Evaluation of Molecular Structural Effects on Needle Coke Mesophase Stacking
Yang Haiyang; Wang Chunlu; Zhou Han; Wang Lixin; Ren Qiang; Fan Qiming
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Molecular simulations were performed to investigate the molecular structural effects on needle coke mesophase stacking. The simulation results showed that the stacking states of anthracene trimer and tetramer accumulations were orderly, while the stacking states of anthracene dimer, pentamer, and hexamer accumulations were disorderly. Anthracene trimer and tetramer in the model compounds were two of the most ideal needle coke mesophase constituents. It was also found that the introduction of methyl side chains in anthracene trimer derivatives was not conducive to the formation of a mesophase crystal. To sum up, the molecules which had similar structures to anthracene trimer or tetramer with no alkyl chains are ideal constituents of needle coke mesophase.
Key words: molecular simulation; needle coke; mesophase; stacking; interlayer spacing
1 Introduction
Needle coke is a carbonaceous material which is often applied to fabricate graphite electrodes and negative electrode materials[1]due to its several unique properties,such as high mechanical strength, small coefficient of thermal expansion, low resistivity, and excellent corrosion resistance. Needle coke is generally formed by liquid-phase carbonization of petroleum or coal tar.Brooks and Taylor[2-3]first obtained mesophase spheres during coal carbonization. It was found that the degree of mesophase development determined the texture of needle coke[4]. Numerous researchers[5-11]have studied the formation process and molecular structures of carbonaceous mesophase. Large molecules of polycyclic aromatic hydrocarbons (PAHs) are formed by aromatic compounds in needle coke feedstock through cracking,dehydrogenation, and condensation reactions during carbonization. These molecules move under the actions of external agitation and thermal motion, and subsequently accumulate into layers under the influence of the van der Waals forces between macromolecules. As the number of layers increases, the intensity of surface tension also increases, and in turn, a spherical crystal is formed in line with the minimum energy principle.
It is generally considered that mesophase molecules possess a lamellar structure. Mochida[12]proposed a“spider”-type mesophase molecular model. Chang[13-15]propounded that mesophase molecules were highly condensed polycyclic aromatic hydrocarbons consisting of several alkyl groups without methylene or aryl structures between aromatic rings. Burgess[16-18]found that the M-50 petroleum pitch monomer was primarily comprised of PAH backbones substituted with 0—4 alkyl(primarily methyl) groups.
However, the molecular constituents of needle coke mesophase are complicated, and fewer literature reports discuss about mesophase stacking. How does mesophase stack? What are the main influencing factors? What are the effects of molecular structure on mesophase stacking?To solve the above problems, molecular simulations were performed in the present research to study different stacking patterns during needle coke mesophase formation.
2 Molecular simulations
2.1 Model compounds
The molecular constituents of needle coke mesophase are complicated, and the molecular structures are not clear now. However, anthracene is not found in fluid catalytic cracking decant oil[19]. Anthracene was a classical kind of PAHs which were used as model compounds of needle coke feedstocks[20-30], and the structures of anthracene oligomers were relatively regular. In the current research,anthracene oligomers and their derivatives were selected as model compounds. Needle coke mesophase stacking with ideal structures were investigated in the following work.
Generally, some model compounds were built based on the degrees of polymerization, such as dimer, trimer,tetramer, pentamer, and hexamer (Figure 1). Other model compounds were built based on the derivative types of anthracene trimer, such as different numbers of side chains (Figure 2).
