas temperature about 1800 ± 200 K was estimated in the cathode-interface region using Boltzmann plotting of the OH(A-X, 0-0) emission band, compared to the 1000 ± 200 K in the anode-solution case.In some sense,this high H2O content is conducive to the capture process of nitrogen; on one hand as a precursor of OH, HO2, and H through dissociation and, on the other hand, allowing pathways forming solvable nitrogen species like HNO2and HNO3in the gaseous phase (R8) [29, 30].These H2O derived species are significantly important for trapping insoluble gaseous species such as NO and NO2with quite small Henry’s Law constants, through the chemical reactions R6-R7 [29, 30]
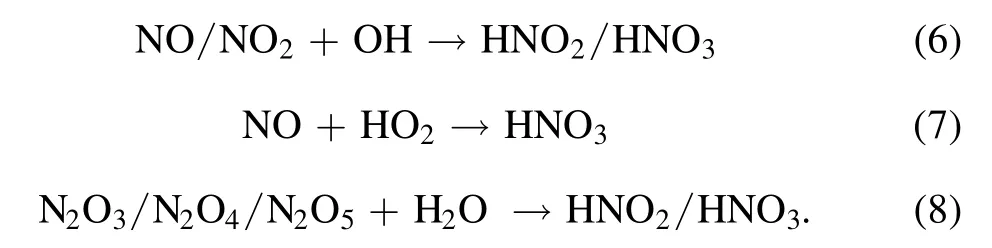
These efficient chemical processes enhance the production of terminal nitrogen-containing species,particularlyin the cathode-solution case.However, the production ofwas quite low and ignorable compared to the amount ofunder the same conditions.It was supposed to be as a result of reaction (3) with H2O2to form ONOOH, as mentioned above.The ONOOH later dissociated fast and converted to nitrate during the 30 min standing of the solution sample.As shown in figure 3,distinct generation of H2O2was observed for liquid used as the cathode, consuming theamount so that it could be ignorable in the following results.In contrast, a detectable amount ofwas stored in the solution due to the absence of H2O2in the anode-solution case, consistent with what was reported by others in similar plasma electrolytic systems [24].Under both polarity conditions, low production ofwas detected, primarily resulting from the scavenging effects ofon solvated electrons through the following reactions (9) and(10),which are vitally important in the formation pathways of NH3through the following reaction (11) according to the work of Hawtof [15]
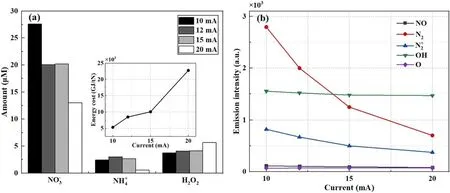
Figure 5.(a)The effect of discharge current on the production of aqueous chemicals by the DC open-air plasma electrolytic system,with the solution as the cathode electrode.(b)Corresponding emission intensities of various concerned gaseous species in the discharge region close to the solution interface: no solution circulation and no airflow, operation time 5 min.
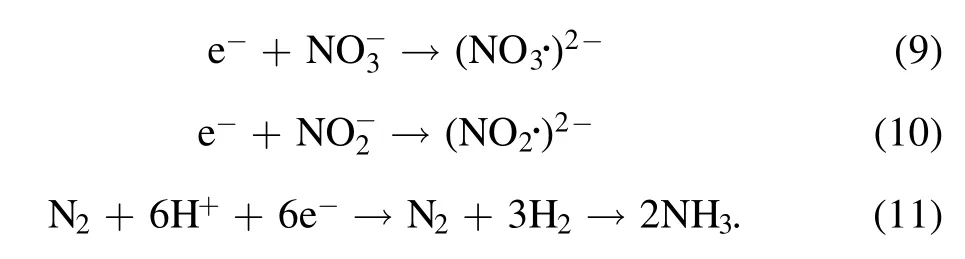
3.2.The effects of discharge current
It was unexpected to observe that increasing the plasma current led to a decrease in the yields of nitrogen chemicals,as shown in figure 5(a).High discharge currents lead to a large number of gas-phase electrons,which could result in the increase in excited N2or solvated electrons through injection from the plasma phase to the solution surface.Based on the above proposed mechanisms, the increase in N-chemical production should be obtained under large discharge currents.However, except for H2O2, here we find that the N-chemical yields exhibit an opposite dependence on the current.This drop in N-chemical yields is supposed to be as a result of the intense water evaporation in the vicinity of the gas/liquid interface due to the increase in currents.In our recent work,the water vapour amount above the solution surface in an argon-flow pin-to-water discharge was measured using an infrared tuneable diode-laser absorption spectroscopy method.The vapour ratio above the gas/liquid interface was higher than 50%under 10 mA discharge current.The vapour pressure in this air plasma electrolytic system is expected to be higher than this level because of heat accumulation without the cooling effects of gas flow or solution circulation.In the argon discharge under 2 slm flow rate,the gas temperature of the plasma region near the interface is about 700 K, much lower than that of the studied air plasma.The dense water vapour will squeeze away air molecules and decrease the ratio of the latter in the plasma column.Particularly, in the gas/liquid interface region, the water vapour concentration is expected to be much higher.A low air content therein might be responsible for the decrease in aqueous N-chemical production.The fast drops in emission intensities of nitrogen or oxygen species in the gas/liquid interface area, as presented in figure 5(b), support the above deductions well.
