Removal of Phenanthrene from Contaminated Soil by Ozonation Process
Yang Yixin; Gao Wenfang; Yang Jingchao; Cao Hongbin
(1. Beijing Key Laboratory of Fuels Cleaning and Advanced Catalytic Emission Reduction Technology,College of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617;2. Beijing Engineering Research Centre of Process Pollution Control and Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190;3. College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124)
Abstract: In order to improve the ozonation efficiency for the remediation of PAHs contaminated soil, the performance experiments were carried out with quartz sand artificially contaminated with phenanthrene. The byproducts of phenanthrene were detected by GC-MS and the toxicity was evaluated by seed germination tests. The influence of the particle size and moisture content of quartz sand on the ozonation efficiency was investigated. In addition, two kinds of real soil was used to compare with the quartz sand. It was revealed that the phenanthrene removal rate reached 96% after 600 minutes by using the ozonation process. Three byproducts of phenanthrene, including 9,10-phenanthrenedione, (1,1’-biphenyl)-2,2’-dicarboxaldehyde, and (1,1’-biphenyl)-2,2’-dicarboxylic acid, were obtained. As proven by seed germination tests, the toxicity of the byproducts was lower than phenanthrene. The phenanthrene was removed more effectively by ozonation in the quartz sand with finer particle size. The ozonation efficiency was significantly improved by increasing the moisture content, which is assumed to be related to the alkalinity of quartz sand.
Key words: ozonation; phenanthrene; quartz sand; soil; remediation
1 Introduction
Soil contamination by anthropogenic polycyclic aromatic hydrocarbons (PAHs) has been a worldwide concern in recent years[1]. Most PAHs are sourced from petroleum catalytic cracking and vehicular emissions[2].The carcinogenicity of PAHs poses a persistent health risk to humans[3-4]. Biological treatment is often limited by the high hydrophobicity and low bioavailability of PAHs[5-6]. Chemical oxidation has emerged as an effective remediation technology for the PAHs contaminated soil[7-9]. Ozonation process is an attractive option, which has been verified to be effective for the decomposition of phenanthrene, anthracene,acenaphthene, fluoranthene, pyrene, chrysene, benzo(a)pyrene, and so on[5,10-18]. The ozonation efficiency is strongly influenced by the soil properties such as porosity, moisture content, and pH conditions[11-21].However, there are some different conclusions because of the various soil matrix and experimental conditions used in previous papers. So further studies are necessary to learn more about the ozonation process for the remediation of PAHs contaminated soil. In the present study, quartz sand was used as the test matrix because of its stability and simple composition.The quartz sand was artificially contaminated with phenanthrene and treated by ozonation process.The byproducts of phenanthrene were identified,and their toxicity was assessed by seed germination tests. The influence of the particle size and moisture content of quartz sand on the ozonation efficiency was investigated, because these two variables could exert great influence on the mass transfer of ozone. In addition, two kinds of real soil was used to compare with the quartz sand to show the influence of soil matrix.
2 Experimental
2.1 Materials
Analytically pure phenan threne and 9 , 1 0 -phenanthrenedione (with a purity of 95%) were purchased from the Alfa Aesar Corp. (1,1’-Biphenyl)-2,2’-dicarboxaldehyde and (1,1’-biphenyl)-2,2’-dicarboxylic acid were obtained from the Tokyo Kasei Kogyo Co., Ltd.Absolute methanol and n-hexane (chromatographically pure) were supplied by the DIKMA Technologies Inc.and used without further purification. All other chemical reagents were supplied by the Sinopharm Chemical Reagent Co., Ltd.
2.2 Soil samples
Unless otherwise specifed, quartz sand with a particle size of 0.40-0.80 mm was used in the experiments. The quartz sand was washed with deionized water and was then oven-dried at 105 °C for 24 hours. Phenanthrene was dissolved in absolute methanol to prepare a stock solution with its concentration equating to 200 mg/L. 100 grams of quartz sand were immersed in 100 mL of stock solution to achieve a loading of 200 mg of phenanthrene per kg of sand. The mixture was then placed in a fume hood and mechanically stirred until methanol was completely evaporated.
