Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks
Gabriela S Kronemberger, Renata Akemi Morais Matsui, Guilherme de Almeida Santos de Castro e Miranda,José Mauro Granjeiro, Leandra Santos Baptista
Gabriela S Kronemberger, Renata Akemi Morais Matsui, Guilherme de Almeida Santos de Castro e Miranda, José Mauro Granjeiro, Leandra Santos Baptista, Laboratory of Tissue Bioengineering, Directory of Metrology Applied to Life Sciences, National Institute of Metrology, Quality and Technology (INMETRO), Duque de Caxias, RJ 25250-020, Brazil
Gabriela S Kronemberger, Leandra Santos Baptista, Post-graduate Program in Translational Biomedicine (Biotrans), Unigranrio, Campus I, Duque de Caxias, RJ 25250-020, Brazil
Renata Akemi Morais Matsui, José Mauro Granjeiro, Leandra Santos Baptista, Post-graduate Program in Biotechnology, National Institute of Metrology, Quality and Technology(INMETRO), Duque de Caxias, RJ 25250-020, Brazil
Guilherme de Almeida Santos de Castro e Miranda, Federal University of Rio de Janeiro(UFRJ), Campus Duque de Caxias, Duque de Caxias, RJ 25250-020, Brazil
José Mauro Granjeiro, Laboratory of Clinical Research in Odontology, Fluminense Federal University (UFF), Niterói 25255-030 Brazil
Leandra Santos Baptista, Multidisciplinary Center for Biological Research (Numpex-Bio),Federal University of Rio de Janeiro (UFRJ) Campus Duque de Caxias, Duque de Caxias, RJ 25245-390, Brazil
Abstract
Key words: Adipose stromal/stem cells; Spheroids; Building-blocks; Bottom-up;Developmental tissue engineering; Cartilage and bone
INTRODUCTION
Classic approaches to tissue engineering rely on scaffold-based strategies, which have limited ability to recapitulate organogenesisin vitro[1,2]. In scaffold-based approaches,limitations are related to the replication of morphological, biomechanical and biochemical signs that occurin vivo, mainly because of the prevalence of cell interactions with scaffolds instead of cell-cell and cell-extracellular matrix interactions found in the natural tissues microenvironment[1]. Other disadvantages of scaffoldbased approaches are (1) The homogeneous distribution of cells to fill the entire area of the scaffold; (2) The final density of the cells reached in the scaffold area; (3) The diffusion of nutrients; and (4) The cost and time to produce a proper design of the desired scaffold to support the desired regenerationin vivo[3-5]. The emerging approach of scaffold-free tissue engineering often relies on the cultivation of cells as spherical clusters known as “spheroids”, which mimic the physiological conditions of tissuesin vitro[6]. During spheroid formation, cells aggregate by cadherins-based interactions in the absence of a fixation medium, in a process known as self-assembly[7]. Spheroids can be used in developmental tissue engineering due to their capacity to form hierarchical tissue structures by recapitulating embryonic processesin vitro.
As a result of their three-dimensional (3D) architecture, spheroids have improved cell biological properties such as increased cell viability and proliferative capacity,more stable morphology and polarization, and improved metabolic functions (as compared to 2D cultures)[2]. Consequently, adult stem cell spheroids show distinct properties suitable for regenerative medicine approaches, such as high adhesion capacity and the secretion of a variety of growth factors[8]. Spheroids can be formed from different cell types, including mature cells[9-11]; however, for tissue engineering approaches, spheroids formed of mesenchymal stromal/stem cells (MSCs) are particularly appealing, due to the regenerative and multipotential properties of these adult stem cells[6].
The subcutaneous adipose tissue is an abundant source of MSCs, recently termed adipose derived stem/stromal cells (ASCs)[12]. Various studies have reported that ASCs have osteogenic[13-17]as well as chondrogenic[18-20]potential for use in scaffoldbased approaches of tissue engineering. In agreement with these studies, ASCs were used successfully in pre-clinical and clinical trials for bone and cartilage repair[21-30].
