C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization
Huai-Chun Yang, Min Zhang, Rui Wu, Hai-Qing Zheng, Li-Ying Zhang, Jing Luo, Li-Li Li, Xi-Quan Hu
Huai-Chun Yang, Rui Wu, Hai-Qing Zheng, Li-Ying Zhang, Jing Luo, Li-Li Li, Xi-Quan Hu,Department of Rehabilitation Medicine, the Third Affiliated Hospital, Sun Yat-sen University,Guangzhou 510000, Guangdong Province, China
Min Zhang, Department of Andrology, the First Affiliated Hospital, Sun Yat-sen University,Guangzhou 510000, Guangdong Province, China
Abstract
Key words: Cognitive impairment; Stroke; Exosomes; C-C chemokine receptor type 2;Microglia/macrophage polarization; Remyelination
INTRODUCTION
Post-stroke cognitive impairment (PSCI) occurs frequently after stroke. The prevalence of PSCI in ischemic stroke patients ranges from 25% to 30%[1], which has been increasing gradually due to the development of modern medicine and the increasing survival rate of stroke patients[2,3]. PSCI imposes a heavy burden on the patients, their families, and societies. However, the treatment of PSCI is still not satisfactory and requires further improvement.
Previous research has shown that mesenchymal stem cell (MSC) therapy faciliates the cognitive recovery after stroke[4,5]. However, the disadvantages of therapies involving MSCs, such as their highin vivoclearance rate after transplantion[6,7], limited capacity to cross blood-brain barrier[8,9], potential immunogenicity[10,11], and unpredictability of cell growth and differentiation[12], have emerged with the development of research. Recent studies have indicated that MSCs mostly act via specific paracrine mechanisms, while exosomes play a key role in the general progress and recovery under conditions of disease[13]. MSC-derived exosomes have displayed positive effects in animal models of various ischemic injuries such as stroke[14],myocardial infarction[15], and renal ischemic injury[16]. To a certain extent, MSC-derived exosomes exert therapeutic effects comparable to those of MSCs and overcome the potential risks and disadvantages associated with MSCs[17,18]. However, there are only a few studies focusing on exosome-based treatments for PSCI.
CC chemokine ligand 2 (CCL2) is highly expressed in the ischemic hemisphere after a stroke; this mediates the migration of C-C chemokine receptor type 2 (CCR2)-positive blood-derived macrophages, thus exacerbating brain tissue damage[19,20].CCR2 knockout mice[21]or CCL2 knockout[22]mice have shown a significant reduction of macrophage proliferation within 2 wk after a stroke, accompanied by neuronal regeneration and decreased infarct volume, suggesting that inhibition of the CCL2/CCR2 axis may play a neuroprotective role after strokes. In addition, CCR2 antagonism[23]or CCR2 knockout[24]can promote the M2 polarization of microglia/macrophages by inhibiting CCR2+ macrophages and improve cognitive impairment in mice with traumatic brain injury.
It is noticeable that in recent years, exosomes secreted by human umbilical cord MSCs (HUC-MSCs) have shown powerful effects on microglia/macrophage activation and polarization in animal models such as the Alzheimer's disease model[25], hypoxic-ischemic encephalopathy model[26], and the peripheral nerve injury model[27]. However, the effects of HUC-MSC-derived exosomes (ExoCtrl) on microglia/macrophage polarization and cognitive function after stroke have not yet been reported. Furthermore, we hypothesize that CCR2-overexpressing HUC-MSCderived exosomes (ExoCCR2) further promote microglia/macrophage M2 polarization by competitively binding to the CCR2 ligand CCL2 and inhibiting the CCL2-mediated infiltration of blood-derived mononuclear macrophages. Particularly, we compared the therapeutic effects of the systemic administration of ExoCCR2and ExoCtrlon PSCI,which will provide new insights into genetically modified exosome-based therapies for PSCI treatment and serve as a preclinical study on cerebral protection after stroke.
MATERIALS AND METHODS
Establishment of the tMCAO model and animals grouping
Adult Sprague-Dawley rats (male, weighing 280-350 g) were underwent the right transient middle cerebral occlusion (tMCAO) for 2 h in accordance with the method as Longaet al[28]described with modifications. Experimental procedures were approved by the Institutional Animal Ethics Committee of Life Sciences School, Sun Yat-sen University. The modified neurological severity score (mNSS) and 2,3,5-Triphenyltetrazolium chloride (TTC) (G3005, Solarbio, China) staining were utilized to confirm the establishment of the tMCAO model. Rats with moderate injury (mNSS values 7-12) were randomly divided into the sham group, tMCAO group, ExoCtrltreatment group, and ExoCCR2treatment group. As described in a previous study, 100μg of the exosomes was dissolved in 500 μL of phosphate-buffered saline (PBS)[29]. One day after operation, the rats from sham and tMCAO groups were injected with 500 μL of PBS, the rats in the ExoCtrland ExoCCR2treatment groups were injected with equal volumes of the respective exosomal solutionsviatail vein injections. BrdU (50 mg/kg/d; B5002, Sigma, United States) was injected intraperitoneally for 14 continuous d one day after the induction of tMCAO.
Transfection of HUC-MSCs with lentiviral vectors and comparison of their biological characteristics
HUC-MSCs were obtained from three healthy donors after they signed the informed consent forms. Briefly, the Wharton gum tissues with blood vessels removed were cut up and digested with collagenase II (1 mg/mL, 234155, Millipore) under 37 °C for 30 min with shaking. The cells were filtered from the suspensions with a cell strainer(diameter 70 μm). The cells were washed with Hank's Balanced Salt Solution(SH30031.02, Hyclone) and cultured in low-glucose DMEM (L-DMEM) (C11885500BT,Gibco) containing 10% fetal bovine serum (04-001-1A, Biological Industries, Israel) in a 5% CO2 incubator.
At passage 3, the HUC-MSCs were transfected with lentiviral vectors expressing both theCCR2andeGFPgenes, and vectors expressing theeGFPgene in accordance with the manufacturer’s instructions. The vector construction is indicated in Supplement Figure 1. Three days after the transfection, the HUC-MSCs transfected with lentiviral vectors encoding CCR2 (namely HUC-MSCsCCR2) or eGFP (namely HUCMSCsCtrl), were sorted using fluorescence-activated cell sorting (Influx, Becton Dickinson). The HUC-MSCsCtrland HUC-MSCsCCR2(passage 6) were identified by microscopic analysis, flow cytometry analysis for detecting the following surface markers: CD13-APC (1:50, 17-0138-41, eBioscience, United States), CD29-APC (1:50,559883, BD Bioscience, United States), CD44-APC (1:50, 559942, BD Bioscience, United States), CD34-PE (1:50, 550761, BD Bioscience, United States), CD45-PE (1:50, 560975,BD Bioscience, United States), CD73-PE (1:50, 60044, Stemcell Technologies, Canada),CD90-PE-Cy7 (1:50, 561558, BD Bioscience, United States), CD105-PerCP-Cy5.5 (1:50,560819, BD Bioscience, USA), HLA-DR-V500 (1:50, 561225, BD Bioscience, United States). Osteogenesis and lipogenesis induction experiments were conducted with modification as described in a previous study[30]. Briefly, for osteogenisis induction experiments, cells were cultured in L-DMEM containing fetal bovine serum (20%),ascorbic acid (100 μg/mL), β-glycerophosphate (10 mmol) and dexamethasone (100 nmol) for three weeks with medium changed every 3 d. For adipogenesis induction experiments, the cells were induced in L-DMEM supplemented with FBS (10%),dexamethasone (100 nmol), indomethacin (0.2 mmol), insulin (10 μg/mL), 3-isobutyl-1-methylxanthine (0.5 mmol). After 3 wk, Osteogenic and adipogenic differentiation were confirmed by oil red O staining and alizarin red staining.
