Molecular Dynamics Simulation on Mobility and Aggregation of Macromolecular Lubricant Oxidation Products and Their Influences on Base Stock
Xia Lei;·Li Yan;·Zhang Hongmei ; Jiang Zhengyi ,4; Long Jun
(1. School of Material and Metallurgy, University of Science and Technology Liaoning, Anshan 114051;2. State Key Laboratory of Metal Material for Marine Equipment and Application, Anshan 114009;3. Iron & Steel Research Institutes of Ansteel Group Corporation, Anshan 114009;4. School of Mechanical, Materials, Mechatronic and Biomedical Engineering,University of Wollongong, Wollongong, NSW 2522, Australia;5. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The mobility and aggregation behavior of macromolecular lubricant oxidation products and their influences on the performance of base stock were probed by molecular dynamics (MD) simulation. The mean square displacement (MSD) of molecules was calculated to explore the mobility of molecules. The distribution appearance of lubricant oxidation products in models was acquired to explore the aggregation of molecules. The results show that the mobility of macromolecular oxidation products is lower than that of base stock. The MSD of macromolecular oxidation products reduces with an increasing macromolecular weight. Macromolecular oxidation products can also decrease the mobility of base stock. The interaction energy between the macromolecules and the base stock soars with the increase of macromolecular weight.Macromolecules with a larger molecular weight can affect more base stock molecules with stronger restriction, which leads to lower mobility of base stock molecules. There are aggregates formed among macromolecular oxidation products, and the molecules in aggregates are connected by hydrogen bonds. The quantity of hydrogen bonds in aggregates is related to temperature.
Key words: molecular dynamics simulation; lubricant; oxidation; aggregation; mobility
1 Introduction
As a kind of protective medium, lubricating oils have been widely used in automobiles and mechanical equipment to reduce friction and wear between friction pairs, so as to save energy and extend the service life of equipment[1]. The performance of lubricating oils will gradually decrease with the increase of service time. The degraded lubricating oils are harmful to the environment,which needs special treatment[2]. With the aggravation of the energy crisis and the increasing awareness of environmental protection, higher requirements have been put forward for the service life of lubricants[3].
There are plenty of reasons for the failure of lubricating oils, among which the oxidation of lubricants is the most important. It is mainly related with the base stock, which consists of paraffins, cycloparaffins, and aromatic hydrocarbons to form the main components of lubricating oils, is prone to oxidation. Many researchers have investigated the mechanism of molecular hydrocarbon oxidation, and most of them consider that it follows the mechanism of free radicals chain reaction[4-5].The oxidation products are mainly organic compounds with hydroxyl, carbonyl and/or carboxyl groups[6-7].They can further react to form macromolecules via hydroxyaldehyde condensation, esterification, and polymerization.
Lubricant oxidation products, especially macromolecular oxidation products, can lead to an increase in the viscosity of lubricating oils[8-9]. The viscosity increase which oils undergo during service is responsible for inferior lowtemperature properties, difficult engine starting, reduced rate of oil feed to lubricated components, premature wear of rubbing parts, and shorter service life of lubricants[9].Lubricant oxidation products can aggregate and form sludge as well. The sludge can be attached to mechanical parts to reduce their heat dissipation, and can also be attached to the inner wall of lubricating system to increase the flow resistance of lubricating oils and even block the oil passage, resulting in insufficient oil supply.In this case, even if lubricating oils still have anti-friction and anti-wear performance, it seems impossible to fully utilize the antiwear and friction-reducing capability of lubricating oils. Hence, it will be considered that lubricating oils have failed and should be replaced. This will not only cause a significant waste of resources, but also produce a lot of pollutants.
Plenty of researches have been conducted to make lubricants have better performance and longer service life.Pfaendtner, et al.[4-5,10]have studied the oxidation process of lubricating oils, which can provide a theoretical basis for inhibiting oxidation. Pfaendtner, et al.[11-13]have investigated the mechanism and activity of antioxidants according to the oxidation theory of lubricants, which is beneficial to the development of superior antioxidants.However, there is little study on how can oxidation products affect the performance of lubricating oils. For example, why does the viscosity of lubricating oils increase after oxidation? Researchers have also made considerable efforts to inhibit the aggregation of lubricant oxidation products, which is a significant cause leading to the formation of sludge[14-15]. The addition of dispersants to lubricating oils is a common practice[1]. However,studies on the aggregation process of lubricant oxidation products are limited. It is of considerable significance to understand the internal cause for viscosity changes of oxidized lubricating oils and the aggregation process of oxidized products. It may be possible to make better use of lubricating oils by taking advantage of the variation of viscosity, if there is a better understanding of the internal cause of viscosity changes in lubricating oils. A preferable comprehension of the aggregation process of lubricating oils oxidation products is conducive to the development of excellent dispersants or other effective control methods for sludge formation.