Two kinds of real soil was collected from a park in Beijing, China. Extraction and analysis showed that the real soil was free from phenanthrene. Particles in a grain size range of 0.40—0.80 mm were sieved out from the real soil and artificially contaminated with phenanthrene in the same way as did the quartz sand.
The mineralogical composition of target soil was determined with an X-ray fluorescence spectrometer(AXIOS, PANalytical B.V., Netherlands), and the total organic carbon (TOC) was measured with an elemental analyzer (Vario EL cube, ELEMENTAR, Germany).The surface area of soil particles was determined by the Brunauer-Emmett-Teller (BET) analysis (Belsorp-max,MicrotracBEL, Japan). The moisture content of soil was calculated by the weight method, which is the mass ratio of moisture and dried soil. The soil samples were baked in the oven at 105 °C for 4 hours. The weight difference between unbaked and baked soil is the mass of moisture.
The soil pH value was measured with a pH meter (FE20,Mettler Toledo, Switzerland). The soil samples were mixed with deionized water in a mass ratio of 1:2.5 and agitated with a magnetic stirrer (DF-101S, Henan Yuhua Instrument Co., Ltd., China) for 1 hour at a speed of 120 r/min to ensure adequate contact. Then the pH value of the filtrate was determined.
2.3 Ozonation
Ozonation was performed in a Pyrex glass column reactor(300 mm in length and 60 mm in internal diameter). A porous ceramic plate was placed horizontally in the glass column to diffuse the ozone gas. In each test, 10 g of soil were heaped onto the ceramic plate without compaction.Ozone gas was produced from ambient air with an ozone generator (DHX-IX, Harbin Jiujiu Electrochemical Engineering Technology Co., Ltd., China). An ozone analyzer (UV-2100, IDEAL, China) was used to measure the ozone feed rate, which was 2.9 mg/min in all experiments. Excess ozone was quenched by a sodium thiosulfate solution. To calculate the consumed ozone dosage, the ozone amount in offgas was measured by the Indigo method[22]. Each experiment was duplicated and the average value was used in the discussion.
2.4 Analysis
The phenanthrene was extracted by methanol with an ultrasound bath (KQ-300DE, Kun Shan Ultrasonic Instruments Co., Ltd, China, with operating conditions covering a frequency of 80 kHz, a power of 1 kW, a temperature of 50 °C, and a time of 60 minutes). The extract was filtered by a 0.2 μm polytetrafluoroethylene filter membrane and measured by a high-performance liquid chromatograph equipped with a reversed-phase C-18 analytical column (Aglient 1260 Infinity, America).The mobile phase was a mixture of methanol (90%) and ultrapure water (10%) at a flow rate of 0.25 mL/min.The column temperature was set at 35 °C. Ultraviolet detection was carried out at 254 nm. Based on the results of recovery experiments, the extraction percentage of phenanthrene by ultrasonic process achieved an over 95% efficiency. All the analytical tests were carried out in duplicate.
The byproducts of phenanthrene were extracted by n-hexane with the same ultrasonic process and identified by gas chromatography-mass spectrometry (GC-MS,Thermo Fisher Trace 1300-ISQ, USA). A TG-5MS silica capillary column (30 m in length × 0.25 mm in diameter× 0.25 μm in film thickness) was employed in GC separation, and a 33:1 split injection was used with the column temperature being heated from 50 °C to 280 °C at a 20 °C/min ramp. Helium (with a purity of 99.999%) as the carrier gas flowed at a rate of 2 mL/min. The detector temperature was 280 °C, and the injector temperature was 270 °C. The MS ionization mode was electron bombardment with an energy of 70 eV. The scanning range (m/z) was 45-500.
The toxicity of phenanthrene and its byproducts was evaluated by seed germination tests with tomato,according to the relevant test method[23]. In this experiment, the quartz sand with a particle size of 0.15-0.18 mm were artificially contaminated by phenanthrene and its byproducts, respectively, with a loading of 100 mg of contaminant per kg of sand. The control experiment was carried out with the clean quartz sand. The incubation process was ended when the seed germination percentage of the control test reached 65%[23].
3 Results and Discussion
3.1 Phenanthrene decomposition
As shown in Figure 1, the phenanthrene was readily decomposed by the ozonation process. The removal rate of phenanthrene reached 96% in 600 minute. As it is an acceptable ozone exposure time, the application of ozonation for phenanthrene removal is feasible.