A slight increase in the amount of aqueous H2O2was obtained with the increase in discharge current, probably resulting from the enhanced effects of ion bombardment or/and dissolution of gaseous H2O2.The latter is strongly dependent on the water vapour pressure above the liquid surface.This dense water vapour might cause a reduction in OH(A-X)emission with the increase in the plasma current,as shown in figure 5(b),since H2O is a highly efficient quencher to the excited OH(A)species.Under high current,an increase in water vapour will squeeze away air molecules in the discharge area and may not contribute much to the O-atom amount as the latter is mainly derived from O2in the air.
On the other side, the energy dissipation by this air/liquid plasma increases fast with the increase in discharge current.As presented in figure 5(a),a low current seems to be favoured for high energy efficiency of the NF process.Therefore, the discharge current was set at 10 mA for the other conditions presented below.
3.3.The effect of solution flow rate
A sharp rise in the amount ofand H2O2was observed in figure 6(a), even with a quite slow flow rate of circulating H2SO4solution, leading to a sudden increase in energy efficiency correspondingly.This enhancement as a result of the solution flow is supposed to be attributed to its induced effects on the whole discharge electrolytic system in both gas and liquid phases.Intense accumulation of species in this thin discharge-solution interface layer with extreme sharp gradients in space were commonly expected in the interactions between plasmas and liquids [20], particularly for short-lived species, such as solvated electronsand OHaq,which were deduced to have ultra-small penetration depths ranging from nm to um [31, 32].For static solutions, abundant residues turned from short-lived species still exist at the solution surface due to their ‘limited’ run-away abilities but ceaseless generations therein [33, 34].At the interface region, part of these residues will take part in the interacting process between the discharge and solution again and convert into other species,which may leave to the open space through heat-induced diffusion,for example,inverse processes of the above R6-R8 reactions.However, with the help of the flowing solution,these backward processes are expected to be reduced and more residual long-lived species are able to be transferred and stored in the solution bulk due to the convection (‘washing away’) effect.
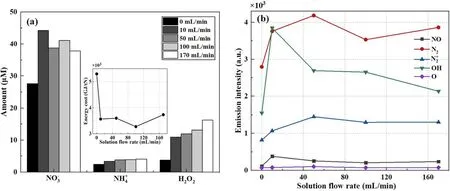
Figure 6.(a)The effect of solution movement on the production of aqueous chemicals by the DC open-air plasma electrolytic system,with the solution as the cathode electrode.(b) Corresponding emission intensities of various concerned gaseous species in the discharge region close to the solution interface: discharge current 10 mA, without airflow, and operation time 5 min.
Under different circulating rates, it is interesting that neither the amounts of nitrate nor the radiation intensities of excited nitrogen species show any apparent variations in the interface region.The gradual growth of the amount of H2O2with the increasing flow rate of the circulating solution, as shown in figure 6(a),proves the above deductions.Hydrogen peroxide is thermodynamically unstable and decomposes to form water and oxygen with aΔHΘof ?2884.5 kJ kg?1[35]
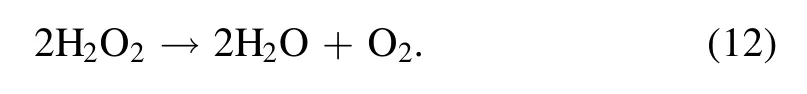
With the increase in the solution flow rate, more H2O2species are saved from the above decomposition process and are reserved in the solution.By comparison, this ‘washing away’ effect induced by the flow of solution is apparently weak for the production of,where a quite slow rising trend was observed.This probably could be attributed to the weak refreshing impact of solution flow on solvated electrons,an essential precursor in the formation pathways of[15].Even with a high flow rate of solution, the solvated electrons still only accumulate in a very local dischargeinteracting area at the interface, due to their extremely short lifetime [32].