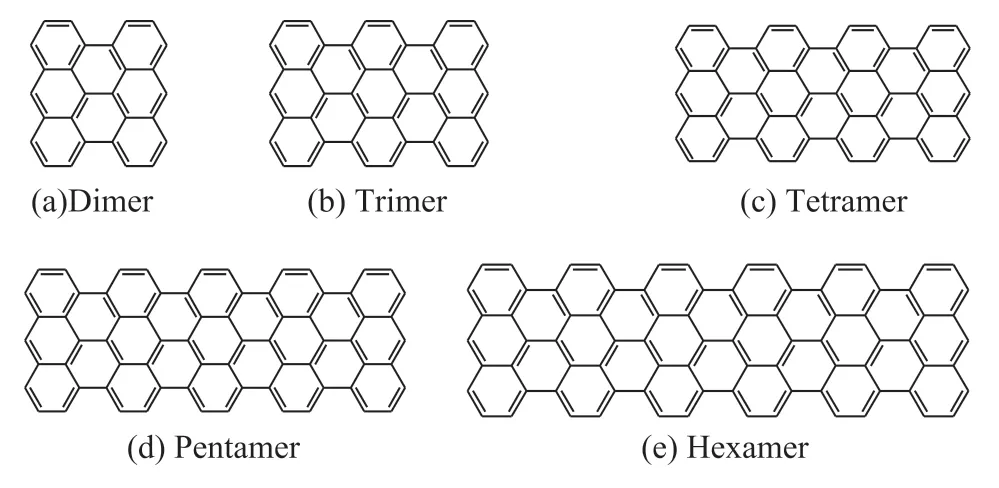
Figure 1 Anthracene oligomers selected as model compounds

Figure 2 Anthracene trimer derivatives selected as model compounds
2.2 Method of investigating the effects of molecular structures on mesophase accumulation
The Amorphous Cell module in Materials Studio 2017 R2(MS) was used to build the periodic models. In order to analyze the effects of molecular structures on mesophase accumulation, nine three-dimensional (3D) periodic models were built accordingly. Except hexamer periodic model, the periodic models consisted of 200 molecules of the models shown in Figure 1 and Figure 2, respectively.Hexamer periodic model consisted of 100 molecules because of its big molecular structure. The initial density of these nine periodic models was set to 0.001 g/cm3. The Forcite module in MS was used to carry out structural relaxation.
As shown in Figure 3, firstly, the canonical ensemble(NVT) was used to simulate dynamics for 200 ps at a temperature of 2 000 K to make the model molecules fully spread out. Then the NVT ensemble annealing was made between 1 500 K and 2 500 K. Secondly, the isothermal isobaric ensemble (NPT) was used to simulate dynamics for 200 ps at 770 K and 1 GPa. The NVT ensemble annealing was carried out between 500 K and 1 000 K. Thirdly, the NPT ensemble was used to simulate dynamics for 200 ps at 500 K and 0.2 MPa. Annealing with the NVT ensemble was carried out between 300 K and 700 K. Fourthly, the NPT ensemble was used to simulate dynamics for 200 ps at 298.15 K and 0.1 MPa.Annealing with the NPT ensemble was carried out between 200 K and 500 K. Lastly, the NPT ensemble was used to simulate dynamics for 200 ps at 298.15 K and 0.1 MPa. The NVT ensemble was used to simulate dynamics for 2 000 ps at 298.15 K.

Figure 3 Structural relaxation steps of periodic models
The time step was 1 fs, the Nose method was used for temperature control, and the Berendsen method was used for pressure control. Compass II was selected for forcefield. Charges were assigned by the selected forcefield. Calculation accuracy was set to medium.
The stable 3D periodic models obtained after structural relaxation were used to analyze the stacking state of mesophase at normal temperature and pressure.
2.3 Interaction energy calculation
For periodic models, the cohesive energy density method in the Forcite module was used to measure the average interaction energy between molecules of the stable 3D periodic models.
For aperiodic models, the geometry optimization method in the Forcite module was adopted to optimize the structure and also to calculate the interaction energy. The cohesive energy was calculated according to Equation (1)[31]:

where, E1and E2refer to the energy of two single molecules, respectively, and Etotalrefers to the total energy of the two molecules combined by π-π interaction.
Compass II was selected for forcefield, whereas Medium was selected for calculation accuracy.
2.4 Analysis of X-ray diffraction spectrograms
The powder diffraction method in the Reflex module of MS was used to calculate and analyze the X-ray diffraction (XRD) spectrograms of the stable 3D periodic models[32]. The interlayer spacing can be calculated according to the Bragg’s law (Equation (2)).

where, d refers to the interlayer spacing, θ refers to the angle between entry ray and crystal face, n refers to the diffraction order, λ refers to the wavelength of X-ray. The X-ray wavelength is 0.1540562 nm. The 2θ value was set at between 20° and 30°. The corresponding interlayer spacing ranged between 0.2976 nm and 0.4436 nm.
As a comparison with XRD calculation results, the interlayer spacing and the 2θ value of graphite crystal is 0.3354 nm and 27.34°, respectively[33]. The mesophase,which has closer interlayer spacing to that of the graphite crystal, is a better precursor of needle coke.
