Efficacy and safety of degarelix in patients with prostate cancer:Results from a phase III study in China
Yingho Sun , Liping Xi , To Xu , J?rn S. Jkosn *,Wiqing Hn , Pr S. S?rnsn Xiofng Wng
a Department of Urology, Changhai Hospital, Second Military Medical University, Shanghai, China
b Department of Urology, First Affiliated Hospital, Zhejiang University, Hangzhou, China
c Department of Urology, Peking University People’s Hospital, Beijing, China
d Global Clinical Research and Development, Ferring Pharmaceuticals A/S, Copenhagen, Denmark
e Department of Urology, Hunan Cancer Hospital, Hunan Province, China
KEYWORDS Degarelix;Goserelin;GnRH antagonist;GnRH agonist;Prostate cancer;China
Abstract Objective: To establish non-inferiority of gonadotropin-releasing hormone degarelix compared with goserelin in suppressing and maintaining castrate testosterone levels from Day 28 to Day 364 in Chinese patients with prostate cancer.Methods: This is an open-label, multi-centre study in which men aged ≥18 years were randomised in a 1:1 ratio to once-a-month subcutaneous injection of either degarelix(240/80 mg)or goserelin (3.6 mg) for 12 months. The primary endpoint was difference in 1-year cumulative probability of suppressing testosterone to ≤0.5 ng/mL. Non-inferiority was to be established if the lower 95%confidence interval(CI)limit for difference in cumulative probability between the treatment arms was greater than -10%. Secondary endpoints included cumulative probability of prostate-specific-antigen-progression-free-survival (PSA-PFS). Safety was also assessed.Results: Baseline demographics and disease characteristics were similar between degarelix(n=142) and goserelin (n=141) treatment arms. The difference in cumulative probability of maintaining castrate levels from Day 28-364 was 3.6% (95% CI:-1.5%, 8.7%), demonstrating non-inferiority of degarelix. The cumulative probability of PSA-PFS at Day 364 was higher for degarelix (82.3%, 95% CI: 74.7%, 87.7%) versus goserelin (71.7%, 95% CI: 63.2%, 78.5%,p=0.038).Adverse events(AEs)were similar between treatment arms,except for more injection site reactions with degarelix versus goserelin.Four(2.8%)and nine(6.4%)patients discontinued due to AEs in degarelix and goserelin groups, respectively.Conclusion: Degarelix was non-inferior to goserelin in achieving and maintaining testosterone suppression at castrate levels during 1-year treatment. PSA-PFS was significantly higher with degarelix, suggesting improved disease control. Both treatments were well tolerated.
1. Introduction
The burden of prostate cancer, which already had an alarming rate in Western countries [1,2], has significantly increased over the past 2 decades in Asia [3]. In the year 2012, a total of 191 054 cases of prostate cancer were reported, accounting for 81 229 deaths in Asia [4]. In China alone, during the period 2009-2011, the estimated prostate cancer incidence and mortality was 60 300 and 26 600,respectively [5].
Testosterone suppression to castrate levels using androgen deprivation therapy (ADT) with gonadotropinreleasing hormone (GnRH) agonist or with the GnRH antagonists is now a common treatment modality for prostate cancer[6].GnRH agonists have a delayed clinical response,and also cause testosterone surges, which may exacerbate the clinical symptoms [7,8]. Though anti-androgens may mitigate the surges,introducing a new drug in combination may lead to more side-effects, as well as added treatment costs [9,10]. GnRH antagonists on the other hand, provide an alternate option of reversible medical castration by causing a rapid and profound testosterone suppression without testosterone surges [9,11].
Degarelix, a third generation GnRH receptor antagonist,is approved in various regions across the globe including the United States, the European Union and Japan, for the treatment of patients with advanced prostate cancer warranting ADT. Previous phase III studies evaluated and demonstrated the clinical efficacy and safety of degarelix in patients with prostate cancer [12-14]. However, these results could not be extrapolated to the Asian population due to the very limited number of patients from Asia included in those studies. This study is part of a continuing confirmation of the efficacy and safety of degarelix in Asia,conducted in the Chinese population.
2. Material and methods
2.1. Patients
Chinese men ≥18 years of age with a histologically confirmed adenocarcinoma of the prostate (all stages),prostate-specific antigen (PSA) level ≥2.0 ng/mL at screening, testosterone level >1.5 ng/mL, and life expectancy of >1 year were included in this study.Key exclusion criteria were previous or current hormonal treatment for prostate cancer (surgical castration or other hormonal manipulation, including GnRH receptor agonists, GnRH receptor antagonists, anti-androgens, estrogens, megestrol acetate, and ketoconazole). However, for patients having undergone prostatectomy, radiotherapy or cryotherapy with curative intention neoadjuvant/adjuvant hormonal therapy for a maximum duration of 6 months was accepted if this treatment had been terminated at least 6 months prior to the screening visit. Other key exclusion criteria were history of any serious or significant health condition,undergoing treatment with 5-alpha reductase inhibitor and/or treatment with any investigational drug within 28 days before enrolling into the study.
2.2. Ethics
The study was approved by the Independent Ethics Committee of People’s Hospital of Peking University (No. 43[2013]) and was conducted in accordance with the Declaration of Helsinki and its amendments, International Council on Harmonisation-Good Clinical Practice Guidelines and in compliance with the approved protocol and applicable regulatory requirements. All patients provided written informed consents before enrolment.
2.3. Study design and settings
This open-label, multi-centre, randomised, parallel-group study was conducted across 25 sites in China from January 2013 to May 2015. Patients were randomised 1:1 to either degarelix or goserelin, stratified by the use of 5-alpha reductase inhibitors in the previous 12 months. Patients received a once-a-month treatment with degarelix or goserelin with 28-day intervals between injections. The total study duration was 364 days. The study design is presented in Fig. 1.