We have studied the aggregation behavior of small molecular oxidation products[16]. This paper mainly focuses on the aggregation behavior of macromolecular oxidation products and their motion, which may be related to the viscosity of lubricating oils.Macromolecules with hydroxyl, carbonyl, and carboxyl groups were used as model molecules of lubricant oxidation products, and the isoparaffin with 26 carbon atoms was employed as the model molecule of base stock. The MD simulation was performed to explore the motion of molecules and the aggregation behavior of oxidation products of lubricants.
2 Computation Details
Base stock mainly consists of paraffins, cycloparaffins,and aromatic hydrocarbons with 20 to 40 carbon atoms.The consumption of the hydrotreated base stock is the largest in all kinds of base stock, and there are more than 90% of saturated hydrocarbons contained in it.The hydrotreated base stock is mainly composed of paraffins and cycloparaffins, among which the content of paraffins is the highest[17]. 11-pentyl heneicosane, a typical paraffin molecular structure, is used as the model molecule of base stock to simplify the model. Lubricant oxidation products are complex mixture of compounds containing polar functional groups. At present, specific structures of them are not clear. Oxidation products are mainly organic compounds with a particular distribution of relative molecular mass. The polar functional groups are mainly hydroxyl, carbonyl, and/or carboxyl groups[6-7]. The macromolecule containing hydroxyl, carbonyl, carboxyl, and ester groups (P-1) was constructed as the model molecule of lubricant macromolecular oxidation products according to characteristics of lubricant oxidation products mentioned in references[6-7]. Structures of base stock and P-1 model molecules are shown in Figure 1. Macromolecular oxidation products with higher molecular weights were obtained by polymerization through the double bond in P-1. P-3, P-5, and P-7 were polymerized by three, five,and seven P-1, respectively.
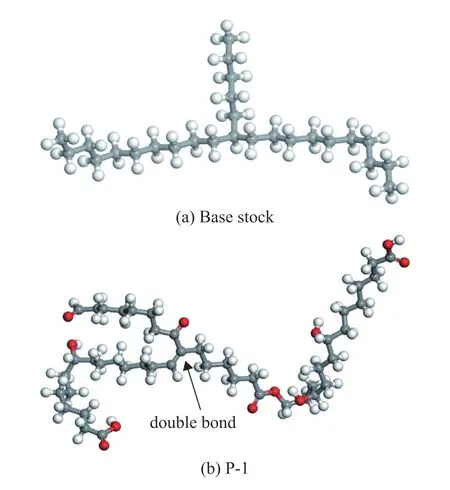
Figure 1 Molecular structures of model molecules—carbon atom;—oxygen atom;—hydrogen atom
All simulations and calculations were performed with Materials Studio 8.0 software. The construction of model molecules and subsequent processing were described in our previous work[16]. The optimized structures of base stock and macromolecules were used to create the model shown in Figure 2. There are 5.2%—10.7% of element O in the sludge collected from engines running at different distances[18]. There are oxidation products with a specific distribution of relative molecular mass in the oxidized lubricant. Small molecular oxidation products are not considered in this paper. The content of element O should be no more than 10.7% in the oxidized lubricant. Therefore, the molar ratio of base stock to macromolecular oxidation products is 30:1 in models, and there are about 1.5%, 3.9%, 5.9%, and 7.4% of element O in lubricant models containing P1, P3, P5, and P7,respectively. The MD simulation was carried out, with the detailed calculation parameters given in the reference[16].The mean square displacement (MSD) analysis was carried out after MD simulation (NVT) to investigate the motion of molecules. The MSD is a technique which determines the mode of displacement of molecules followed over time. In particular, it can help to determine whether the molecule is freely diffusing, being transported, or bound in its movement[19-21]. In addition,The MSD analysis can derive an estimate of the molecular movement. It can be obtained directly from molecular positions in MD simulation, which is shown in Equation(1). In an equilibrium ensemble, the average squared displacement must be independent of the time t, and can be averaged out, leaving the MSD over an interval Δt:

Figure 2 Model of lubricants with macromolecularoxidation products—carbon atom;—oxygen atom;—hydrogen atom
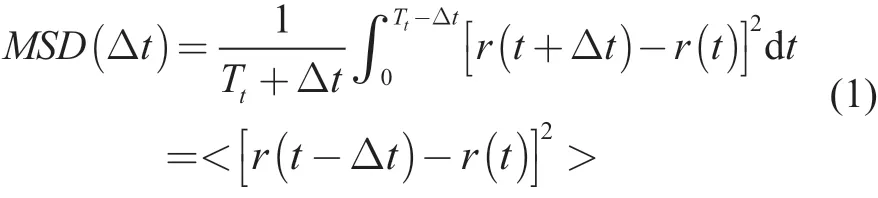
where Ttis the total simulation time; r(t) is the position of molecules at time t; r(t+Δt) is the position of molecules after an interval Δt, the squared displacement of molecules during that interval is [r(t+Δt)-r(t)]2, and < >is the average of all atoms in the model. If there are N equivalent molecules, the MSD can be averaged further:

3 Results and Discussion
3.1 MSD of molecules
The model containing only base stock and the models containing base stock and different kinds of macromolecular oxidation products (P-1, P-3, P-5, and P-7) were simulated for 1 000 ps at the temperature of 25 °C, 100 °C, and 200 °C, respectively, to study the mobility of molecules. The MSD of molecules was analyzed after MD simulation, with the results shown in Figure 3. As shown in Figure 3, the MSD of macromolecular oxidation products (P-1, P-3, P-5,and P-7) is smaller than that of base stock at different temperatures, and it reduces with the increase of macromolecular weight. It may be the reason for the viscosity increase when macromolecular oxidation products are formed in lubricants. The MSD of molecules increases with an increasing temperature, indicating that there is a larger motion ability for molecules at higher temperatures, which may be the reason why the viscosity decreases with the increase of temperature[22-23].However, the amount of MSD growth with temperature is different in macromolecular oxidation products with different molecular weights. There is a smaller increase for the molecule with a more considerable molecular weight. Therefore, the effect of macromolecular oxidation products with a larger molecular weight on the viscosity of lubricating oils may be greater. The MSD of molecules is related to molecular weight and temperature. For the base stock and all kinds of macromolecular oxidation products, the MSD of them at 100 °C is about twice that of molecules at 25 °C, while the MSD of them at 200 °C is about five times that of molecules at 25 °C.
The formation of macromolecular oxidation products in lubricants may influence the performance of base stock as well. The MSD analysis of base stock without and with different macromolecular oxidation products at a temperature of 25 °C, 100 °C, and 200 °C,respectively, was carried out to investigate the effect of macromolecular oxidation products on the mobility of base stock. The results are shown in Figure 4. It manifests that the MSD of base stock increases with a rising temperature, irrspective of the existence of macromolecular oxidation products in the lubricating oils,and the formation of macromolecular oxidation products in lubricants will change the MSD of base stock. Figure 4(a) and 4(b) show that the MSD of base stock decreases at 25 °C and 100 °C when there are macromolecular oxidation products in lubricants, and the MSD of base stock falls off with the increase of macromolecular weight. It indicates that macromolecular oxidation products will restrict the movement of base stock, and the restriction on oxidation products with a larger molecular weight is stronger. However, the MSD of base stock without macromolecular oxidation products and with P-1 and P-3 is similar at 200 °C, while that of base stock with P-5 and P-7 is smaller (Figure 4(c)). It manifests that there is little influence on the mobility of base stock when P-1 and P-3 are formed in lubricants, while P-5 and P-7 can obviously restrict the movement of base stock,at a temperature of 200 °C. In summary, the formation of macromolecular oxidation products will influence the movement of base stock, and the effect is stronger with the increase in molecular weight of macromolecular oxidation products. P-1 and P-3 have little restriction on the motion of base stock at 200 °C. In order to study the cause of this phenomenon, the interaction energy between different types of macromolecular oxidation products and base stock was calculated according to Equation (3):
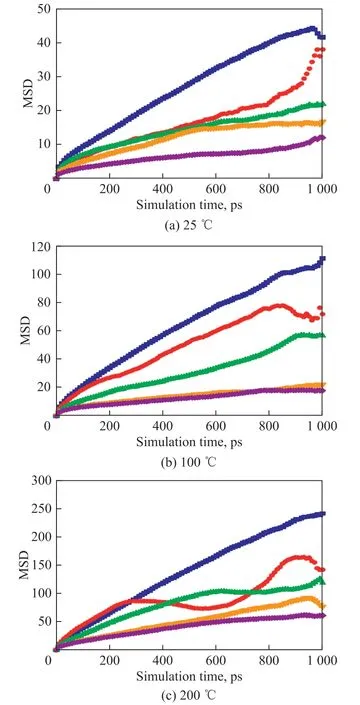
Figure 3 MSD of base stock without macromolecules and macromolecular oxidation products at:(a) 25 °C, (b) 100 °C, (c) 200 °C■—base stock; ●—P-1; ▲—P-3; ▼—P-5; ◆—P-7

where Einteris the interaction energy between macromolecular oxidation products and base stock; EP-Bis the energy of the model containing both macromolecular oxidation products and base stock; EPand EBare the energy of macromolecular oxidation products and base stock, respectively. The results are shown in Table 1.