On the other hand, a cooling effect was induced in the interface region if the solution was circulating.The temperature of the bulk solution after 10 min discharge dropped from 33 °C to 29.9 °C when the circulating rate of the solution changed from 0 to 10 ml min?1, and gradually declined to 26.5°C at a maximum flow rate of 170 ml min?1of the used peristaltic pump.Also, a slight reduction in gas temperature of about 120 K in the local discharge area near the interface was observed while the solution was circulated, even with a slow flow rate of 10 ml min?1, through the same Trof the OH (A,v′=0,J≤7) approach.Correspondingly, the temperature of the electrolyte interface is expected to drop as well due to the refreshing effect of the flowing aqueous surface.As a result, water evaporation is supposed to be weakened compared to that of the static solution.This coincides well with the observed decreasing trend of OH(A) emission intensity,as shown in figure 6(b).Correspondingly,a decline in gaseous H2O2formation is supposed in the circulating solution due to a reduction in water vapour induced by the above cooling effects.However,this reduction of H2O2might be compensated excessively by the above positive ‘washing away’ impact, namely decreasing the H2O2decomposition process,and still result in an enhanced reservation of H2O2in the solution bulk with the increase in the solution flow rate.
The applied DC voltage almost remained constant,while the flow rate of the H2SO4solution varied and a comparable energy efficiency of NF in the range of 3250 to 3750 GJ/tN was achieved, much lower than 5000 GJ/tN under the static solution conditions.Based on the above deductions, two positive effects are supposed to be induced by the solution circulation, namely more long-lived residues, including N chemicals and H2O2saved in the solution, and more air molecules remaining in the discharge area to generate more NOxbecause of the drop in water evaporation and water accumulation near the interface.Therefore, more aqueous N chemicals are produced and saved in the solution phase,finally resulting in a sharp increase in NF energy efficiency.
3.4.The effect of airflow rate
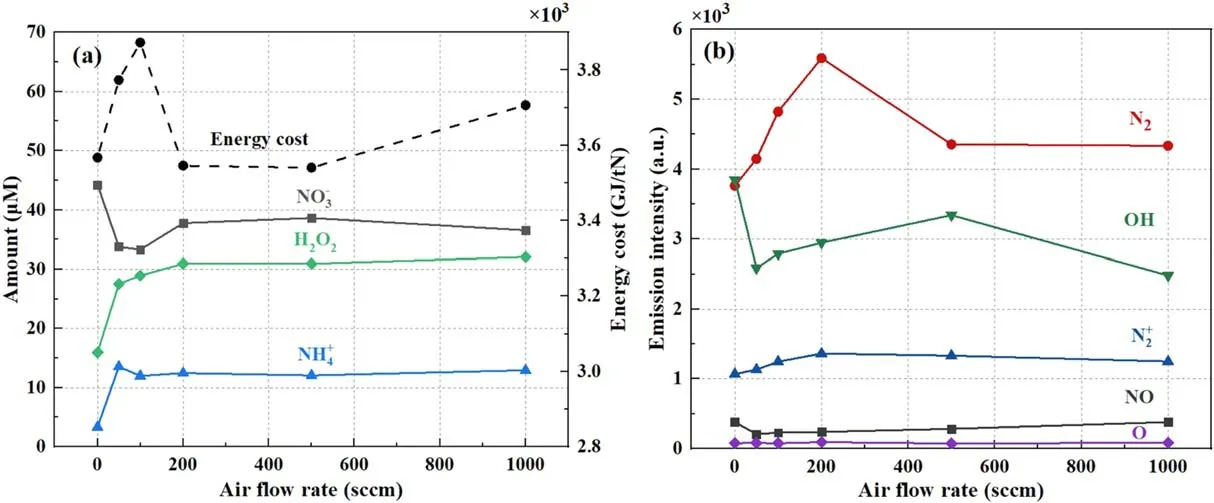
Figure 7.(a) The effect of airflow rate on the production of aqueous chemicals by the DC open-air plasma electrolytic system, with the solution as the cathode electrode.(b)Corresponding emission intensities of various concerned gaseous species in the discharge region close to the solution interface with a discharge current of 10 mA, operation time of 5 min, and solution flow rate of 10 ml min?1.