2.4. Study treatments
Degarelix was administered as a deep subcutaneous (s.c.)injection in the abdominal region, at a starting dose of 240 mg (40 mg/mL) at Day 0, followed by 12 monthly (28-day intervals) maintenance doses of 80 mg (20 mg/mL).
Goserelin (Zoladex? 3.6 mg sustained-release depot,supplied as Safe System?) was administered s.c. into the anterior abdominal wall as 12 monthly (28-day intervals)doses. Patients could also receive anti-androgen treatment, bicalutamide 50 mg/day, starting with the first goserelin dose, and for a maximum of 28 days as flare protection, at the discretion of the investigator.
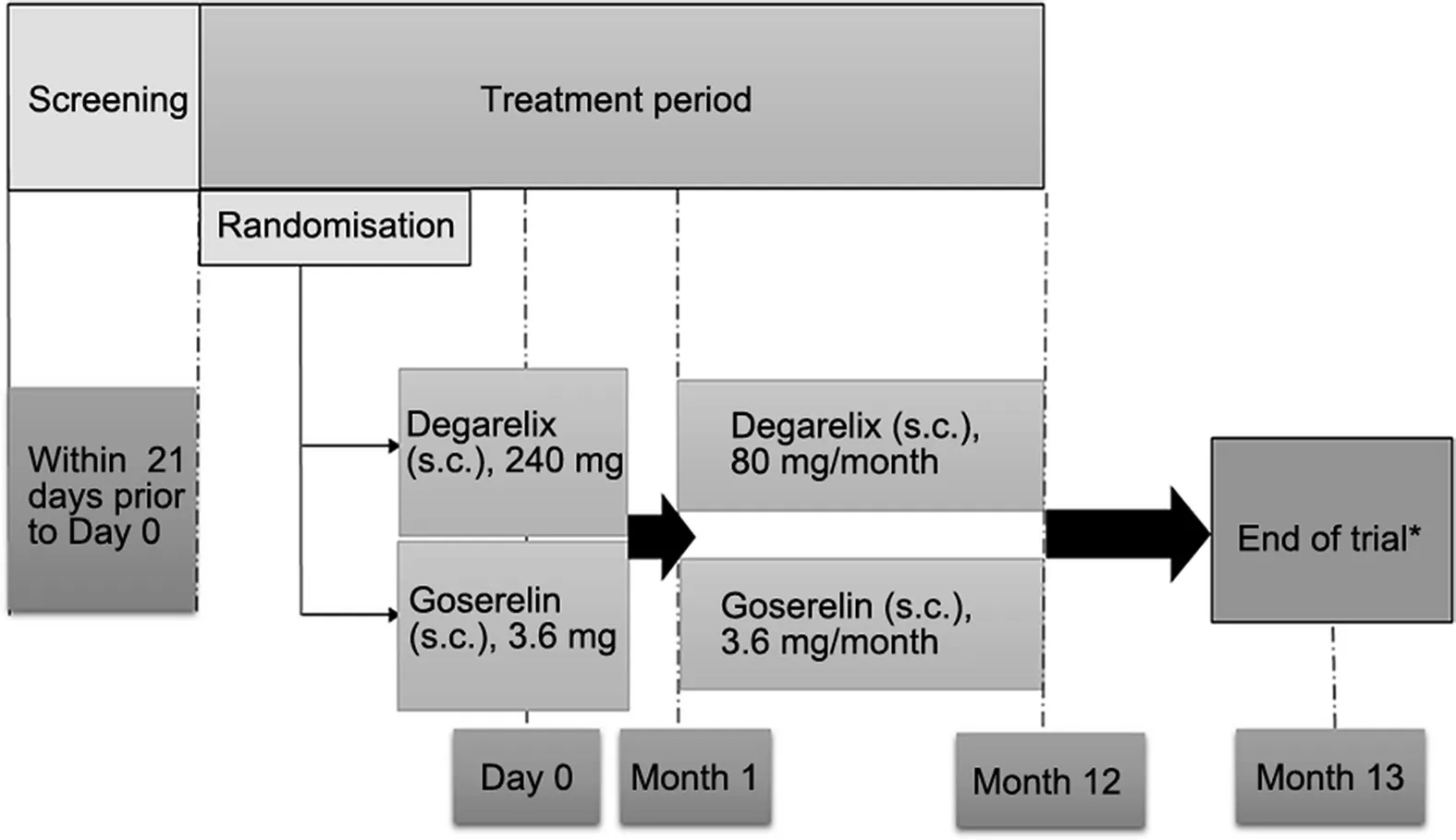
Figure 1 Study design. *End of study visit was conducted after 12 months(364 days of treatment).Discontinued patients were called in for end of study assessments after a decision of discontinuation was taken; s.c, subcutaneous.
2.5. Study endpoints
The primary endpoint of the study was the difference in the cumulative probability of testosterone at castrate level(≤0.5 ng/mL) from Day 28 to Day 364 between patients treated with the degarelix and goserelin.
Secondary endpoints were cumulative probabilities of testosterone at castrate level from Day 56 to Day 364, no PSA failure, PSA-progression-free survival (PSA-PFS), and PFS. PSA failure was defined as two consecutive assessments at least 2 weeks apart with an increase of 50%and at least 5 ng/mL increase compared to nadir. PSA-PFS was defined as PSA failure or death from any cause, whichever is first. PFS was defined as PSA failure, death from any cause, or introduction of additional therapy related to prostate cancer, whichever is first. Also evaluated was proportion of patients with testosterone levels ≤0.5 ng/mL at Day 3, and at each subsequent visit, serum levels of testosterone and PSA over time; and percentage change in PSA from baseline to Day 28. Changes in health-related quality of life (HRQoL) were measured by European Organization for Research and Treatment of Cancer (EORTC)QLQ-C30 and lower urinary tract symptoms (LUTS) were measured by International Prostate Symptom Score (IPSS).