Exosome isolation and identification
The isolation of exosome was performed according to a previous study[31]. Briefly, the exosomes were collected by differential ultracentrifugation, and their morphology was analyzed by transmission electron microscopy. The distribution of the exosomes based on their diameters was performed using a qNano?system (Izon Science,Oxford, United Kingdom). Western blotting was used to detect the CCR2 expression and the exosome-specific markers CD9, CD63, and CD81.
Enzyme-linked immunosorbent assay (ELISA)
To test the CCL2-binding capacity of the exosomes, ExoCtrland ExoCCR2were coincubated with recombinant rat CCL2 (100 ng/well, 400-12, PeproTech, United States). Differential ultracentrifugation was performed to obtain exosome-free supernatants. ELISA kits (CSB-E07429r, Cusabio Biotech, China) were utilized to detect the CCL2-binding capacity of ExoCCR2and ExoCtrl, according to the protocol of manufacturer.
Cognitive function test
The Morris water maze test was conducted as our previous study described[32]. The test was carried out at 23 d after the induction of tMCAO. The rats were first subjected to five consecutive days of the place navigation test. On day 6, a spatial probe test (60 s) was performed under the same condition without platform. During the test, the latency to the platform and the time recorded in the target quadrants were analysed.The mNSS values were recorded at 1, 4, 14, and 28 d after exosome treatment, as described previously[33]. The rats were tested by an individual blinded to the grouping for three times, and the means of the mNSS results were recorded. The normal score is 0, while the maximal deficit score is 18. Rats with mNSS values ranging from 7-12 were included in the study.
Western blotting
Western blotting was conducted in accordance with the protocol as our previous study described[34]. First, the proteins were obtained from the ischemic cerebral hemisphere or cultured cells by treatment with the kit of protein extraction (KeyGen BioTech, China) according to the protocol of manufacturer. The protein samples were loaded onto 10% polyacrylamide gels and electrophoresed under 120V voltage; the resultant bands were transferred onto polyvinylidene difluoride membranes. Next,the polyvinylidene difluoride membranes were incubated with rabbit anti-CD9(1:2000, ab92726, Abcam, United Kingdom), rabbit anti-CD63 (1:10000, 25682-1-AP,ProteinTech, United States), rabbit anti-CD81 (1:1000, ab109201, Abcam, United States), rabbit anti-CCR2 (1:1000, DF2711, Affinity Biosciences, United States), rabbit anti-CCL2 (1:1000, ab25124, Abcam, United States), mouse anti-iba-1 (1:500, MABN92,Millipore, United States), rabbit anti-NF-κB (1:1000, ab16502, Abcam, United States),mouse anti-CD68 (1:1000, ab201340, Abcam, United States), rabbit anti-myelin basic protein (anti-MBP) (1:200, ab40390, Abcam, United States), and rabbit anti-β-actin(1:1000, #3700, Cell Signaling Technology, United States) antibodies at 4 °C overnight,and then with peroxidase-conjugated secondary antibodies at 37 °C for 1 h. The protein bands were developed using a specific chromogenic substrate (ECL, KeyGen BioTech, China), according to the manufacturer’s instructions.
RN A isolation, reverse transcription, and real-time PCR
Total RNA from the ischemic cerebral hemispheres or cultured cells was extracted by TRIzol (Invitrogen, United States), according to the protocol of manufacturer. Reverse transcription for synthesizing the cDNA was performed using the PrimeScript? RT Master Mix (Takara, Japan), according to the manufacturer's instructions. The resulting cDNA was then subjected to quantitative real-time PCR for the evaluation of the relative mRNA levels. The real-time PCR amplifications were performed with a final reaction volume of 20 μL using the TB Green? Premix Ex Taq? II kit (Takara,Japan), according to the manufacturer's instructions. The reaction mixtures were preheated at 95°C (30 s) for one cycle and then amplified at 95°C (5 s) and 60°C (34 s)for 40 cycles. The Ct (threshold cycle) value of each sample was analyzed by the 2-Ctmethod, and the mRNA expression levels of the target genes were normalized to the expression level of β-actin to obtain the relative expression levels. The sequences of the used primers are as follows (Table 1).
Immunofluorescence
Frozen sections for immunofluorescence staining were prepared as described in our previous study[34]. First, the frozen sections were treated for 5 min with hot EDTAcitrate buffer (95 °C) (P0085, Beyotime Biotechnology, China) for antigen retrieval,followed by treatment with a blocking reagent (Beyotime Biotechnology, China) for 1 h at 25 °C. Then, the sections were incubated with mouse anti-iba1 (1:200, MABN92,Millipore, United States), rabbit anti-CD206 (1:200, ab64693, Abcam, United States),rabbit anti-CD16 (1:100, ab211151, Abcam, United States), and rabbit anti-MBP (1:200,ab40390, Abcam, United States) antibodies overnight at 4 °C. The sections were rinsed in PBS for 5 min each for three times, and were then incubated with goat anti-mouse secondary antibodies and goat anti-rabbit secondary antibodies for 1 h at 25 °C.Fluorescence signals were detected using a confocal laser scanning microscope(Dragonfly, Oxford Instruments, United Kingdom). For Brdu/NG2 double immunostaining, rabbit anti-NG2 (1:200, AB5320, Millipore, United States) and rat anti-BrdU (1:200, ab6326, Abcam, United Kingdom) antibodies were used according the protocol described in our previous study[32].
Transwell assays
The transwell assay was performed for examining the migration of mouse macrophages (raw 264.7 cells, CC9001, CELLCOOK, China), according to a previous study[35]. The macrophage suspension (106/mL, 100 μL) was transferred into the upper transwell chamber (pore size of 8 μm; Corning, United States). Cells from the CCL2 control, CCL2 + ExoCtrland CCL2 + ExoCCR2groups, which were subjected to different treatments, were added into the lower transwell chamber. After co-incubation for 16 h at 37 °C, the macrophages remained in the upper transwell chamber were scraped.The membranes were fixed using 4% paraformaldehyde and stained with DAPI(F6057, Sigma, United States). The macrophages that remained in the lower chamber were observed using a fluorescence microscope (Leica DM6B, Germany).