3 Results and Discussion
Aromatic hydrocarbons could accumulate in four main ways as a result of π-π interactions[34], viz.: (a) parallel face-to-face “sandwich” (S), (b) parallel displaced (PD),(c) perpendicular T-shaped (T), and (d) perpendicular Y-shaped (Y) (Figure 4).
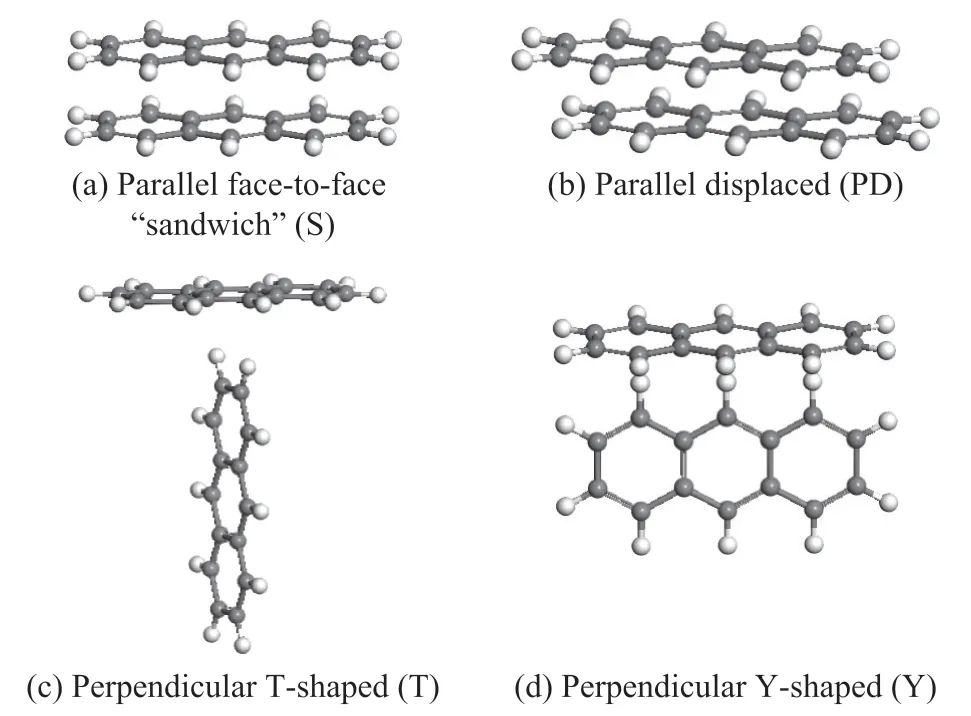
Figure 4 Configurations of benzene dimer stacking
3.1 Influences of polymerization degree on mesophase accumulation
Anthracene dimer, trimer, tetramer, pentamer, and hexamer were used as the model compounds. The accumulation states are displayed in Figure 5. The corresponding XRD spectrograms of anthracene oligomers are presented in Figure 6. The property parameters of each oligomer are exhibited in Table 1.
It can be clearly seen from Figure 5 that the anthracene oligomers mainly accumulated in the parallel displaced mode; however, different amount of perpendicular T-shaped and perpendicular Y-shaped accumulations were also observed. It is noticeable from Table 1 that the molecular weight, the C/H atom ratio, the density ofaccumulation, and the cohesive energy density gradually increased with an increasing polymerization degree, since the value of d002decreased first and then increased with an increasing polymerization degree. The interlayer spacings of anthracene trimer and tetramer accumulations were the shortest among the oligomers,
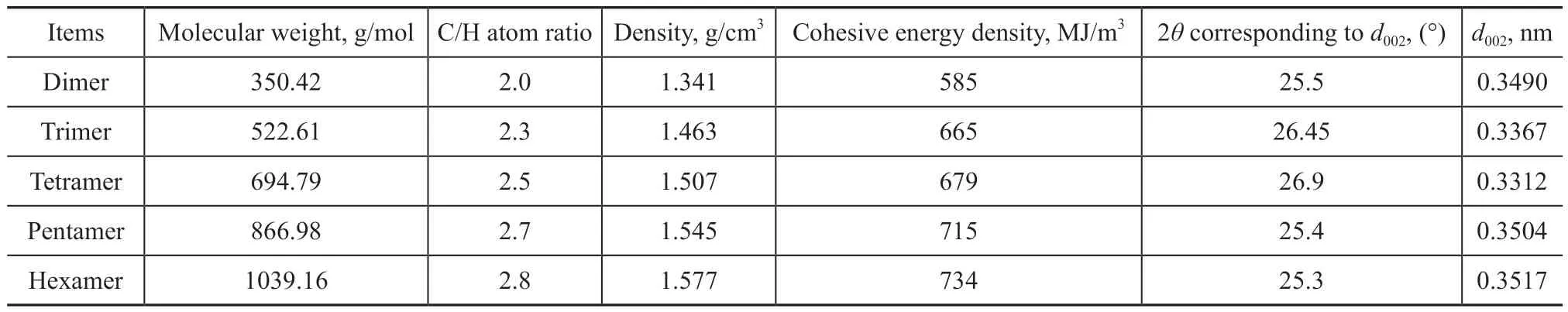
Table 1 Properties of anthracene oligomers

Figure 5 Accumulation states of anthracene oligomers

Figure 6 XRD spectrograms of anthracene oligomers accumulations
In comparison to other oligomers, the anthracene dimer had smaller molecular mass, less aromatic rings, and weaker intermolecular force. The intermolecular binding force of anthracene dimer was weak in movement.When two PAH molecules were stacked on top of each other, more number of aromatic rings could be stacked,leading to stronger interaction force. The maximum energy for S, PD, T-shaped and Y-shaped interactions of aperiodic models was calculated as 79.6, 81.7, 30.4,and 31.7 kJ/mol, respectively. The interaction energy of anthracene dimer in parallel stacking was higher than that in perpendicular stacking. This is quite different from benzene, which has only one aromatic ring. In parallel stacking, the PD mode was tighter than the S mode,thereby leading to higher interaction energy. Therefore,anthracene dimer mainly accumulated in the PD mode.