Safety was evaluated by recording adverse events(AEs),and other laboratory parameters. The AEs were presented by Medical Dictionary for Regulatory Activities.
2.6. Study assessments
Blood samples for analysis of testosterone and PSA were collected at baseline (visit 1) followed by 15 visits,including the end of trial visit, separated by 28 days.Blood sampling was done pre-dose at dosing visits preferably in the morning hours (i.e.8:00 am to 11:00 am). Serum testosterone levels were determined using a validated liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS, ICON Laboratory Services [Tianjin] and Covance Pharmaceuticals R&D [Shanghai] Co., Ltd., China)method. Serum PSA levels were determined using a chemiluminescent immunoassay method. The EORTC QLQC30 questionnaire was completed at baseline (Day 0), Day 28, 84, 168 and 364, and contained a total of 30 questions,assessed five functional scales, three symptom scales and six single items. The IPSS questionnaire contained seven questions regarding incomplete emptying, frequency,intermittency, urgency, weak stream, straining, and nocturia.Each question was assigned a score of 0-5.A score of“0”corresponds to a response of“not at all”for the first six symptoms and “none” for nocturia, and a score of “5”corresponds to a response of “almost always” for the first six symptoms and “five times or more” for nocturia. Safety was monitored throughout the study period.
Locally advanced prostate cancer, as per the protocol,was defined as tumour growth beyond the organ/organ capsule, infiltration of surrounding structures and lymph node metastases. Further, to assess metastatic disease patients had a bone scan.
2.7. Statistical methods
The sample size was determined based on the assumption of a 95% 1-year testosterone suppression rate for both degarelix and goserelin, and for a 92% power of the study,120 patients per treatment group were required considering a constant drop-out rate of 20% per annum.
In line with International Council for Harmonisation guideline(E9)on statistical principles for clinical trials[15],the primary (non-inferiority) analysis was performed using both the full analysis set (FAS) and per protocol (PP) analysis set, and both were assessed equally important.
The FAS was defined as the data of all patients included in the intention-to-treat (ITT), who received at least one dose of study drug, and had at least one efficacy assessment. The PP analysis set was defined as the patients included in the FAS without any major protocol deviations.Safety was evaluated in the safety analysis set, and comprised of patients who received at least one dose of study drug.
The 1-year cumulative probability of testosterone levels below castrate level(≤0.5 ng/mL)was estimated using the Kaplan-Meier (KM) method using testosterone measurements every 4 weeks (at Day 28 to Day 364). The standard error (SE) of the mean of this estimate was based on Greenwood’s formula. The two-sided 95% confidence interval(CI)for the suppression probability was based on the log-log transformation, Greenwood’s formula, and asymptotic maximum likelihood theory. The two-sided 95% CI of the difference between degarelix and goserelin in cumulative suppression rate probabilities from Day 28 to Day 364 was constructed using the pooled SEs. If the lower limit of this CI was >-10%, the non-inferiority of degarelix to goserelin was confirmed.However,if the lower limit of this CI was >0%, superiority could have been declared. In case of failure to calculate the CIs and SEs while achieving a 0%or a 100% response rate, CIs were calculated using the Clopper-Pearson interval, (0, 3.69/N) for 0% observed responders, and (1-3.69/N, 1) for 100% responders, where N is the number of completers and 3.69=-lnα/2 with α=0.05. When estimating the SE of the KM estimate in the case of a 0% or a 100% response, the SE of the mean of the KM estimate was set to1/2×3/N,where 3/N corresponds to the one-sided 95% Clopper-Pearson CI with a 0% or 100%response.
The median percentage change from baseline to Day 28 in PSA was presented for both treatment groups, and comparisons between the treatment groups were made using the Wilcoxon test (α=0.05, two-sided). Cumulative probabilities of no PSA failure, PSA-PFS and PFS were also estimated using the KM method.
Categorical data were summarised as counts and percentages, while descriptive statistics were presented for continuous data; the data were tabulated by treatment group and visit. For laboratory efficacy parameters(testosterone and PSA) with reported values below the lower limit of quantification(LLOQ),a value of1/2LLOQ was used in the calculations. Drop-outs were accounted for by the KM approach,as censored observations.Drop-outs were censored at the time of their last testosterone assessment;missing values after Day 28 were imputed as ≤0.5 ng/mL provided all other testosterone assessment were less than 0.5 ng/mL, including missing values at Day 364. If one or both values before and after the missing value was greater than 0.5 ng/mL, the patient was considered an endpoint failure at the first assessment above 0.5 ng/mL.
For the secondary endpoints related to PSA, there was an additional analyses according to whether or not the patient was previously treated with a 5-alpha reductase inhibitor. The KM analysis of cumulative probability of no PSA failure was performed for the subgroups defined by the previous inhibitor use.