Despite the osteogenic and chondrogenic potential of ASCs, the use of spheroids of ASCs (or other MSCs) in bone tissue engineering is still in its infancy[31]. Saburinaet al[32]reported that ASC spheroids express osteoblast markers such as osteocalcin and osteopontin, and have angiogenic potential and calcium deposits. In addition, the use of scaffold-free 3D culture, including spheroids, in chondrogenesis studies led to the identification of important molecular markers of cartilage formation[33-35]. Spheroids have the capacity to express crucial extracellular matrix molecules such as collagen type II, tenascin-C, collagen type IX and aggrecan[36], recapitulating cartilage formation. Spheroids also secrete COMP (cartilage oligomeric matrix), a thrombospondin family protein (TSP-5)[37,38]recognized as a biomarker for chondrogenesis[39].
Our research group recently reported the production of a stably engineered cartilage using ASC spheroids under chondrogenic and hypoxic conditions[35]. In addition, we have recently established a hypertrophic engineered cartilage(manuscript submitted) for future use in bone engineering[31]. Although hypertrophic cartilage has been used as a template for osteogenesisin vivobased on different strategies[40-42], hypertrophic cartilage made from spheroids has not been tested in this context.
Finally, spheroids have already been used as building blocks for the biofabrication of different tissues (such as nervous and cardiac tissues) and recent data support their potential use in 3D bioprinting approaches[43,44].
USE OF SPHEROIDS FOR CARTILAGE AND BONE ENGINEERING
Cultures of MSCs as 2D monolayers are widely used and suitable for cell expansion.However, 2D cultures have numerous limitations; in particular, these cultures have limited cell differentiation potential[45,46].In vivo, stem cells dwell in specific compartments of tissue microenvironments known as “niches”, which regulate cell physiology[47]. In 3D cultures - particularly in scaffold-free strategies such as spheroids(Figure 1) - niche conditions can be recreated[48].
The use of 3D scaffold-free cultures, including spheroids, as anin vitrocartilage model has been widely explored in the past few years[49,50], because the hypoxia gradient found inside spheroids mimics the microenvironment of native cartilage,favoring the differentiation of MSCs and ASCs down the chondrogenic pathway[51].
Yoonet al[52]showed that ASCs in 3D cultures have improved chondrogenic potential when compared with monolayers. More recently, Occhettaet al[53]showed that the downregulation of bone morphogenetic protein (BMP) signaling in bone marrow MSCs guides embryonic progenitors towards articular cartilage formation,and is responsible for stable chondrogenesis, protecting against vessel invasion and,consequently, bone formation. In a co-culture approach, spheroids composed of a mixture of chondrocytes and ASCs had upregulated expression of chondrogenic markers[54]. Importantly, Dikinaet al[55]successfully used a modular system based on MSC spheroids to engineer cartilaginous scaffold-free tissue for tracheal tissue replacement. MSC differentiation was optimized by delivering TGF-β entrapped in gelatin microspheres, and MSC spheroids were guided to form a cartilaginous tube structure with mechanical properties similar to those of native trachea.
Our research group isolated and characterized human cartilage progenitor cells(CPCs) capable of spontaneous chondrogenesisin vitro[56]in the absence of exogenous stimuli of chondrogenic growth factors, when using a 3D scaffold and serum-free culture[57]. Recently, we modified our 3D culture system to a scalable methodology using micro-molded, non-adhesive hydrogel[58]. This methodology prevents cell attachment, and encourages cell-cell interactions, improving chondrogenic differentiation[59]. The micro-molded hydrogel strategy showed promising results not only for chondrogenesis, but also for the formation of spheroids of homogeneous size and shape, and with high cell viability[58].
ASC spheroids made using micro-molded hydrogel are also homogeneous in size,shape and have increased cell viability[35]. Induced ASC spheroids showed a chondrogenic potential similar to that of CPC spheroids, as validated by proteomic analysis of spheroid culture supernatants, known as “secretomes”[35]. Whenin vivo,these spheroids might be able to secrete cartilage specific extracellular molecular proteins and bioactive molecules, in order to promote the formation of cartilage tissue(Figure 2). Interestingly, our secretome data on differentiated ASC spheroids revealed the absence of collagen type X, a classic marker of chondrocyte hypertrophy[60].Furthermore, comparative secretome analysis revealed that induced ASC spheroids secreted higher levels of the chondrogenesis biomarkers collagen type II and COMP than CPC spheroids. Induced ASC spheroids also had increased secretion of a new biomarker of chondrogenesis - TSP-1[35]- an anti-angiogenic protein recently described as anti-hypertrophic[61].