The calculated results indicate that the interaction energy between macromolecular oxidation products and base stock increases with an increasing molecular weight of macromolecular oxidation products, when the distance between them is constant. The interaction energy between P-1 and base stock is only 3.18 kJ/mol, while that between P-3 and base stock is 14.81 kJ/mol. There is similar interaction energy for P-5 and P-7, which is equal to 28.61 kJ/mol and 28.74 kJ/mol, respectively.The interaction energy between P-5 and base stock reduces with the increase of the distance between them.It manifests that macromolecular oxidation products can affect the base stock within a certain distance, and the influence will decline when the distance becomes longer.The interaction energy between P-5 and base stock with a distance of 1.8 nm is even larger than that between P-1 and base stock with a distance of 1.5 nm, which indicates that P-5 has a more substantial effect on base stock over a broader range than P-1. The interaction energy between macromolecular oxidation products and base stock rises with an increasing molecular weight. However, there is a similar scenario for P-5 and P-7. It may be owing to the reason that there are some atoms in macromolecular oxidation products, which have little effect on base stock when the molecular weight of macromolecular oxidation products increases to a certain extent. These atoms are far away from base stock. So, the interaction energy between macromolecular oxidation products and base stock stops to increase markedly with the increase in molecular weight of macromolecular oxidation products.
The interaction energy between P-1 and base stock is quite small (Table 1). Besides, the MSD of P-1 is just a little bit smaller than that of base stock (Figure 3), which results in the MSD of base stock with P-1 to be similar to that of base stock without macromolecular oxidation products (Figure 4). There is an obviously lower mobility for macromolecular oxidation products with a higher molecular weight (Figure 3). Moreover, they have greater interaction energy with base stock, and they can influence the base stock in a wider extent (Table 1). Therefore,macromolecules with a larger molecular weight can restrict the movement of base stock more effectively, even at high temperatures (Figure 4).
3.2 Aggregation of macromolecular oxidation products
To investigate the aggregation of macromolecular oxidation products, a 2 000 ps MD simulation was carried out for the model containing base stock and P-5.The results show that there are hydrogen bonds formed within and between P-5 in the simulation process. Thequantity of hydrogen bonds of P-5 in four model cells over simulation time is counted, which is shown in Figure 5. Figure 5(a) manifests that there is a little increase for the number of hydrogen bonds within P-5 in the first 400 ps at a temperature of 25 °C. It decreases at the 400-800 ps and then grows. There is a fluctuating variation in the number of hydrogen bonds at 100 °C, and the general trend is negative. The quantity of hydrogen bonds within P-5 reduces in the first 0-1 200 ps and then rises at 200 °C. Figure 5(b) shows that the quantity of hydrogen bonds between P-5 is rising in the first 0 ps to 800 ps and then remains the same at 25 °C. It increases in the first 400 ps and keeps constant from 400 ps to 1 200 ps,and then rises at the temperature of 100 °C, while there is an irregular fluctuating variation for the quantity of hydrogen bonds between P-5 at 200 °C. There are always some hydrogen bonds between P-5 in the model at 25 °C and 100 °C. It indicates that stable aggregation occurs between P-5 under these conditions. Sometimes, there are a large amount of hydrogen bonds between P-5 in the model at 200 °C (800 ps). However, there are very few(400 ps) or no hydrogen bonds (2 000 ps) at some other times, which indicates that there is no stable aggregates formed at the temperature of 200 °C.

Table 1 Interaction energy between macromolecular oxidation products and base stock
The structure of aggregates formed by P-5 at a temperature of 100 °C after the 2 000 ps simulation is shown in Figure 6. In can be seen from Figure 6 that the molecules in aggregates are connected by hydrogen bonds. The hydrogen bonds are mainly formed between carboxyl,hydroxyl, and carbonyl groups in P-5. Hydrogen bond is usually a weak interaction between the electron deficient H atom, which is called the proton donors, and the electron rich atom or atom groups, which are known as the proton receptors[24]. There are proton receptors in P-5, and they may be oxygen atoms in carboxyl, hydroxyl, aldehyde,and carbonyl groups. Hydrogen atoms in carboxyl and hydroxyl groups can act as proton donors. Therefore,hydrogen bonds can be formed within and between P-5.