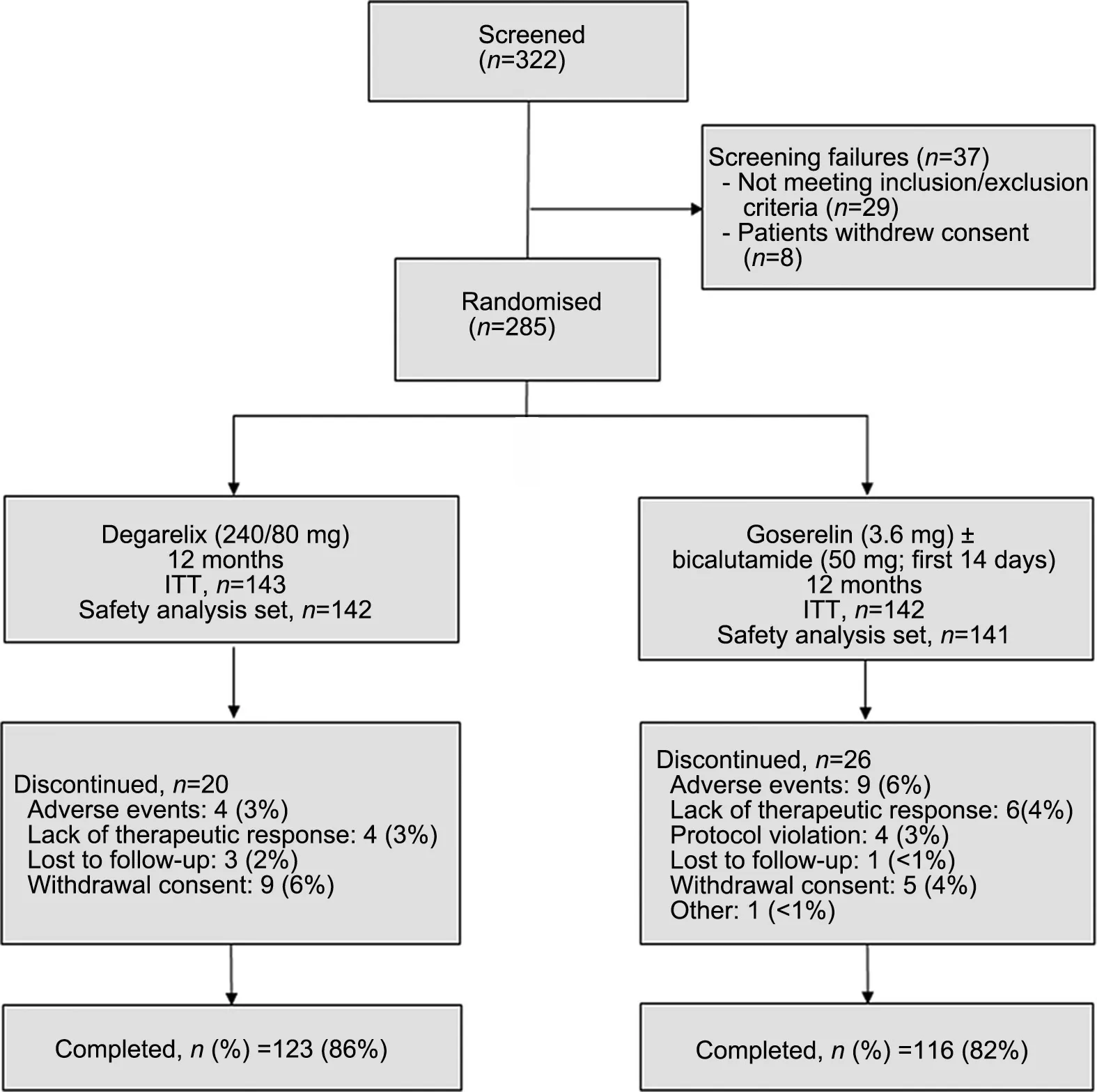
Figure 2 Patient disposition. ITT, intention-to-treat analysis set.
3. Results
Of the 322 patients screened, 285 patients were randomised(143:Degarelix;142:Goserelin,ITT analysis set),and 239 patients completed the study (123: Degarelix; 116:Goserelin). The most common reasons for discontinuation were AEs, consent withdrawal, and lack of therapeutic response (Fig. 2). The median age of the patients was 74 years(range:47-91 years).The two treatment groups were comparable for the baseline and disease characteristics(Table 1).
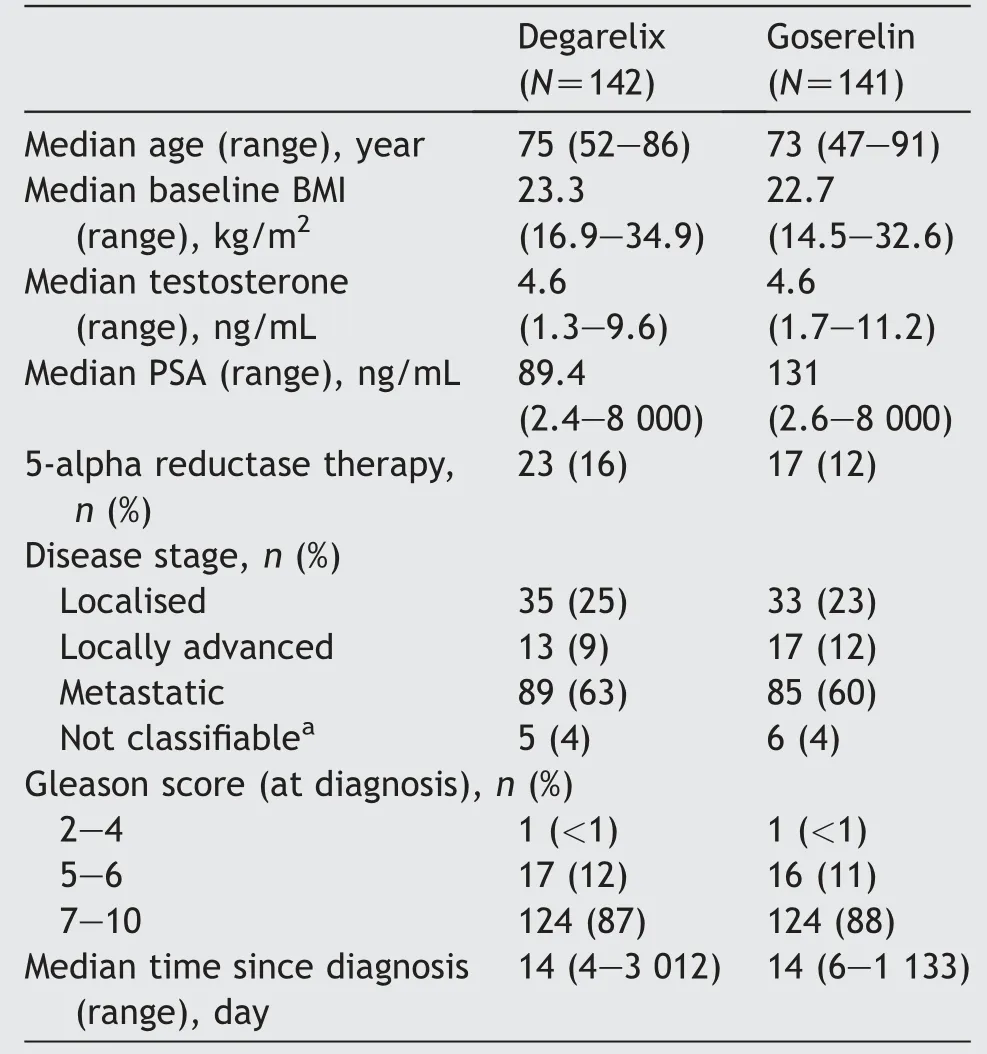
Table 1 Baseline characteristics-full analysis set.