Statistical Analysis
The results were expressed as the mean ± standard error of mean (SEM). SPSS22.0 for Windows was applied for the statistical analysis. One-way Analysis of Variance(ANOVA), followed by Least Significant Difference (LSD)-ttest procedure or Student’sTtest, was applied for comparing the statistical differences.P< 0.05 was statistically significant.
RESULTS
CCR2-overexpressing HUC-MSCs load the CCR2 receptor into their exosomes
Cultured human MSCs express extremely low levels of the CCR2 receptor during continuous passage[30]. This result was consistent with that of the study by Huanget al[30], as indicated by flow cytometry, Western blotting, and quantitative real-time PCR(qRT-PCR) analyses, which indicated that the HUC-MSCsCtrl(passage 6), following the fluorescent-activated cell sorting analysis, showed a low CCR2 protein and mRNA expression. Moreover, the CCR2 protein and mRNA expression in HUC-MSCsCCR2increased significantly (Figure 1A-1D). Since HUC-MSCs are characterized by specific surface markers such as CD13, CD29, CD44, CD34, CD45, CD73, CD90, CD105, HLADR[36,37], and the osteogenesis and lipogenesis capacity[38], we checked the biological characteristics changes by flow cytometry analysis, and osteogenesis and lipogenesis induction experiments. The results showed CCR2 overexpression had no significant effects on the biological characteristics of the HUC-MSCs (Supplementary Figure 2).The morphology and diameter distribution of ExoCtrland ExoCCR2were confirmed using transmission electron microscopy and the qNano?system (Izon Science, Oxford,United Kingdom), respectively; there was no significant difference between the ExoCtrland ExoCCR2(Figure 1E, 1F). Since exosomes are characterized by specific marker CD9,CD63, and CD81[38,39], we investigated the expressions of them by Western blotting.The results indicated both ExoCtrland ExoCCR2expressed CD9, CD63, and CD81(Figure 1G); however, ExoCCR2expressed high amounts of CCR2, while ExoCtrlexpressed extremely low amounts of CCR2 (Figure 1H).
To further compare the CCL2-binding capacity of ExoCCR2and ExoCtrl, ELISA wasperformed. The results suggested that ExoCCR2bound significantly to CCL2, compared to ExoCtrl, while ExoCtrlshowed little CCL2-binding capacity, compared to the case for the CCL2 control group (Figure 1J).
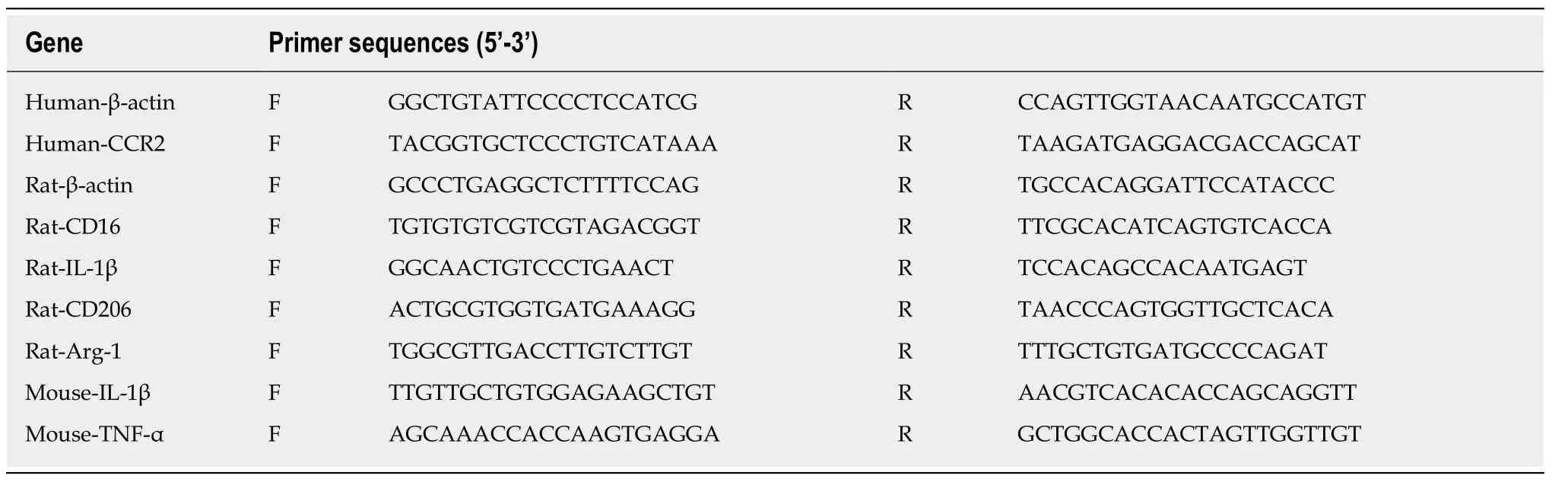
Table 1 Lists of the sequences of the used primers
ExoCCR2 showed more beneficial effects against PSCI than ExoCtrl
The Morris water maze is a common tool for performing cognition tests in animals with experimental stroke[40,41](Figure 2A). The establishment of tMCAO were confirmed mNSS behavioral test and TTC staining at 1 d after surgery, as indicated in Supplementary Figure 3. Compared with the tMCAO group, the rats in both the ExoCCR2and ExoCtrltreatment groups showed a significant decrease in the escape latency spent finding the platform (indicating spatial learning) from day 4 and day 5 during the navigation test. The latency spent finding the platform in case of the animals from the ExoCCR2treatment group further decreased significantly compared to the case for the animals from the ExoCtrltreatment group at day 4 and day 5 during the navigation test (Figure 2B). During the spatial probe test, the rats from both the ExoCCR2treatment and ExoCtrltreatment groups showed a significant increase in the time spent in the target quadrant (indicating spatial memory). Moreover, the rats from the ExoCCR2treatment group showed a further improvement with regards to the time spent in the target quadrant, compared to those from the ExoCtrltreatment group(Figure 2C). At the same time, the mNSS values of the rats in the ExoCCR2and ExoCtrltreatment groups decreased significantly compared to those of the rats from the tMCAO group; the mNSS values of the rats from the ExoCCR2treatment group showed a further decrease compared to those of the rats from the ExoCtrltreatment group(Figure 2D).
ExoCCR2 showed more beneficial effects with regards to oligodendrogenesis and remyelination than ExoCtrl
Oligodendrogenesis and remyelination contribute to the recovery from PSCI[42,43].Therefore, we examined the fluorescence intensity of MBP indicating the integrity of myelination and the number of BrdU+/NG2+ cells indicating the proliferation status of oligodendrocyte around the ischemic area by immunofluorescence staining; the expression of the MBP protein extracted from the ischemic hemispheres was quantified by Western blotting analysis. Compared to the samples obtained from rats in the tMCAO group, samples from the rats subjected to the ExoCtrland ExoCCR2treatments exhibited increased fluorescence intensity and protein expression of MBP at day 28 after tMCAO. Moreover, ExoCCR2treatment showed superior effects on the fluorescence intensity and protein expression of MBP compared to that showed by ExoCtrltreatment (Figure 3A-D). Compared to the samples from rats in the tMCAO group, the samples obtained from rats in both the ExoCtrland ExoCCR2treatment groups showed an increased number of BrdU+/NG2+ cells around the ischemic area at day 28 after tMCAO. Moreover, the changes in samples obtained from rats in the ExoCCR2treatment group were more enhanced than those in the samples obtained from rats in the ExoCtrltreatment group (Figure 3E, 3F).