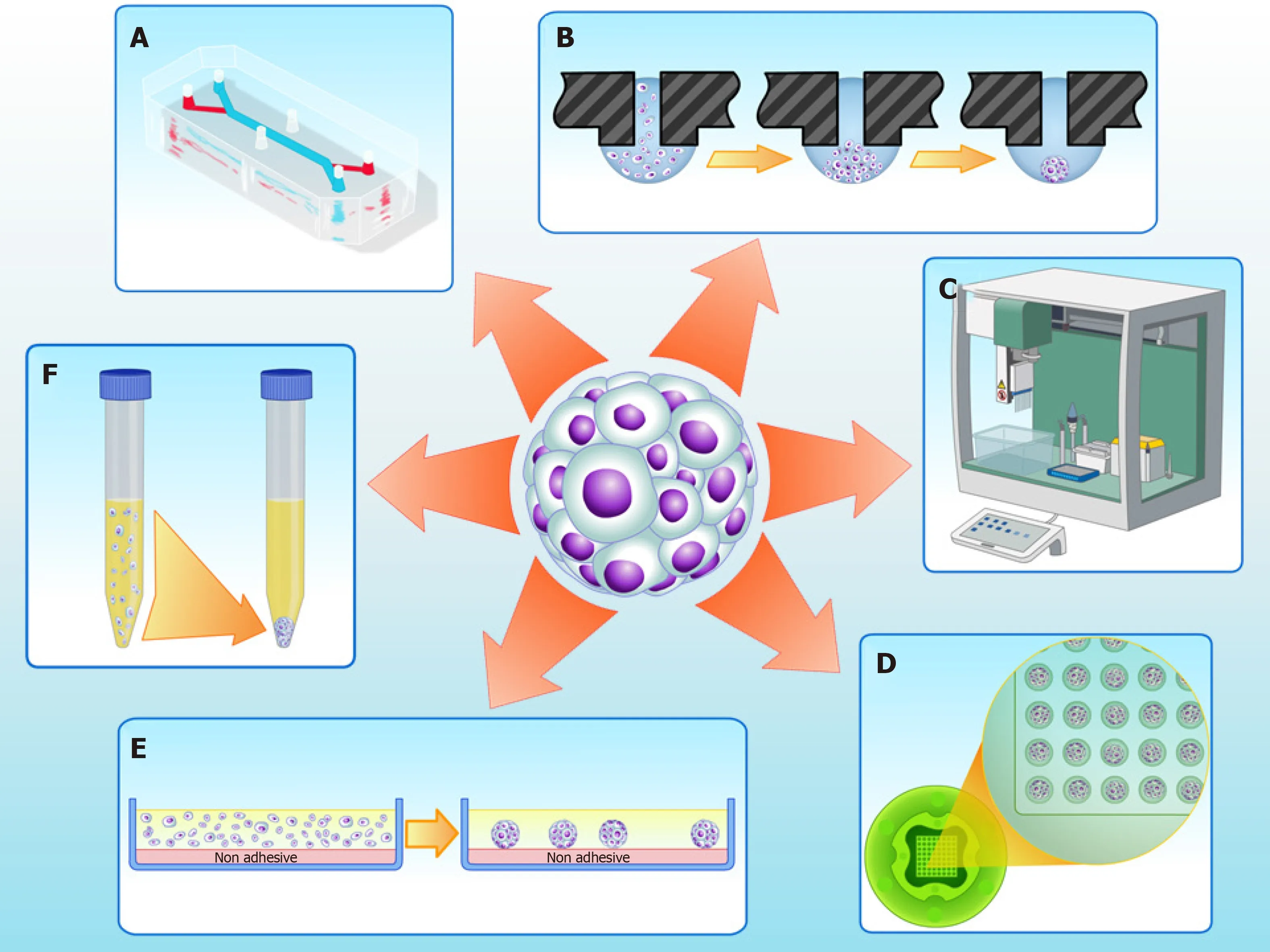
Figure 1 Three-dimensional cell culture techniques to fabricate spheroids in vitro. A: Microfluidics; B: Hanging drop; C: Automatic platforms; D: Non-adherent agarose micromolded hydrogel produced from a silicone mold (3D Petri dish?, Microtissues); E: Non adhesive surfaces; F: Pellet culture.