Figure 5 Quantities of hydrogen bonds over simulation time within P-5 and between P-5■—25 °C; ●—100 °C; ▲—200 °C
The stability of hydrogen bonds and the formation rate of hydrogen bonds are related to the mobility of molecules that have proton donors and/or proton receptors (P-5).Hydrogen bonds in the model may form and break up dynamically, and they can form only when polar molecules are at certain distances and in proper angles[25]. The maximum hydrogen-acceptor distance is 0.25 nm and the minimum donor-hydrogen-acceptor angle is 90.0° in this study. The stronger motion ability of molecules is beneficial for them to moving to the appropriate position and angle for forming hydrogen bonds. Therefore, hydrogen bonds will form more rapidly when the mobility of molecules is enhanced. The motion ability of P-5 at 100 °C is better than that of P-5 at the temperature of 25 °C (Figure 3), which can result in more hydrogen bonds in the model and a faster formation of hydrogen bonds between P-5 at 100 °C (Figure 5(b)). Hydrogen bond is a kind of weak interaction, and it is less restrictive to the molecules or atoms that can form hydrogen bonds. Therefore, hydrogen bonds tend to break down with the increase of molecular mobility. The motion ability of atoms in P-5 may be so strong at 200 °C that hydrogen bonds are less stable, which leads to fluctuating variation in the quantity of hydrogen bonds (Figure 5(b)).Hydrogen bonds between lubricating oil oxidation products may promote the formation of sludge. The formation of hydrogen bonds is difficult at high temperature. However,the sludge can form easily under this condition, which seems to be contradictory. Therefore, there may be some other influencing factors for the formation of sludge besides hydrogen bonds.

Figure 6 Hydrogen bonds formed between P-5 at a temperature of 100 °C after a 2 000 ps simulation—carbon atom;—oxygen atom;—hydrogen atom;—hydrogen bond
4 Conclusions
The MD simulations were employed to investigate the mobility and aggregation behavior of macromolecular lubricating oils oxidation products in the base stock and their influence on the performance of base stock. The results can be summarized as follows:
(1) The MSD of macromolecular oxidation products is smaller than that of base stock at different temperatures.Besides, the MSD of base stock reduces with the existence of macromolecular oxidation products in lubricants, which may be the reason for the viscosity increase of oxidized lubricants.
(2) The MSD of macromolecular oxidation products declines with the increase of their molecular weight.There is more tremendous interaction energy between the macromolecular oxidation products with higher molecular weights and the base stock, and they can restrict the movement of base stock more effectively, which can result in a smaller MSD of base stock.
(3) Macromolecular oxidation products can form aggregates by hydrogen bonds. Aggregates are stable at 25 °C and 100 °C, while hydrogen bonds between macromolecular oxidation products may break down at 200 °C, which may be related to different motion abilities of macromolecules at various temperatures.
Acknowledgments: The authors are grateful for the calculation support of the Key Laboratory of Molecular Oil Refining of the Research Institute of Petroleum Processing in SINOPEC and the financial supports from the Natural Science Foundation of China(NSFC; No.51671100), the State Key Laboratory of Metal Material for Marine Equipment and Application-School of Material and Metallurgy, University of Science and Technology Liaoning Co-project (No. SKLMEA-USTLN 201905), the University of Science and Technology Liaoning Talent Project(No.601010314) and the University of Science and Technology Liaoning Young Teachers Fund (No.2019QN08).
- 中國煉油與石油化工的其它文章
- Multiphase Flow Simulation of New Vapor Distributor in Dividing Wall Column and Control Mechanism
- Evaluation of Molecular Structural Effects on Needle Coke Mesophase Stacking
- A Simple and Highly-Efficient Approach for Construction of 2D Nanostructured H-BN/WS2 Heterojunction through Hydrothermal Method-Assisted Exfoliation and Their Friction Performance in Grease
- Influence of Two Preparation Methods on Rheological Properties of Lithium Grease
- Removal of Phenanthrene from Contaminated Soil by Ozonation Process
- Effects of Sampling Conditions on Composition and Emission Characteristics of Volatile Organic Compounds in Process Units from Different Refineries