ExoCCR2 promoted microglia/macrophage M2 polarization and inhibited microglia/macrophage M1 polarization in vivo compared to that by ExoCtrl
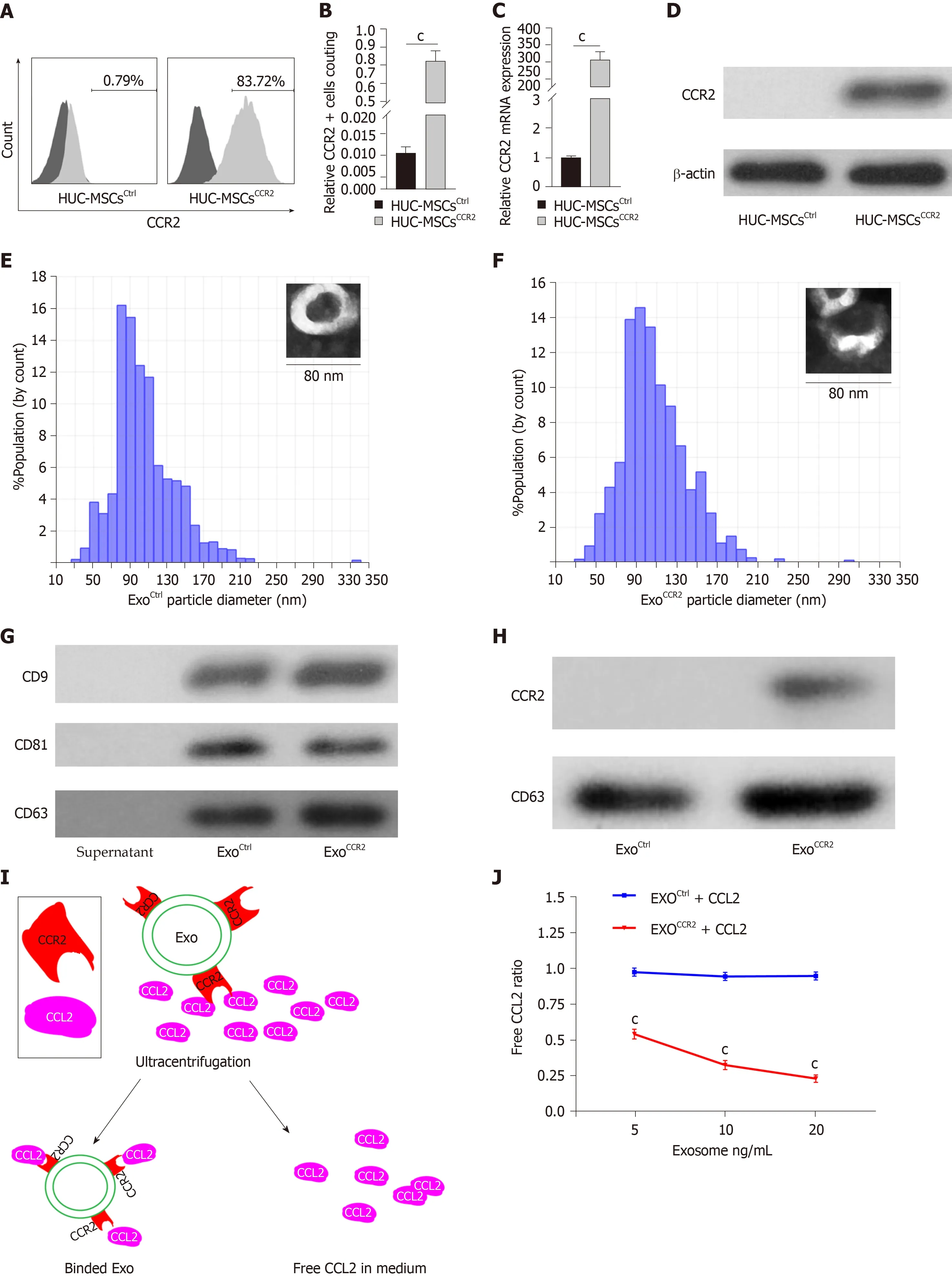
Figure 1 Human umbilical cord mesenchymal stem cellsCCR2 load the C-C chemokine receptor type 2 receptor into their exosomes. A, B: Flow cytometry analysis of the C-C chemokine receptor type 2 (CCR2) receptor on human umbilical cord mesenchymal stem cells (HUC-MSCs)Ctrl and HUC-MSCsCCR2, n = 3, cP <0.001; C: CCR2 mRNA expression in HUC-MSCsCtrlvs HUC-MSCsCCR2, n = 3, cP < 0.001; D: Western blotting analysis for the quantification of the CCR2 expression in HUC-MSCsCtrlvs HUC-MSCsCCR2, n = 3; E, F: Analysis of the exosomal morphology and diameter distribution of ExoCtrl and ExoCCR2 using transmission electron microscopy and the qNano? system, respectively, n = 3; G: Western blotting analysis for the detection of the exosomal specific markers CD9, CD63, and CD81 in ExoCtrl and ExoCCR2, n = 3; H: Western blotting analysis for the quantification of the exosomal CCR2 expression in the ExoCtrl and ExoCCR2 samples, n = 3; I:Schematic diagram describing the extraction of the exosomes from the medium; J: Detection of the CCL2-binding ability of the exosomes by ELISA, n = 3, cP < 0.001.