3.1. Efficacy
Degarelix was non-inferior to goserelin in achieving and maintaining serum testosterone suppression at castrate levels from Day 28 to Day 364 (Fig. 3). The difference between the two treatment groups was 3.6% (95% CI: -1.5%,8.7%), and the lower limit of the CI (for the difference in probability) was higher than the pre-defined threshold of >-10%. The individual cumulative probabilities of maintaining castrate testosterone levels over a period of 1 year was 97.0% (95% CI: 92.3%, 98.9%) for degarelix and 93.4% (95% CI: 87.7%, 96.5%) for goserelin. The sensitivity analysis in patients who did not receive a previous treatment with 5-alpha reductase inhibitor demonstrated noninferiority of degarelix to goserelin. However, in the subgroup previously treated with 5-alpha reductase inhibitor,the point estimate of the difference in suppression rates was-4.5%,but due to the low number of patients(n=40),non-inferiority could not be demonstrated.The cumulative probability of achieving and maintaining serum testosterone suppression at castrate levels from Day 56 to Day 364 was also comparable between degarelix and goserelin(97.0% [95% CI: 92.3%, 98.9%] and 95.5% [95% CI: 90.2%,97.9%], respectively).
Testosterone levels were rapidly suppressed to castrate levels with degarelix (0.25 ng/mL) at Day 3 compared with goserelin (p<0.0001), and 96% of patients achieved castrate levels of testosterone in the degarelix group compared to none in the goserelin group (p<0.0001). The time concentration curve of testosterone with respect to degarelix and goserelin is presented in Fig. 4. In the goserelin group, there was a 53% increase in the testosterone levels from the baseline to Day 3 (4.58 ng/mL and 7.31 ng/mL). After Day 3, the proportion of patients achieving testosterone castrate levels was similar in both groups, though median testosterone levels were higher in the goserelin group as compared with the degarelix group(0.05 ng/mL [range, 0.05-0.38 ng/mL] and 0.112 ng/mL[range, 0.05-9.92 ng/mL], respectively). Median levels of testosterone remained suppressed for both degarelix and goserelin groups until the end of the study on Day 364.Similar results were observed in the PP analysis set.
Treatment with degarelix resulted in a rapid and more profound PSA suppression from baseline to Day 3 versus goserelin group (22.20% versus 8.65% reduction in PSA).However, by Day 28, the treatment groups had a 91%reduction from baseline in PSA levels, which continued to be similar throughout the treatment period in the two treatment groups (Fig. 5). A previous treatment/no treatment with 5-alpha reductase inhibitor did not impact the PSA reduction.The cumulative probability of no PSA failure from Day 0 to Day 364 for the degarelix and the goserelin group was 82.8% (95% CI: 75.2%, 88.2%) and 73.4% (95% CI:64.9%, 80.1%), respectively. Subgroup analysis showed that PSA failure occurred more frequently in patients with a high PSA level at baseline, and those with metastatic disease.On the other hand,patients previously treated with 5-alpha reductase inhibitor had a lower PSA failure rate. The cumulative probability of PSA-PFS at Day 364 was significantly higher for degarelix as compared with goserelin(p=0.038).Furthermore, the cumulative probability of PFS showed a favourable trend for degarelix in terms of disease control(Table 2).
The improvement in HRQoL was comparable between the two treatment groups. Further, the results of the IPSS questionnaire demonstrated a relief in urinary symptoms at all visits as compared to the baseline in both treatment groups. The mean changes in IPSS score from baseline to Day 364 were -5.92 and -5.24 in the degarelix group and the goserelin group, respectively. The mean changes from baseline to Day 364 in IPSS-QoL scores were -1.17 and-1.35 in the degarelix and the goserelin group,respectively.