While several studies have reported successful chondrogenic differentiation using spheroids, only a few studies have reported the use MSC or ASC spheroids in bone engineering[31]. Osteogenic differentiation is commonly reached by the addition of inducers to the culture medium. Hildebrandtet al[62]showed that MSC spheroids induced using dexamethasone, ascorbic acid and β-glycerophosphate had a widespread distribution of collagen type I, the main collagen found in bone extracellular matrix. In addition, Shenet al[63]reported that ASC spheroids induced into the osteogenic pathway using a cocktail of vitamin D3, ascorbic acid,dexamethasone and β-glycerophosphate developed calcium deposits (stained with Alizarin red); these deposits were associated preferentially with the inner spheroid cells. In agreement with these data, Gurumurthyet al[64]observed that growth as spheroids improved the synthesis of calcium deposits by ASCs. In that study, ASC spheroids and monolayers were maintained in medium containing ascorbic acid,dexamethasone, β-glycerophosphate and 10% fetal bovine serum. Recently, Rumińskiet al[65], compared the osteogenic potential of ASCs by culturing them as monolayer,spheroids or seeded in a scaffold. The results showed that ASCs spheroids presented an up-regulation of osteogenic markers. In addition, after the induction of cells to later osteogenic differentiation events, cells dissociated from spheroids produced mineral and osteocalcin. In this study, ASCs spheroids were kept in a medium containing the inducing factors 10 nmol dexamethasone, 50 μg/mL ascorbic acid 2-phosphate and 3 mmol NaH2PO4. During the induction of later differentiation events, the medium was supplemented with 10 nmol 1α,25-dihydroxyvitamin D3.
BMP-7 stimulates bone metabolism, as well as modulating the proliferation and differentiation of MSCs into bone tissue cells[66]. According to our preliminary results,ASC spheroids induced using BMP-7 had calcium deposits, they were negative for typical bone extracellular matrix components, showing a restricted area of positivity for osteocalcin. Nevertheless, even in the absence of BMP-7, ASC spheroids had strongin situstaining for collagen type X, a classic early marker of hypertrophic chondrocytes[60,67], the precursors of endochondral ossification.
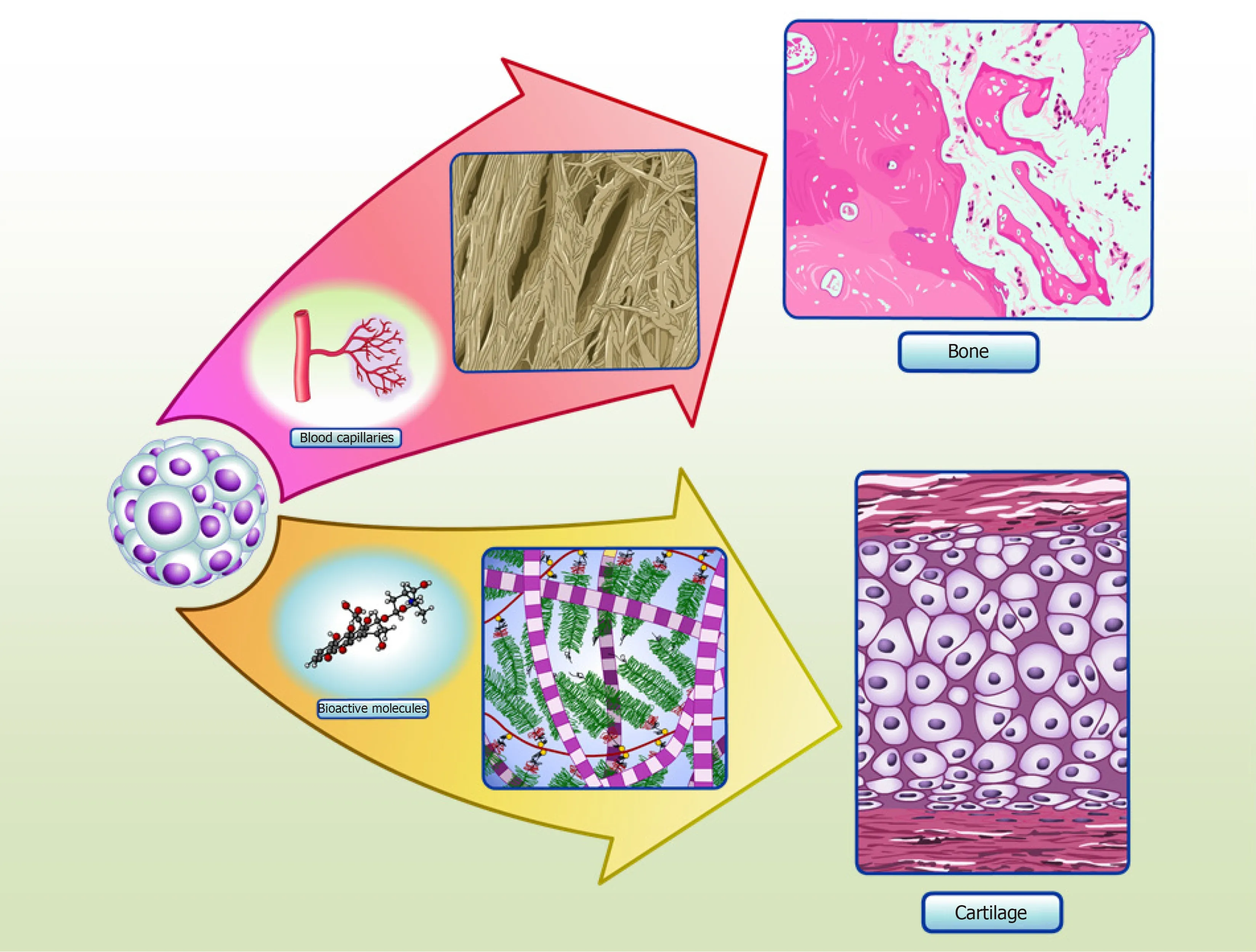