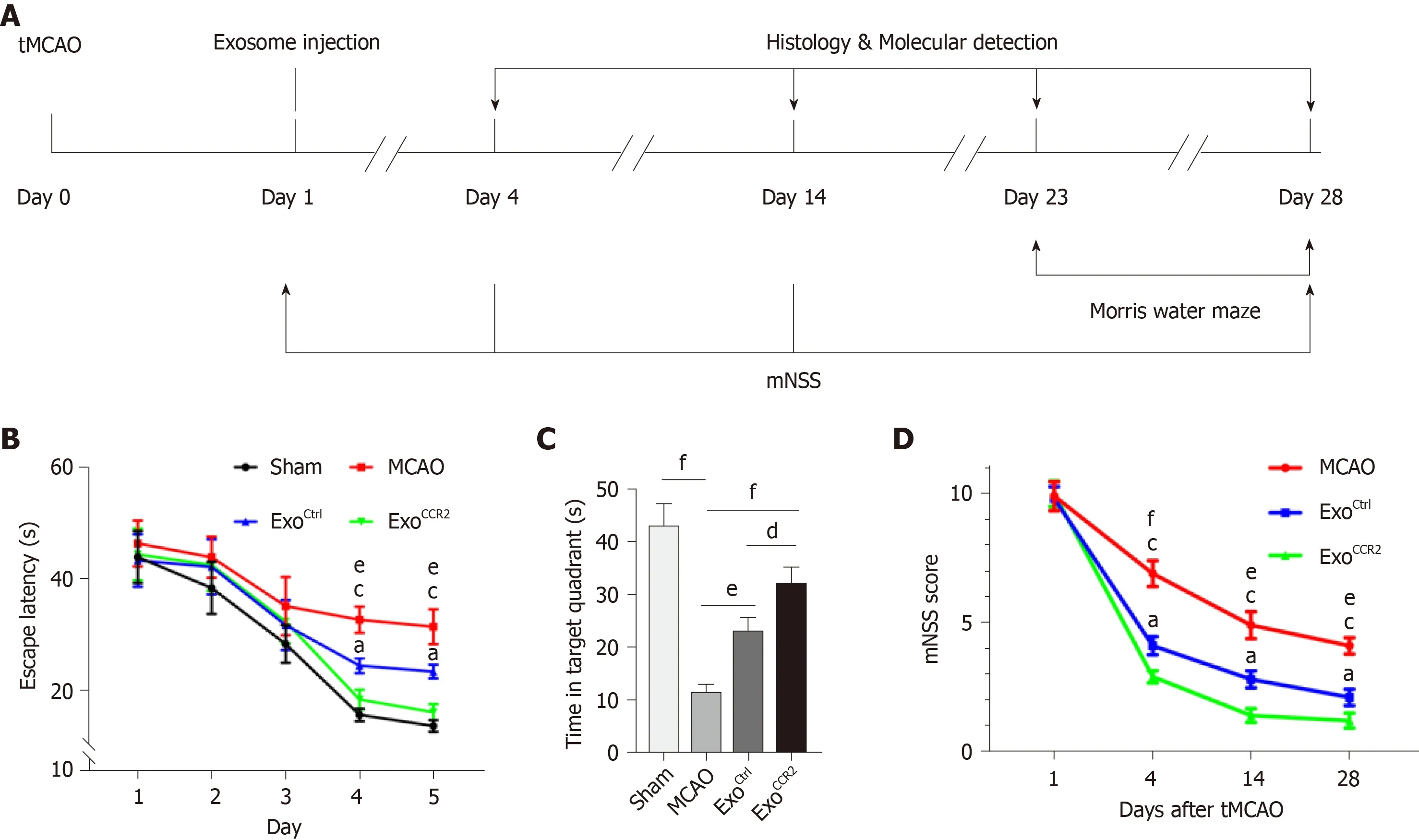
Figure 2 ExoCCR2 improved the spatial learning and memory at day 28 after transient middle cerebral occlusion compared to ExoCtrl. A: Experimental schedule to observe the effects of exosomes on rats with transient middle cerebral occlusion (tMCAO); B: Effect of exosomes on the mean escape latency to find the platform in each group. n = 10, eP < 0.01, ExoCtrl vs tMCAO, cP < 0.001, ExoCCR2 vs tMCAO, aP < 0.05, ExoCCR2 vs ExoCtrl; C: Effect of exosomes on the time spent in the target quadrant in case of rats from each group. n = 10. dP < 0.05, eP < 0.01, fP < 0.001; D: Effect of exosomes on the mNSS values of rats from each group. n =10. eP < 0.01, ExoCtrl vs tMCAO, fP < 0.001, ExoCtrl vs tMCAO, cP < 0.001, ExoCCR2 vs tMCAO, aP < 0.05, ExoCCR2 vs ExoCtrl.
Since microglia/macrophage polarization plays an important role in the process of oligodendrogenesis and remyelination after stroke[40,44], we performed qRT-PCR analysis to quantify the mRNA levels of the M1 markers CD16 and IL-1β and the M2 markers CD206 and Arg-1; we also performed immunofluorescence staining to detect CD16/iba-1 and CD206/iba-1, to compare the effects of ExoCtrland ExoCCR2on microglia/macrophage polarization. The CD16 and IL-1β mRNA expression levels in samples obtained from rats after ExoCCR2and ExoCtrltreatment decreased significantly and the mRNA expression levels of CD206 and Arg-1 increased significantly compared to those in samples obtained from rats in the tMCAO group at day 4 and day 14 after tMCAO. The changes in rats from the ExoCCR2treatment group were more enhanced compared to those in rats from the ExoCtrltreatment group (Figure 4A-H).These results were validated by immunofluorescence staining for CD16/iba-1 and CD206/iba-1 at day 14 after tMCAO (Figure 4I, 4J).
ExoCCR2 suppressed CCL2-induced macrophage migration and activation in vivo and in vitro compared to ExoCtrl
In pathological conditions such as cerebral ischemia, numerous CCR2+ blood-derived macrophages migrate into the ischemic area due to the highin situexpression of CCL2[19,20], which plays a critical role in microglia/macrophage activation and polarization. Downregulation of the CCL2/CCR2 axis inhibits mononuclear macrophage infiltration, which reduces the over-activation and M1 polarization of microglia/macrophages and promotes the alternative M2 activation of microglia/macrophages[21-23,45]. Therefore, we examined the expression of the CCL2,nuclear factor kappa B (NF-κB), ionized calcium-binding adapter molecule 1 (iba-1),and CD68 proteins by Western blotting analysis. The results showed that the expression levels of CCL2, NF-κB, iba-1, and CD68 in samples obtained from rats in the ExoCCR2and ExoCtrltreatment groups decreased significantly compared to the samples obtained from rats in the tMCAO group; additionally, the changes in samples from rats in the ExoCCR2treatment group were more enhanced compared to those in samples from rats in the ExoCtrltreatment group (Figure 5A-E).
To further confirm these resultsin vitro, a transwell assay for quantifying the number of migrated macrophages, qRT-PCR analysis for quantifying the mRNA expression levels of IL-1β and tumor necrosis factor α (TNF-α), and Western blotting analysis for quantifying the NF-κB protein expression were performed to evaluate the effects of ExoCCR2and ExoCtrlon the migration and activation of macrophagesin vitro.The results indicated that ExoCCR2treatment significantly inhibited macrophage infiltration, and reduced the mRNA expression levels of IL-1β and TNF-α and the expression levels of the NF-κB protein, compared to the cells from the ExoCtrltreatment and CCL2 control group; on the contrary, ExoCtrlshowed no significant effects on macrophage migration, the mRNA expression levels of IL-1β and TNF-α,and the expression levels of the NF-κB protein, compared to the case for cells from the CCL2 control group (P> 0.05) (Figure 5F-5K).