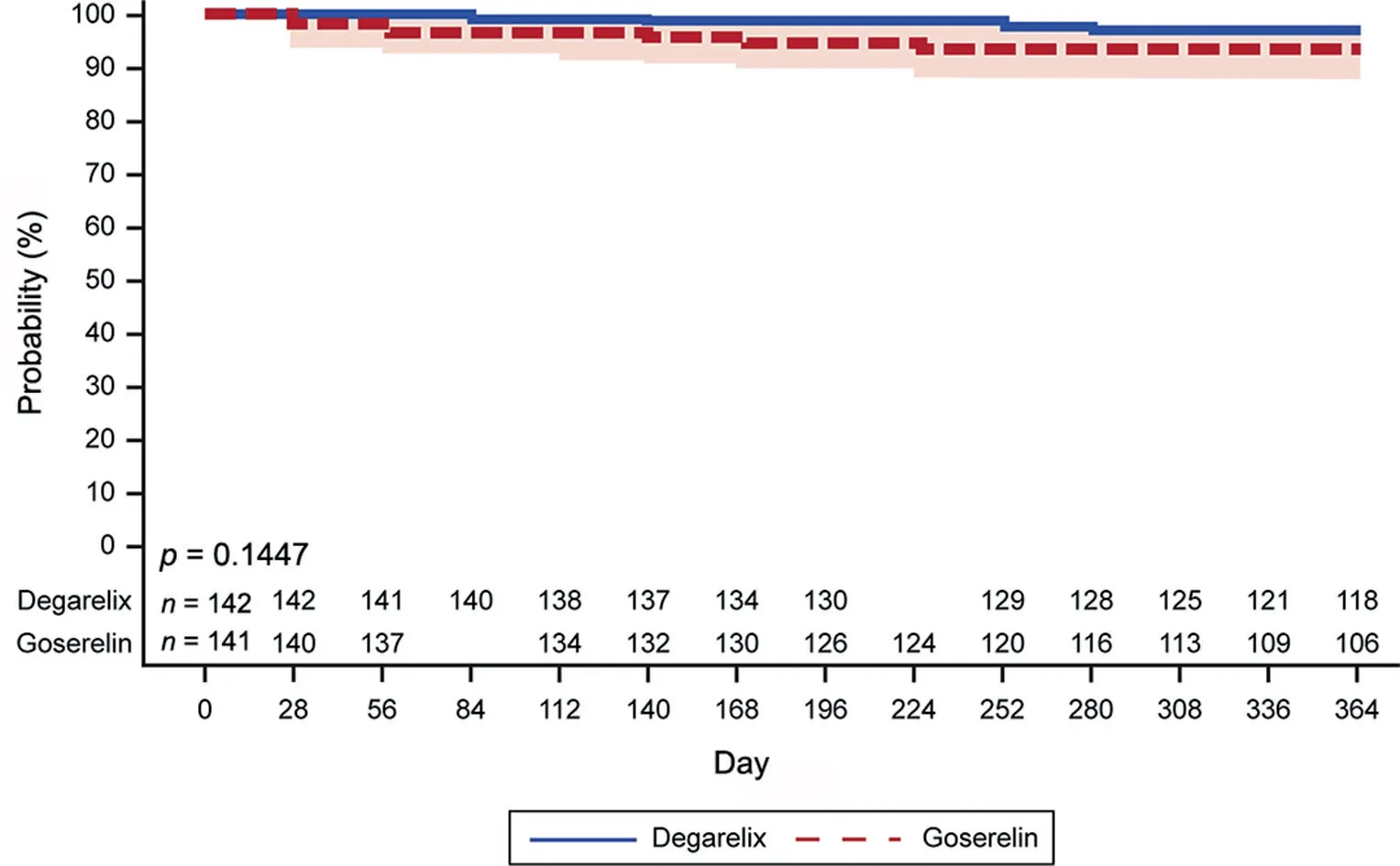
Figure 3 Cumulative probability of testosterone at castrate level (≤0.5 ng/mL) from Day 28 to Day 364.

Figure 4 Time concentration curve of testosterone: Median values (interquartile range).
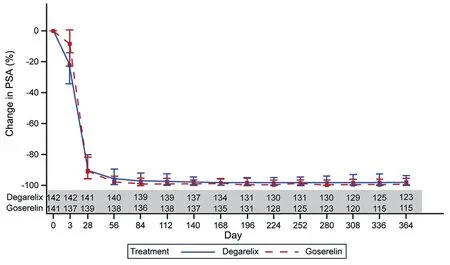
Figure 5 Percentage change from baseline in PSA: Median values (interquartile range). PSA, prostate-specific antigen.
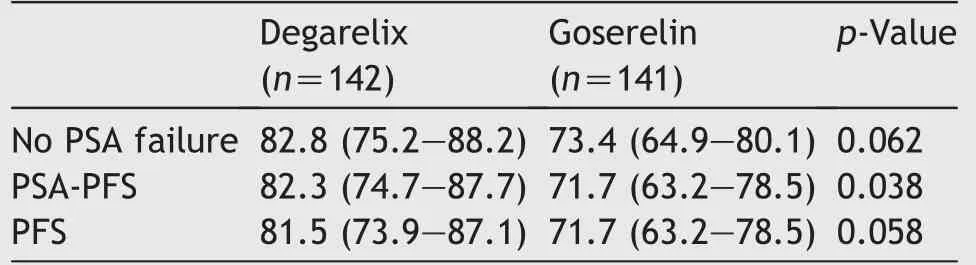
Table 2 Estimate of disease progression at Day 364-full analysis set.
3.2. Safety
Most of the AEs were mild to moderate in intensity. The incidence of treatment-emergent AEs was higher with degarelix than with goserelin (76.1% and 58.9%, respectively) (Table 3). Predominantly general disorders and administration site conditions were reported in 52.1%of the degarelix patients. Most injection site reactions (35.0%)occurred after the first dose of degarelix and 29%following the other dosing intervals (Table 3). There were 13 discontinuations due to an AE(four in the degarelix and nine in the goserelin group),however,none of these were assessed as treatment-related and none of the discontinuations were due to injection site reactions.
No marked trends were noted in data stratified by baseline PSA category and previous use of 5-alpha reductase inhibitors. Patients with metastatic disease reported higher incidence of severe AEs in the goserelin group as compared to the degarelix group (14.1% versus 6.7%,respectively).