In comparison to other oligomers, anthracene trimer and tetramer had moderate molecular mass, moderate intermolecular force and smaller amplitude of molecular distortion during vibration. The intermolecular force of anthracene trimer and tetramer was much stronger than that of anthracene dimer, hence, their intermolecular binding force was strong in movement. The calculated maximum interaction energy between two anthracene trimer molecules in S, PD, T-shaped and Y-shaped mode was 138.2, 145.9, 41.1, and 56.3 kJ/mol, respectively.The maximum interaction energy between two anthracene tetramer molecules in S, PD, T-shaped and Y-shaped mode was calculated as 196.8 kJ/mol, 201.1 kJ/mol,36.2 kJ/mol, and 76.7 kJ/mol, respectively. The molecular interaction energy of anthracene trimer and tetramer in parallel stacking was higher than that in perpendicular stacking. In parallel stacking, the PD mode was tighter than the S mode, thereby leading to higher interaction energy. Therefore, the anthracene trimer and tetramer mainly accumulated in the PD mode. According to XRD analysis (Figure 6), the 2θ value corresponding to the strongest peak of tetramer was the highest among all oligomers. It indicated that the interlayer spacing of the tetramer was the smallest and its accumulation degree was the most compact. The trimer ranked only second to the tetramer.
In comparison to other oligomers, anthracene pentamer and hexamer had bigger molecular mass, more aromatic rings, and stronger intermolecular force.Their intermolecular binding force was very strong in movement. The maximum interaction energy between two anthracene pentamer molecules in S, PD, T-shaped and Y-shaped mode was calculated as 254.9, 261.1, 36.2,and 98.3 kJ/mol, respectively. The maximum interaction energy between two anthracene hexamer molecules in S, PD, T-shaped and Y-shaped mode was calculated as 314.6, 320.6, 45.7, and 122.9 kJ/mol, respectively. The molecular interaction energy of anthracene pentamer and hexamer in parallel stacking was higher than that in perpendicular stacking. In parallel stacking, the PD mode was tighter than the S mode, hence causing higher interaction energy. Therefore, the anthracene pentamer and hexamer mainly accumulated in the PD mode. In addition, it is possible for a molecule to stack with other molecules in several different ways simultaneously.Hence, this kind of accumulation like the pentamer and the hexamer would not lead to orderly but chaotic mesophase accumulation.
To sum up, the anthracene trimer and tetramer had the most orderly stacking forms, and their crystals were the most regular among all oligomers; the interlayer spacings of the anthracene trimer and tetramer accumulations were the shortest, and the accumulation degrees were the most compact, while the degrees of graphitization were the highest.