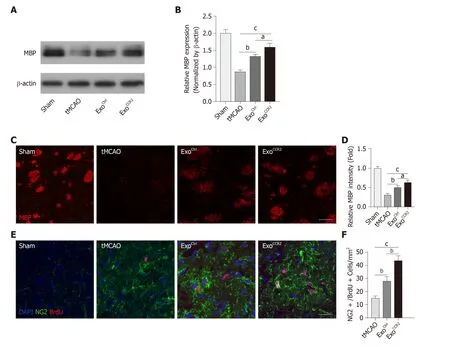
Figure 3 ExoCCR2 exerts superior beneficial effects on remyelination and oligodendrogenesis at day 28 after transient middle cerebral occlusion compared to ExoCtrl. A, B: Western blotting analysis of the MBP expression in samples from rats in each group. n = 5, aP < 0.05, bP < 0.01, cP < 0.001; C, D: Analysis of MBP fluorescence intensity in samples from rats in each group. Scale bar = 50 μm, n = 6, aP < 0.05, bP < 0.01, cP < 0.001; E, F: NG2+/ BrdU+ cell colocalization count by immunofluorescence staining. Scale bar = 50 μm, n = 6. bP < 0.01, cP < 0.001.
DISCUSSION
With increasing studies seeking to isolate the specific paracrine factors that mediate the therapeutic effects of MSCs, the therapeutic efficacy of exosomes derived from their parent cells has been found to be comparable to that of MSC therapies[13,14].Intravenous administration of MSC-derived exosomes to a rodent model of stroke or a rodent model of traumatic brain injury has been shown to substantially promote white matter damage repair, thereby improving the behavioral and cognitive outcomes[29,46]. Moreover, genetically modified exosomes such miR-17-92- or miR-133b-overexpressing exosomes have been found to enhance the therapeutic effects of exosome-based treatment in a model of experimental stroke[47,48]. Exosome-mediated intercellular communications via the transfer of exosomal proteins or RNAs between the source and target cells have been extensively evaluated[49]. However, only a few studies have focused on the surface receptors on exosomes. Ciulloet al[50]have found that treatment with C-X-C motif chemokine receptor 4 (CXCR4)-overexpressing exosomes showed more beneficial outcomes in a myocardial infarction animal model than the treatment with control exosomes, suggesting that the receptors on exosomes may also contribute to their therapeutic effects. Shenet al[35]have found that CCR2-positive exosomes suppress macrophage migration and alleviate ischemic renal injury. Since Huanget al[30]have demonstrated that CCR2-overexpressing MSCs enhance the therapeutic effects of MSC treatment in rats with tMCAO, we further explored whether exosomes derived from CCR2-overexpressing MSCs can show enhanced therapeutic effects. The results indicate that HUC-MSCsCtrland their secreted ExoCtrlexpressed low amounts of CCR2, while HUC-MSCsCCR2and ExoCCR2showed a high expression of CCR2. Moreover, the results showed that ExoCCR2showed significant binding capacity to the ligand CCL2in vitrocompared to ExoCtrl; this is consistent with the results of the study by Shenet al[35], Based on this finding, we hypothesize that when present on exosomes, the CCR2 receptor may exert more powerful therapeutic effects for the treatment of PSCI.
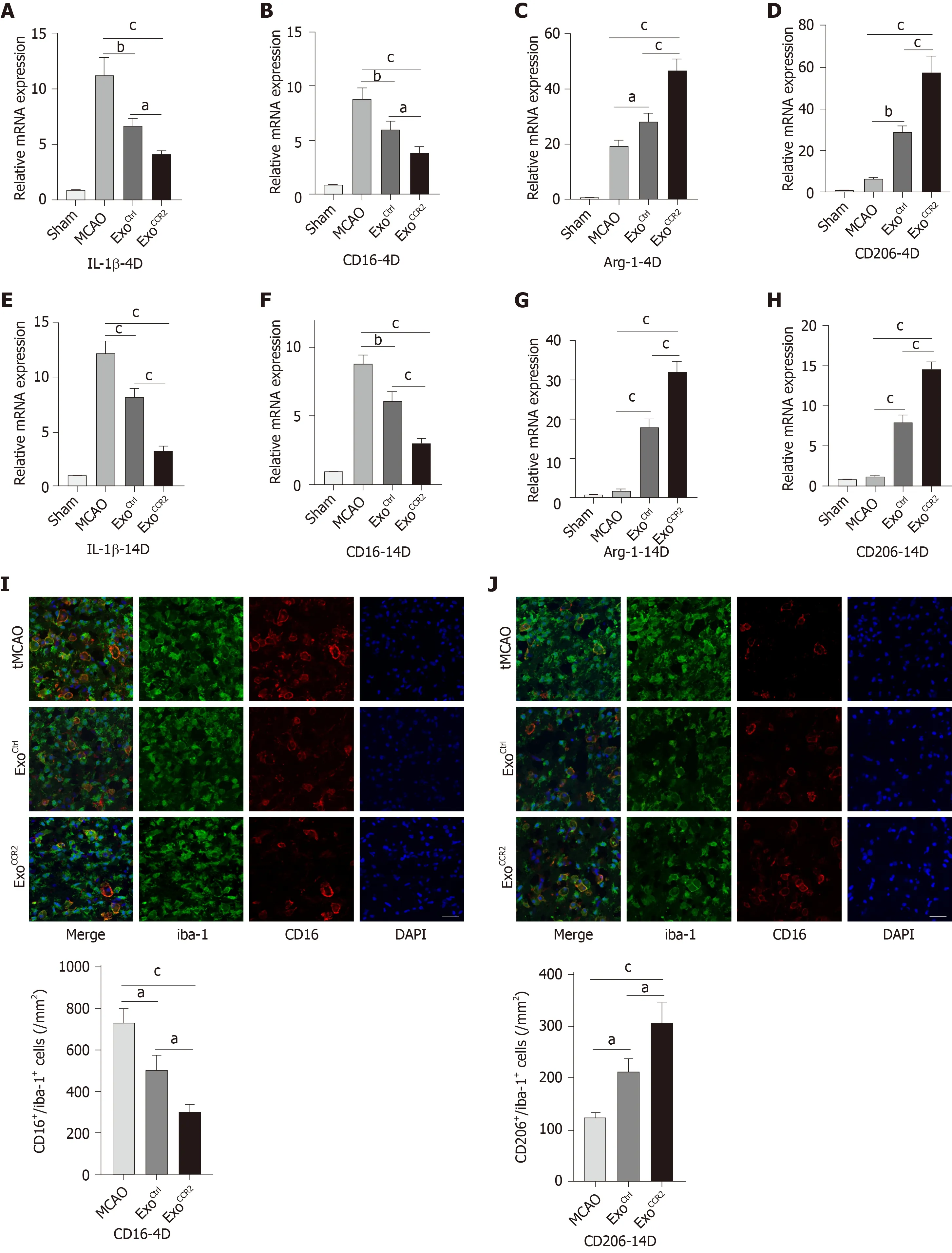
Figure 4 ExoCCR2 drove microglia/macrophage M2 polarization and inhibited microglia/macrophage M1 polarization at day 4 and day 14 after transient middle cerebral occlusion compared to ExoCtrl. A-D: Relative CD16, IL-1β, CD206, and Arg-1 mRNA expression changes in samples obtained from rats in each group on day 4 after transient middle cerebral occlusion (tMCAO), n = 6, aP < 0.05, bP < 0.01, cP < 0.001; E-H: Relative CD16, IL-1β, CD206, and Arg-1 mRNA expression changes in samples obtained from rats in each group on day 14 after tMCAO, n = 6, bP < 0.01, cP < 0.001; I: CD16/iba-1 immunofluorescence staining and cell colocalization counts 14 d after tMCAO. Scale bar = 50 μm, n = 6, aP < 0.05, cP < 0.001; J: CT206/iba-1 immunofluorescence staining and cell colocalization counts 14 d after tMCAO. Scale bar = 50 μm, n = 6, aP < 0.05, cP < 0.001.