It is interesting to show that the production of the above aqueous chemicals exhibited distinct responses if a flowing air fluent was applied as the discharge gas.As presented in figure 7(a),the yield of nitrate dropped immediately,and even a low airflow (50 sccm) was blown to the liquid surface.It increased slightly if the airflow rate was raised to 200 sccm and remained almost constant.However, the other two species, namelyand H2O2, displayed a reverse trend compared to nitrate.Their production was enhanced by the flowing air channel.The observed different responses of the three chemicals are supposed to be as a result of the reduction of water vapour above the liquid surface, if a gas flow was applied.This ‘blow-away’ effect of water vapour by a gas flow has been confirmed through a laser diagnostic technique in a similar pin-to-water discharge in our recent work.The decrease in OH(A-X) emission intensity, as shown in figure 7(b), also implies a drop in water content due to the blow-away effect.Correspondingly, reaction processes involving H2O or its derivates will decrease,apparently due to the reduction in water content above the gas/water interface,e.g.the above trapping processes R6-R8 of nitrogen.This finally results in a decline in the aqueous nitrate generation.
The enhanced production of aqueous H2O2indicates that its dominant formation process occurs in the liquid phase but not the gas discharge.That is to say, the dissolution of gaseous H2O2produced above the liquid surface contributes unimportantly to the yield of H2O2in the solution, since the former is strongly dependent on the H2O amount in the gaseous plasma.Similar deductions have been proved and reported by others in plasma/liquid electrolytic systems[36, 37], where the combination of OH radicals produced in the water surface layer through the important ion bombardment effect was deduced to be responsible for the aqueous H2O2formation[36].With the help of gas flow,which blows off the accumulated dense water vapour above the solution surface,more ions of air molecules are able to fly to strike the interface and enhance the bombardment process.This directly raises the production of aqueous OH, which immediately combines to form H2O2in the interface region.The rise in emission intensities of the nitrogen species N2(C)and(B),as presented in figure 7(b), also implies that more energetic species, including ions and excited species, are flying to the solution below.
Although the energy efficiency of NF by the studied air/water plasma electrolytic system was enhanced by varying the operational conditions,such as cathode polarity or circulation of the solution, it is still much lower compared to the HB process, even one order of magnitude lower than that by the warm dry air glow discharge[12].This low energy efficiency is supposed to be induced by the effect of abundant water vapour above the interface, which plays a barrier role impeding the process between air molecules and plasma electrons.At the gas/water interface region, energy is consumed significantly by processes like dissociation, ro-vibrational excitation of H2O evaporated from the water surface.Nevertheless,this type of air/water plasma system can still be an alternative option for directly synthesized nitrogen fertilizer for crops in agriculture, due to its simple configuration,easy scale-up, and bactericidal ability.
4.Conclusions
Fixation of nitrogen in air gas by distributed and renewable energy-driven plasma sources could be an important routine that complements the traditional HB technique.Particularly,direct syntheses of aqueous N chemicals like ammonia,nitrate, and nitrite, easily absorbed by plants, are favourable.For this purpose, a catalyst-free air gas/liquid plasma electrolytic system was investigated and assessed based on NF energy efficiency.The impacts of controllable parameters,including discharge current and flow statuses of air gas and solution, were studied.Applying a circulating solution could enhance the yield of nitrogen products and, correspondingly,achieve a higher energy efficiency.Increasing the discharge current or blowing an airflow do not lead to positive effects on the NF process,or even result in an adverse impact.From the point of application,although the energy efficiency is still one order of magnitude lower than that of dry nitrogen synthesis (around 300 GJ/tN) by warm gaseous discharge,direct formation of aqueous nitrogen fertilizer by the studied simple and low-cost air/liquid plasma electrolytic system is an alternative approach in agriculture, not to mention the bactericidal effect of plasma-activated solutions.Also, this pin-to-water configuration is easily designed in the form of an array to scale up in application.
Acknowledgments
This work was partly supported by National Natural Science Foundation of China (No.11975061), the Technology Innovation and Application Development Project of Chongqing (No.cstc2019jscx-msxmX0041),the Construction Committee Project of Chongqing(No.2018-1-3-6),and the Fundamental Research Funds for the Central Universities (No.2019CDQYDQ034).
 Plasma Science and Technology2021年3期
Plasma Science and Technology2021年3期
- Plasma Science and Technology的其它文章
- Numerical simulation of laser-induced plasma in background gas considering multiple interaction processes
- Kinetic-theory-based investigation of electronegative plasma-wall transition with two populations of electrons
- Investigation of the fast magnetosonic wave excited by the Alfvén wave phase mixing by using the Hall-MHD model in inhomogeneous plasma
- The theoretical study on intermittency and propagation of geodesic acoustic mode in L-mode discharge near tokamak edge
- Experimental study of sheath potential coefficient in the J-TEXT tokamak
- Nonlinear evolution and secondary island formation of the double tearing mode in a hybrid simulation