Figure 1 Phenanthrene removal rate as a function of ozone exposure time(ozone flow: 2.9 mg/min)
Three byproducts of phenanthrene were identified by GC-MS analysis, namely 9,10-phenanthrenedione,(1,1’-biphenyl)-2,2’-dicarboxaldehyde, and(1,1’-biphenyl)-2,2’-dicarboxylic acid, as shown in Figure 2. Judging from the perspective of molecular structure, 9,10-phenanthrenedione is the initial product of phenanthrene oxidation. The positions 9, 10 of phenanthrene have a lower localization energy, which often results in the directive attack by oxidant at these locations[24]. Most likely the ozone molecule was added onto the positions 9 and 10 to form a primary ozonide and was then subsequently transformed into 9,10-phenanthrenedione[25]. The 9,10 C-C bond was broken by further oxidation to produce biphenyl aldehydes and biphenyl carboxylic acids. The relative intensity of the byproducts in GC-MS spectra versus ozonation time is illustrated in Figure 3. The curves rise upward at first and then go down, which indicates that the byproducts may be further decomposed.

Figure 2 GC-MS spectra of the byproducts of phenanthrene

Figure 3 Relative intensity of phenanthrene byproducts in GC-MS spectra versus ozonation time■—(1,1'-Biphenyl)-2,2'-dicarboxaldehyde;●—(1,l'-Biphenyl)-2,2'-dicarboxylic acid; ▲—9,l0-Phenanthrenedione

Table 1 Seed germination test results of phenanthrene and its byproducts

Figure 4 Effect of particle size of quartz sand on phenanthrene removal rate(ozone flow: 2.9 mg/min; exposure time: 5 minutes)
Seed germination tests were carried out to compare the toxicity of phenanthrene with its byproducts. The result is shown in Table 1. The seed germination percentage for phenanthrene was 46.6%, as a contrast to 65% in the control sand. A decrease by 18% indicated the inhibitive effect of phenanthrene on the seed germination. The seed germination percentage for the byproducts was higher than phenanthrene, which meant the less toxicity of the byproducts.
3.2 Effect of the soil particle size
The influence of the particle size of quartz sand on the ozonation process was illustrated in Figure 4. The phenanthrene decomposition was more efficient in quartz sand with finer particle size. After 5 minutes of ozonation, the phenanthrene removal rate reached 21% in quartz sand with a particle size range of 0.40―0.80 mm but was 62% for the quartz sand with a particle size range of 0.15―0.18 mm. The consumed ozone dosage was calculated, with the result illustrated in Figure 5. The value is 0.47 and 0.89 mg of ozone per gram of sand for the particle size range of 0.40―0.80 mm and 0.15―0.18 mm, respectively. This means that the utilization factor of ozone is higher in the quartz sand with finer particle size. The reason may be related to the larger specific surface area of the finer quartz sand, which resulted in an increased ozone availability because of the larger gas-solid interfacial area[26-27].
3.3 Effect of the soil moisture
The superiority of gaseous ozone over aqueous ozone for the remediation of PAHs contaminated soil was reported in previous research papers[11-12,14,18]. The ozone concentration in gaseous phase is several times higher than that obtainable in aqueous solution[11]. Moreover,the higher diffusivity of gaseous ozone could have easier access to PAHs than the aqueous ozone[12]. As a result, the ozonation efficiency was decreased by increasing the soil moisture content.

Figure 5 Consumed ozone dosage per gram of quartz sand with different particle size(ozone flow: 2.9 mg/min; exposure time: 5 minutes)
However, the experimental result in the present study is contrary to previous papers. As shown in Figure 6, the presence of moisture was positive for the degradation of phenanthrene. This unusual result may be related to the production of hydroxyl radicals from ozone decomposition in aqueous solution. Since hydroxyl ions are the primary initiators of the chain reactions of ozone decomposition, the ozonation efficiency is strongly influenced by the pH value in aqueous solution[28].According to the previous papers, the soil pH value is also an important factor for the ozonation process. For example, the pH value of Boyndie soil and Cruden Bay soil was 3.61 and 6.03, respectively, and phenanthrene was inefficiently removed by ozonation if the water-holding capacity of soil was increased[12]. Less efficient PAHs removal was observed when water was added into the treated sand soil and peat soil with a pH value of 6.4 and 6.7, respectively[19]. The pyrene removal rate in sandy-loam with a pH value of 6.2 decreased when the moisture content was increased from zero to 5% or 10%[18]. In the above-mentioned studies, the soil samples were all slightly acidic and the water could bring about a negative effect on the ozonation process. However, the presence of water promoted anthracene degradation in the sand with a pH value of 7.3, despite a lag in degradation caused by the mass transfer resistance of water film[16].It was deduced that the sand particles were surrounded by water film and the ozone mass transfer was blocked,which delayed the anthracene degradation in the first 15 minutes. After the ozone gas was dissolved in water film, both the removal of anthracene and the formation of byproducts were promoted. It is notable that phthalic acid, the main byproduct of anthracene, was formed after 20 minutes in the moist sand but was generated after 120 minutes in the baked sand. The pH value of quartz sand used in the present study was 7.9 (as shown in Table 2),and the positive effect of the soil moisture on ozonation efficiency was significant even in the initial mass transfer stage. It is assumed that a weakly alkaline liquid phase formed by the moisture in the quartz sand pores is favorable to ozone decomposition. So the dissolution of ozone gas could be accelerated because of the continuous upset of the gas-liquid dissolution equilibrium by ozone decomposition, which contributes to overcoming the mass transfer resistance caused by the moisture. Moreover,the degradation of phenanthrene may also be improved by hydroxyl radicals formed from ozone decomposition in the aqueous phase[18]. Therefore, it is suggested that the moisture is favorable to the ozonation process for the alkaline soil and unfavorable for the acidic soil.
3.4 Ozonation experiments with real soil
Two kinds of real soil was used to compare with the quartz sand. The soil properties are listed in Table 2.They are mainly composed of aluminosilicate and some metals such as iron, calcium, potassium, and sodium. The pH value of soil I and soil Ⅱ is 7.8 and 6.5, respectively,which can be a contrast of weak alkaline to weak acidic environment. Experimental results about the phenanthrene removal by ozonation are illustrated in Figure 7.