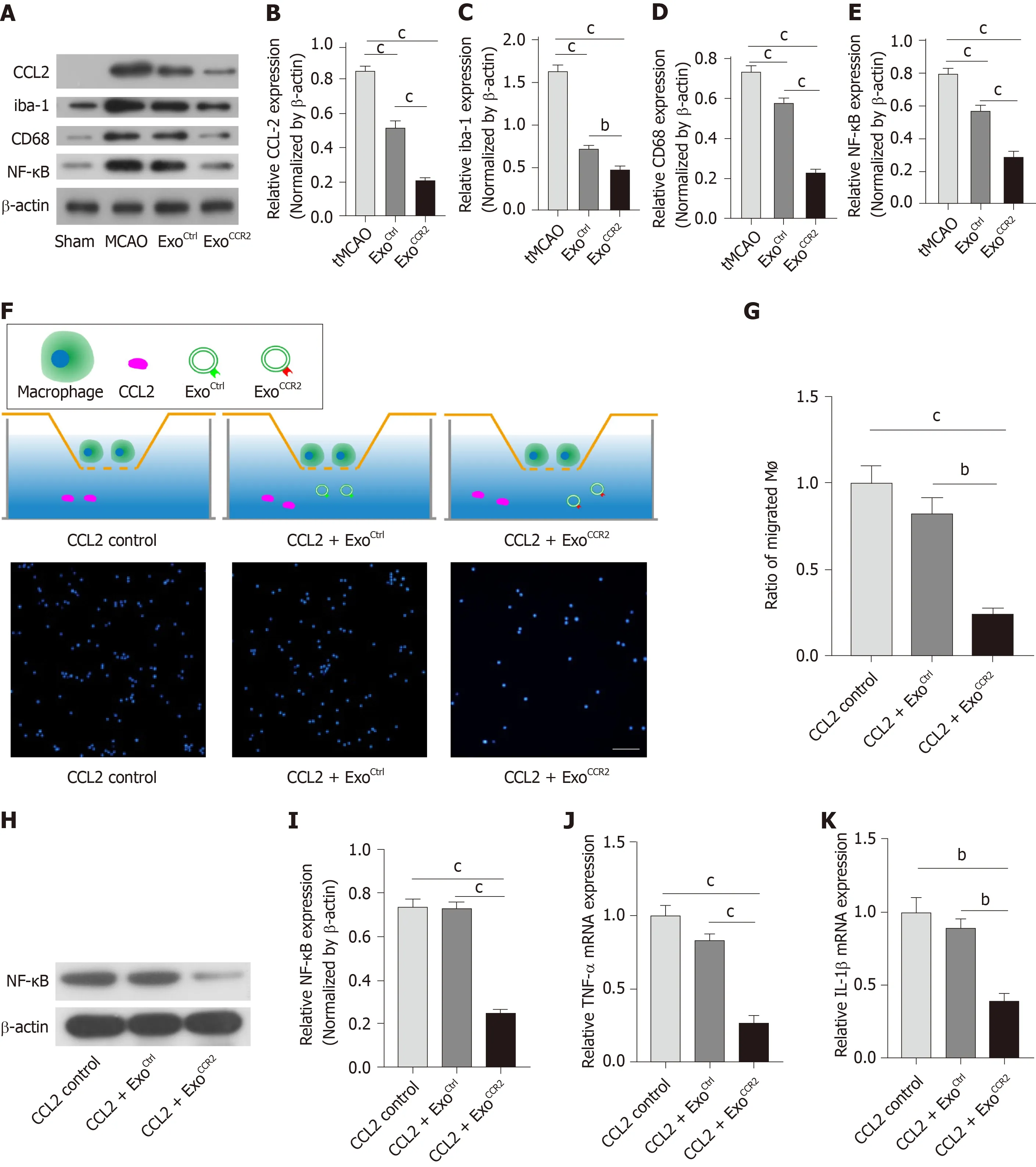
Figure 5 ExoCCR2 showed more powerful effects on CCL2-induced macrophage migration and activation in vivo and in vitro than ExoCtrl. A-E: Comparison of the expression levels of the CCL2, iba-1, CD68, and NF-κB proteins in samples from rats in each group (in vivo) at day 4 after transient middle cerebral occlusion, n =6, bP < 0.01, cP < 0.001; F: Schematic diagram of the transwell experiment. Immunofluorescence detection of the migrated macrophages in case of each treatment group (in vitro), n = 3; scale bar = 200 μm, bP < 0.01, cP < 0.001; D-K: Comparison of the mRNA expression levels of TNF-α and IL-1β and the expression levels of the NF-κB protein in cells from each group (in vitro), n = 3, bP < 0.01, cP < 0.001.
MSC-based treatments have been evaluated to promote cognitive recovery in an animal model of stroke[4]or traumatic brain injury[51]. Previous studies have indicated that exosome treatment can promote the repair of white matter damage after stroke and facilitate the recovery of neurological function after stroke[48,52]. Exosomes produced by MSCs mediate several therapeutic effects of MSCs; however, reports about the effects of exosome treatment on cognitive impairment after stroke are rare.Since HUC-MSC-derived exosomes have shown potent effects on microglial activation and polarization in animal models such as the hypoxic-ischemic encephalopathy model[26]and the peripheral nerve injury model[27], and improved the cognitive function in an Alzheimer's disease model by modulating microglial polarization[25], we utilized HUC-MSC-derived exosomes in this study. The results showed that the spatial learning and memory in the rats from the ExoCtrland ExoCCR2treatment groups were significantly better than those in the rats from the tMCAO group; additionally, ExoCCR2treatment significantly promoted the recovery of spatial learning and memory in rats, compared to that by ExoCtrltreatment.
Although PSCI is a heterogeneous disease, white matter damage is the most common pathological change observed in almost all cases of vascular dementia[53]and most types of stroke[54]. Both basic medical studies and clinical studies have suggested that white matter damage after stroke is highly correlated with PSCI[43,55,56]. In the acute phase of stroke, oligodendrocyte damage causes the demyelination of the white matter, leading to neurotransmission disorders. During the recovery phase of stroke,oligodendrocytes and their precursor cells proliferate and differentiate into mature oligodendrocytes, which play a key role in remyelination[57]. Thus, facilitating the proliferation of oligodendrocytes and their precursor cells promotes remyelination and cognitive function after stroke[58]. Our finding is consistent with that of Xinet al[48,52], who also found that exosomes promote oligodendrogenesis and remyelination following experimental stroke. Another important finding is that ExoCCR2treatment further promoted oligodendrogenesis and remyelination, compared with ExoCtrltreatment. These results indicate that ExoCCR2treatment notably promoted the recovery from PSCI by enhancing oligodendrogenesis and remyelination compared to that by ExoCtrltreatment.