3.2 Influences of side chains on mesophase stacking
Derivatives of anthracene trimer with different numbers of side chains were used as the model compounds. The influences of side chains on mesophase stacking were investigated in detail in the current sub-section. The accumulation states of anthracene trimer derivatives with different numbers of methyl side chains are displayed in Figure 7, and the corresponding XRD spectrograms are presented in Figure 8. The property parameters of anthracene trimer derivatives with different numbers of methyl side chains are presented in Table 2.
It can be seen from Table 2 that the C/H atom ratio,the density of accumulation, the cohesive energy density, and 2θ corresponding to d002decreased with an increasing number of methyl side chains. It indicated that the introduction of methyl side chains made the intermolecular force weak.
It can be seen from Figure 7 that the anthracene trimer derivatives with different number of methyl side chains mainly accumulated in the parallel displaced mode;however, a small amount of perpendicular Y-shaped accumulation was also observed.
As shown in Figure 8, the 2θ value corresponding to the strongest peak of anthracene trimer with no side chain was the highest among all derivatives with side chains.The introduction of methyl side chains in anthracene trimer significantly reduced the viscosity and facilitated the molecular movement[15]. However, the hybrid type of carbon atom in alkyl side chain of mesophase molecule was the sp3hybridization. The carbon atom in methyl side chain would maintain a certain angle with the planar part of the molecule after mesophase solidification. Alkyl side chains in mesophase molecules generated a relatively loose stacking structure, which was not conducive to the formation of a mesophase crystal.
4 Conclusions
Molecular simulations were used to study the phenomenonof molecular stacking which was difficult to observe in experiments. Anthracene oligomers and anthracene trimer derivatives were used as the model compounds.

Table 2 Properties of anthracene trimer derivatives with methyl side chains
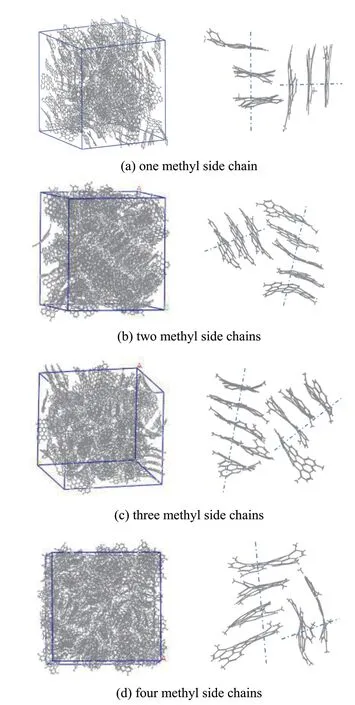
Figure 7 Accumulation states of anthracene trimer derivatives with different numbers of methyl side chains

Figure 8 XRD spectrograms of accumulations of anthracene trimer derivatives with different number of methyl side chains
Anthracene trimer and tetramer had the most orderly stacking forms, and their crystals were the most regular among all oligomers, while the interlayer spacings of anthracene trimer and tetramer accumulations were the shortest, the accumulation degrees were the most compact,and the degrees of graphitization were the highest.
The introduction of methyl side chains in anthracene trimer is not conducive to the formation of a mesophase crystal since alkyl side chains generate a relatively loose stacking structure.
Molecular structures of needle coke mesophase before being solidified should theoretically be close to the anthracene trimer or tetramer in the model compounds with no alkyl side chains by screening raw materials and controlling the production condition.
Acknowledgments: Funding provided by the Molecular Simulation Key Laboratory at SINOPEC Research Institute of Petroleum Processing is gratefully acknowledged.
- 中國煉油與石油化工的其它文章
- Multiphase Flow Simulation of New Vapor Distributor in Dividing Wall Column and Control Mechanism
- Molecular Dynamics Simulation on Mobility and Aggregation of Macromolecular Lubricant Oxidation Products and Their Influences on Base Stock
- A Simple and Highly-Efficient Approach for Construction of 2D Nanostructured H-BN/WS2 Heterojunction through Hydrothermal Method-Assisted Exfoliation and Their Friction Performance in Grease
- Influence of Two Preparation Methods on Rheological Properties of Lithium Grease
- Removal of Phenanthrene from Contaminated Soil by Ozonation Process
- Effects of Sampling Conditions on Composition and Emission Characteristics of Volatile Organic Compounds in Process Units from Different Refineries