There were 14 serious AEs(SAEs)reported by 12 patients(8.5%) treated with degarelix and 27 SAEs reported by 18(12.8%) patients treated with goserelin. The most common SAEs were cardiac disorders,which occurred in five patients(3.5%)in the degarelix group and two patients(1.4%)in the goserelin group.Two patients each in the degarelix and the goserelin group had SAEs assessed as treatment-related(acute kidney injury and lung infection possibly related to degarelix and femur fracture and haematuria possibly related to goserelin). Two patients (1.4%) receiving degarelix had increased PSA levels that were reported as SAEs,of which one SAE led to withdrawal of the patient from the study. There were no other notable AEs.
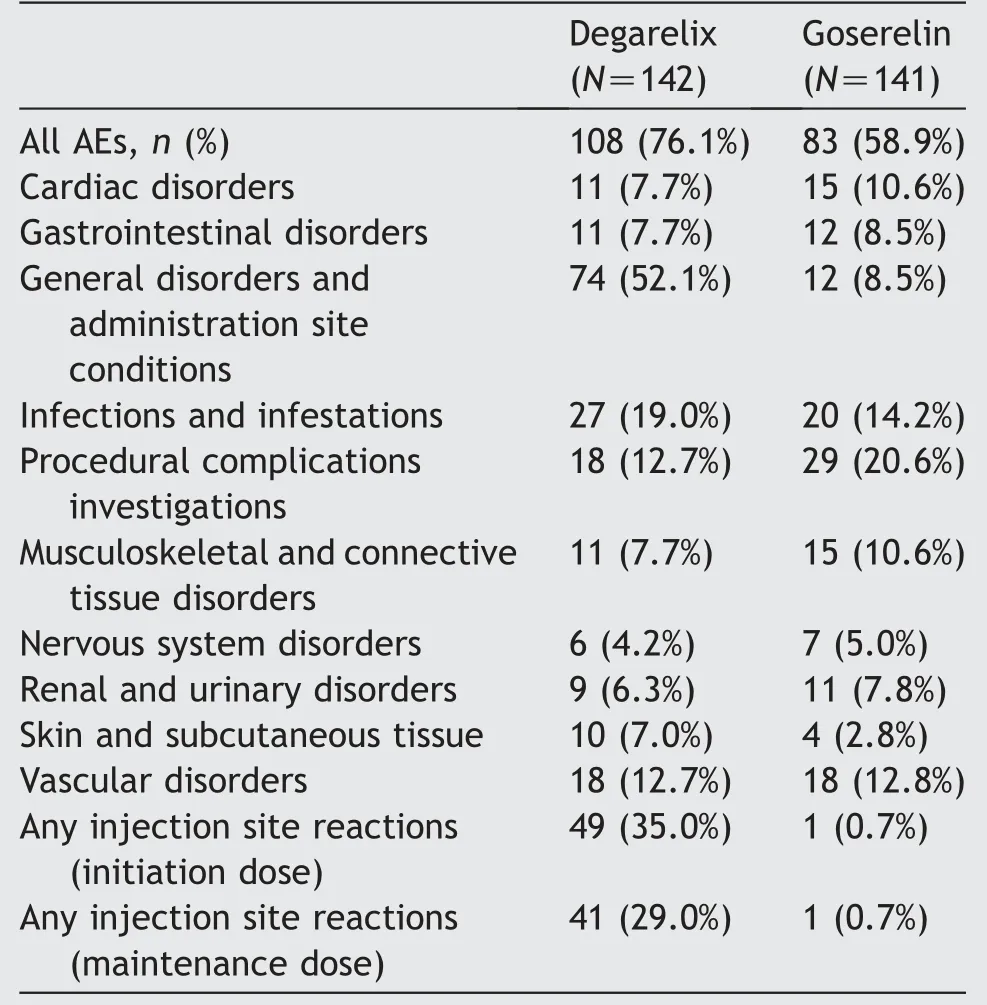
Table 3 Treatment-emergent adverse events-safety analysis set (5% in either group).
4. Discussion
The data from the current study demonstrated that degarelix is non-inferior to goserelin in achieving and maintaining serum testosterone suppression at castrate levels over a period of 1 year in Chinese patients with prostate cancer.This reinstates the non-inferior efficacy of degarelix versus goserelin with degarelix eliciting a rapid and sustained testosterone suppression without testosterone surge.