Microglia, which are the resident macrophages in the central nervous system, as well as blood-derived macrophages, activate and display dynamic M1 and M2 polarization after stroke[59]. Since activated microglia and blood-derived macrophages are similar with regards to morphology and biological function, and co-express iba-1,CD11b, and F4/80, many scholars have referred to activated microglia and bloodderived macrophages as the same group of cells[60,61]. M1 microglia/macrophage polarization deteriorates oligodendrogenesis and white matter damage by releasing inducible nitric oxide synthase and pro-inflammatory factors such CD16, IL-1β, and TNF-α, while M2 microglia/macrophage polarization facilitates oligodendrogenesis and white matter repair by releasing the mannose receptor CD206 and antiinflammatory factors such as IL-10, Ym-1 and Arg-1 and engulfing tissue fragments after stroke[40,43,62]. Promoting M2 polarization and inhibiting M1 polarization boosts oligodendrogenesis and remyelination[63,64], and facilitates the recovery from PSCI[40].The results of our study show that both the ExoCtrland ExoCCR2treatments promoted M2 microglia/macrophage polarization and inhibited M1 microglia/macrophage polarization, compared to the case for the rats in the tMCAO group, and ExoCCR2showed enhanced effects compared to ExoCtrl. Therefore, the enhanced beneficial effects of ExoCCR2against PSCI may be related to their more effective regulation of microglial polarization-mediated oligodendrogenesis and remyelination.
It is well-known that CCL2 is expressed in high amounts in the ischemic hemisphere after stroke, which mediates the infiltration of CCR2+ mononuclear macrophages into the ischemic site and aggravates the excessive activation and M1 polarization of microglia/macrophages[23,45]. Therefore, we postulate that CCR2-overexpressing exosomes may function as endogenous CCL2 sponges binding to these ligands, block the over-infiltration of macrophages, and subsequently inhibit the excessive activation and M1 polarization of microglia/macrophages. These results support the findings from previous studies, which have reported that MSC-derived exosomes downregulate CCL2 overexpression[65]and microglia/macrophage overactivation[27]; ExoCtrlsignificantly downregulated the expression of CCL2, iba-1,CD68, and NF-κBin vivo, compared to the case for rats in the tMCAO group.Moreover, ExoCCR2further downregulated the expression of CCL2, iba-1, CD68, and NF-κB. To verify thisin vivofinding,in vitroexperiments were performed, which showed that ExoCCR2bound significantly to CCL2in vitrocompared with ExoCtrl, while ExoCtrlshowed a low degree of binding to CCL2. Meanwhile, ExoCCR2significantly inhibitedin vitromacrophage infiltration and the release of inflammatory factors, and reduced the NF-κB expression, compared to ExoCtrl. Therefore, CCR2 molecules on exosomes may function as endogenous CCL2 sponges that bind to these ligands and inhibit the infiltration of macrophages and the subsequent over-activation and M1 polarization of microglia/macrophages.
In conclusion, the present study demonstrated that both ExoCtrland ExoCCR2improved the cognitive function in rats after ischemic stroke by promoting M2 microglia/macrophage polarization, thereby enhancing oligodendrogenesis and remyelination. Furthermore, this study is the first to provide evidence that ExoCCR2have enhanced beneficial effects compared to ExoCtrl, partially due to the action of CCR2 molecules as endogenous CCL2 sponges, whereby they bind to these ligands and inhibit the infiltration and activation of macrophages. Since we utilized human MSC-derived exosomes, our research serves as a pre-clinical study; further studies on stroke patients are required to confirm our hypothesis.
ARTICLE HIGHLIGHTS
Research background
Post-stroke cognitive impairment (PSCI) is a common sequela of stroke with considerable impact on the health well-being and quality of life to patients, and poses significant financial burden on society. Exosomes have been shown to possess therapeutic effects that are comparable to the mesenchymal stromal cells. However, few studies have focused on the effects of exosomes derived from human umbilical cord mesenchymal stem cells (HUC-MSCs) (ExoCtrl) on PSCI.Here in this study, we aimed to explore the if exosomes derived from C-C chemokine receptor type 2 (CCR2)-overexpressing HUC-MSCs (ExoCCR2) have any therapeutic effects on PSCI, and clarify the possible underlying mechanisms.Research motivation
Effective treatment strategies for PSCI in stroke patients are an unmet clinical need.
Research objectives
In the present study, we aimed to: (1) Investigate whether CCR2 over-expressing exosomes possess improved therapeutic effects on PSCI; and (2) The possible underlying mechanisms involved in the therapeutic benefits of exosomes.
Research methods
The morphology of ExoCtrland ExoCCR2were determined by transmission electron microscopy and qNano? particles analyzer; the CCR2 expression in the ExoCtrland ExoCCR2was evaluated by Western blotting; the binding capacity of exosomes to CC chemokine ligand 2 (CCL2) in vivo was examined by ELISA; the effects of ExoCtrland ExoCCR2on PSCI in experimental stroke rats were assessed by Morris water maze. Remyelination and oligodendrogenesis was analyzed by Western blotting and immunofluorescence microscopy, and microglia/macrophage polarization were investigated by qRT-PCR and immunofluorescence imaging. The infiltration and activation of hematogenous macrophages were analyzed by transwell migration analysis and Western blotting.
Research results
CCR2-overexpressing HUC-MSCs could deliver CCR2 receptor rich exosomes. There were not significant difference in the size and morphology between ExoCtrland ExoCCR2. ExoCCR2showed more powerful binding capacity to CCL2, while ExoCtrlhardly bound to CCL2. ExoCCR2enhanced the beneficial effects of ExoCtrlon PSCI through further promoting microglia/macrophage polarization-mediated oligodendrogenesis and remyelination. Compared with ExoCtrl, ExoCCR2showed more powerful suppression on CCL2-induced macrophage migration and activation in vivo and in vitro.
Research conclusions
CCR2 over-expressing on exosomes showed enhanced therapeutic benefits on PSCI through more powerful modulation on microglia/macrophage polarization-mediated oligodendrogenesis and remyelination. The additional therapeutic effect maybe related to the suppression on CCL2-induced macrophage infiltration and activation.
Research perspectives
Our study provides great insight in the application of stem cells-based therapies for neural degenerative disorders. Comparisons of the therapeutic effects of ExoCtrland ExoCCR2on more clinically relevant animal models of stroke are warranted.
ACKNOWLEDGEMENTS
Authors are grateful to the generous support of the Center for Stem Cell Biology and Tissue Engineering, Sun Yat-sen University.
 World Journal of Stem Cells2020年2期
World Journal of Stem Cells2020年2期
- World Journal of Stem Cells的其它文章
- Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective
- Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks
- Clonal isolation of endothelial colony-forming cells from early gestation chorionic villi of human placenta for fetal tissue regeneration
- Comparison between the therapeutic effects of differentiated and undifferentiated Wharton's jelly mesenchymal stem cells in rats with streptozotocin-induced diabetes