Figure 2 Spheroids in cartilage and bone tissue microenvironments. Adipose stem/stromal cells (ASCs) spheroids delivered to defect area in vivo can secrete bioactive molecules and synthetize typical extracellular matrix proteins of cartilage, promoting regeneration. Our group have detected collagen type II and COMP in the secretome of in vitro ASCs spheroids, as the production of typical extracellular matrix proteins of cartilage in situ[35]. ASCs spheroids delivered to critical-size bone defects can stimulate angiogenesis by secretion of vascular endothelial growth factor (VEGF) and synthesize bone typical extracellular matrix proteins, promoting regeneration. Our group have detected higher levels of VEGF secreted in ASCs spheroids induced to form a hypertrophic cartilage in vitro, as the production of typical extracellular matrix proteins of bone in situ (manuscript submitted).
In agreement with the intrinsic capacity of ASC/MSC spheroids to form hypertrophic chondrocytes suggested by our preliminary results, Muragliaet al[68]reported a transition from chondrogenesis to osteogenesis in human MSC spheroids produced using the pellet technique. Initially, a chondrogenic induction medium was used, composed of human TGF-β1 and dexamethasone. At the end of four weeks, the medium was replaced with osteogenesis inducing factors (β-glycerophosphate and dexamethasone) for an additional three weeks. The authors found crystallization inside the spheroids, together with remodeling from a typical cartilage extracellular matrix to bone. Table 1 summarizesin vitroandin vivostudies with ASCs spheroids for cartilage and bone engineering.
USE OF SPHEROIDS FOR THE DEVELOPMENTAL ENGINEERING OF CARTILAGE AND BONE
Developmental engineering for bone tissue formationin vitroaims to recapitulate the stages of bone development that occurin vivo[76,77]. Chondrogenesis is the primordial stage of skeletal development, involving the migration and recruitment of MSCs, the condensation of progenitor cells, and the differentiation and maturation of chondrocytes, which culminate in the formation of cartilage and bone, during endochondral ossification[78,79]. Fell[80]first described one of the early events in chondrogenesis: The aggregation of chondroprogenitor MSCs that leads to pre-cartilage condensation. This process depends on cell-cell and cell-matrix interactions,and is associated with intense changes in cytoskeletal architecture[81].