Figure 6 Effect of moisture content of quartz sand on phenanthrene removal rate(ozone flow: 2.9 mg/min; exposure time: 5 minutes)

Table 2 The properties of soil samples
The natural moisture content is 4.4% for Soil I and 3.9%for Soil Ⅱ. After 5 minutes of ozonation, the phenanthrene removal rate reached 28% for Soil I and 19% for SoilⅡ. The better ozonation efficiency for Soil I may result from its favorably higher BET surface area and lower TOC content than Soil Ⅱ, as shown in Table 2. Increasing the soil moisture content promoted the phenanthrene degradation for Soil I (alkaline pH) but inhibited it for Soil Ⅱ (acidic pH). The phenanthrene removal rate increased from 28% to 71% when the moisture content of Soil I was increased from 4.4% to 100%. While, for SoilⅡ, the phenanthrene removal rate decreased from 19%to 6%, if the moisture content was increased from 3.9%to 10%. The results are consistent with the hypothesis that the effect of soil moisture content on ozonation efficiency is related to the soil pH value. If the soil pH value is in a weak alkaline range, it is possible to increase the ozonation efficiency by increasing the soil moisture content. From this point of view, the application mode of ozone can be selected as gaseous or aqueous according to the soil pH value.
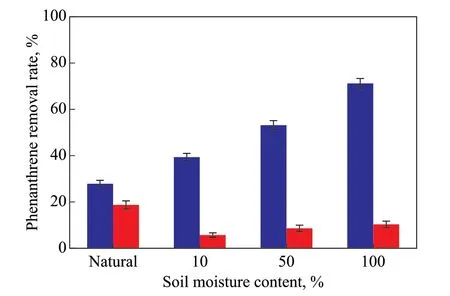
Figure 7 The phenanthrene removal rate of real soil with different moisture content(ozone flow: 2.9 mg/min; exposure time: 5 minutes)■—Soil I; ■—Soil II
4 Conclusions
Ozonation was effective in degrading phenanthrene in contaminated soil. In quartz sand with a particle size range of 0.40-0.80 mm, the phenanthrene removal rate reached 96% after 600 minutes of ozonation with an ozone feed rate of 2.9 mg/min. The ozonation efficiency was increased with the decrease in the particle size of the quartz sand. The improvement in the ozonation efficiency was ascribed to the larger interfacial area of the finer quartz sand. Three phenanthrene decomposition byproducts were detected,namely 9,10-phenanthrenedione, (1,1’-biphenyl)-2,2’-dicarboxaldehyde, and (1,1’-biphenyl)-2,2’-dicarboxylic acid. Seed germination tests showed that the toxicity of the phenanthrene byproducts is lower than the parent compound. Unexpectedly, the ozonation efficiency was markedly improved by increasing the soil moisture content. The relationship between the effect of soil moisture on ozonation efficiency and soil pH value was noticed. Upon combining with the previous studies, it is suggested that the soil moisture is advantageous to the ozonation process for alkaline soil and disadvantageous for acidic soil. Experimental results about two kinds of real soil with different pH values are consistent with the above assumption.
Acknowledgment: Appreciation and acknowledgment are given to the National Natural Science Foundation of China(No. 51508353 and No. 21676027), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality(IDHT20180508).
- 中國煉油與石油化工的其它文章
- Multiphase Flow Simulation of New Vapor Distributor in Dividing Wall Column and Control Mechanism
- Molecular Dynamics Simulation on Mobility and Aggregation of Macromolecular Lubricant Oxidation Products and Their Influences on Base Stock
- Evaluation of Molecular Structural Effects on Needle Coke Mesophase Stacking
- A Simple and Highly-Efficient Approach for Construction of 2D Nanostructured H-BN/WS2 Heterojunction through Hydrothermal Method-Assisted Exfoliation and Their Friction Performance in Grease
- Influence of Two Preparation Methods on Rheological Properties of Lithium Grease
- Effects of Sampling Conditions on Composition and Emission Characteristics of Volatile Organic Compounds in Process Units from Different Refineries