The main objective of any ADT is to rapidly suppress testosterone to castrate levels as elevated testosterone levels are associated with an increased risk of progression of prostate cancer [16]. Importantly, serum testosterone levels as low as 0.25 ng/mL indicate a good prognosis in patients with prostate cancer receiving ADT [17]. The current study demonstrated that degarelix was non-inferior to goserelin in achieving and maintaining castrate levels of testosterone, where >95% patients achieved testosterone levels <0.5 ng/mL as early as Day 3 with degarelix treatment, which is in-line with the mechanism of action of degarelix. On the contrary, in the goserelin group, there was a testosterone surge at Day 3. This has a clinical significance as a testosterone surge causes a transitory exacerbation of clinical symptoms or flares [18]. However,patients could receive anti-androgen treatment for flare protection, starting with the first goserelin dose, for a maximum of 28 days, if needed, which was >50% in the goserelin group in this study. The current study also demonstrated that 97% of the patients in the degarelix group achieved and maintained testosterone castrate levels for 364 days in the study, where >60% of the patients had metastatic disease. Similar results were observed in a previous pivotal study in a non-Asian population, where only 20% patients had a metastatic disease [12]. This substantiates the efficacy of degarelix in patients with metastatic disease. PSA suppression is a surrogate marker for disease monitoring and predicting PFS in patients with prostate cancer [19]. Failure to reach PSA nadir of≤4.0 ng/mL in patients with metastatic disease 7 months after initiation of therapy has been associated with poor prognosis with a median survival of 1 year [20]. Furthermore, it has been observed that despite testosterone suppression, some of the patients undergoing ADT will eventually progress to castration resistant prostate cancer[21].As noted in this study,biochemical evidence of clinical improvement was demonstrated with a similar and quick suppression in PSA levels in both treatment groups with a median 90% reduction from baseline at Day 28 and further median decreases of 97% and 98% at Days 84 and Day 364,respectively. The overall PSA suppression in the current study was similar to that observed in the non-Asian patients in the CS21 study[12,22].Furthermore,a subgroup analysis based on baseline PSA levels and disease stage showed that PSA failure was more frequent in patients with baseline PSA>50 ng/mL, and those with metastatic disease. This was expected as patients with advanced prostate cancer and with a high baseline PSA level are more likely to have clinical progression of prostate cancer [19]. Importantly,the study results demonstrated supportive evidence of disease control in favour of degarelix as PSA-PFS for degarelix was statistically significant as compared with goserelin. Moreover, a favourable trend for degarelix over goserelin was observed in terms of PSA suppression. The events attributed to death, or overall disease progression were few, explaining the similar favourable trend for degarelix for PSA-PFS and PFS.
Patients with prostate cancer often report a poor HRQoL due to LUTS [25], and addressing the same has its own clinical significance. The current study showed that both treatments similarly helped in alleviating LUTS. Similar results were noted in a previous study where an improvement in EORTC-QLQ scores and thereby improved HRQoL was observed following treatment with degarelix [23].
One year treatment with degarelix and goserelin was anticipated to have adverse reactions associated with testosterone suppression such as hot flush, loss of libido,impotence, and increased sweating [10,24]. However, very low incidence of AEs related to sexual dysfunction or sweating were reported,with most of the AEs being mild or moderate in severity and were comparable between the two groups. The injection site reactions were the most commonly reported AEs in the degarelix group. Similar to the previous study with degarelix [12], injection site reactions were transient and slowly decreased over time(initiation dose to maintenance dose, a trend consistent with once-monthly regimen of degarelix) and did not lead to any discontinuations in the study. Hypersensitivity reactions due to a histaminergic reaction as noted in a previous study with the GnRH antagonist abarelix[25]were not reported in the current study,suggesting degarelix has only weak histamine releasing properties. Overall, the safety and tolerability profile of the degarelix was in-line with previous studies including that for the cardiovascular events[12,26].The current study had one major limitation,i.e.an open-label design. However,it should also be noted that this was unavoidable due to different dosing regimens and formulations for the two treatments. Since this was a known factor that could bias the results, central randomisation was used to minimise selection bias. Importantly,the data for the objective primary endpoint,i.e.serum testosterone levels,were kept blinded for the sponsor.The strength of this study was the stratified randomisation of the patients by PSA category and previous treatment with 5-alpha reductase inhibitors. This allowed the investigator to evaluate efficacy of degarelix irrespective of previous treatment or disease state because it was anticipated that many of the patients would be receiving 5-alpha reductase inhibitors within the past year.
5. Conclusio n
The results from this randomised open-label comparative study demonstrated that degarelix once-a-month dosing regimen was efficacious and non-inferior to once-a-month goserelin in achieving and maintaining testosterone suppression at castrate levels with an acceptable safety profile in Chinese patients with prostate cancer.Also,PSA-PFS,the endpoint for disease progression,was significantly higher in the degarelix group which suggests an improved disease control. However, sufficiently powered prospective randomised trial is required to demonstrate if these results translates into survival benefit.
Author contributions
Study concept and design:Yinghao Sun, Liping Xie, Tao Xu,J?rn S. Jakobsen, Weiqing Han, Xiaofeng Wang.
Data acquisition: Yinghao Sun, Liping Xie, Tao Xu, J?rn S.
Jakobsen, Weiqing Han, Per S. S?rensen, Xiaofeng Wang.
Data analysis: Yinghao Sun, Liping Xie, Tao Xu, J?rn S.
Jakobsen, Weiqing Han, Per S. S?rensen, Xiaofeng Wang.
Drafting of manuscript: Yinghao Sun, Liping Xie, Tao Xu,J?rn S. Jakobsen, Weiqing Han, Per S. S?rensen, Xiaofeng Wang.
Critical revision of the manuscript:Yinghao Sun,Liping Xie,Tao Xu, J?rn S. Jakobsen, Weiqing Han, Per S. S?rensen,Xiaofeng Wang.
Statistical analysis: Per S. S?rensen.
Conflicts of interest
Yinghao Sun, Tao Xu and Xiaofeng Wang has no conflict of interest to declare. Liping Xie has serve as a consultant to Ferring.J?rn S.Jakobsen and Per S.S?rensen currently are the employees of Ferring Pharmaceuticals. This trial was sponsored by Ferring Pharmaceuticals. Medical writing support was provided by Mohit Joshi and Sakshi Jindal(Tata Consultancy Services), funded by Ferring.
 Asian Journal of Urology2020年3期
Asian Journal of Urology2020年3期
- Asian Journal of Urology的其它文章
- Androgen receptor: Functional roles and facets of regulation in urology
- Mesenteric metastases from mature teratoma of the testis: A case report
- Intractable hematuria due to giant prostatic hyperplasia effectively treated with prostatic artery embolization
- Sheathless and fluoroscopy-free retrograde intrarenal surgery: An attractive way of renal stone management in high-volume stone centers
- Survival after radical cystectomy for bladder cancer: Multicenter comparison between minimally invasive and open approaches
- Androgen receptor in bladder cancer: A promising therapeutic target