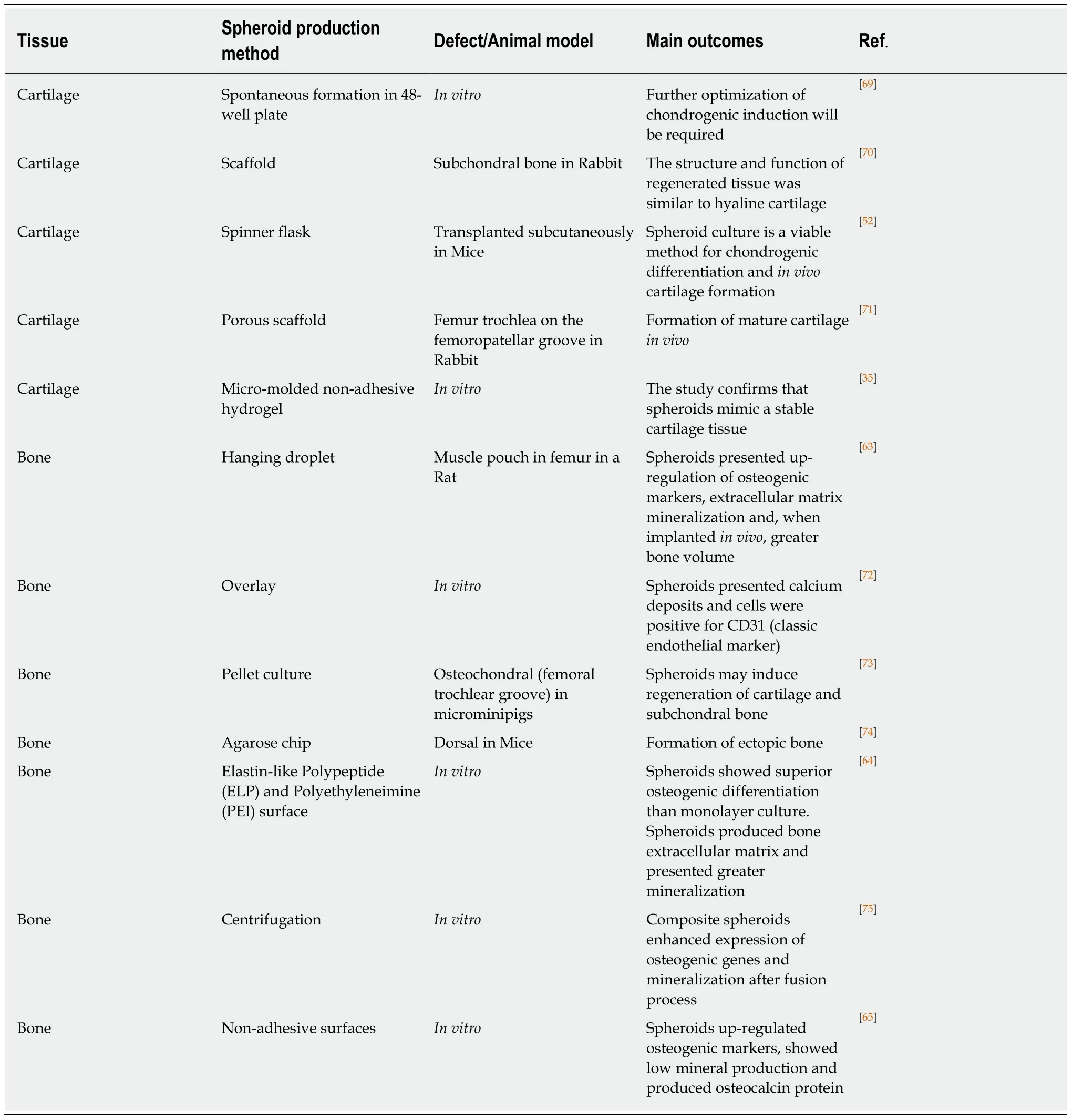
Table 1 In vitro and in vivo studies with adipose derived stem/stromal cells spheroids for cartilage and bone engineering
Bone engineering studies rely on mimicking endochondral ossification, the main mechanism of bone regeneration/repair after injury or fractures[82]. Endochondral ossification is tightly coordinated by cellular and molecular mechanisms[77]. MSCs initially condense and differentiate into chondrocytes, forming a hyaline cartilaginous matrix template that is subsequently replaced by vascularized bone tissue[83].
All hypertrophic cartilage-associated molecular events seem to occur in ASC spheroids, suggesting that these cells can be used to faithfully recapitulate endochondral ossificationin vivo[73]. According to our preliminary results, these main events can also be recapitulatedin vitrofrom ASC spheroids induced down the chondrogenic and osteogenic pathways (manuscript submitted). The stage of precartilage condensation is closely linked to an increase in hyaluronidase activity and to the appearance of cell adhesion molecules, mainly cadherins[36,84]. In spheroid formation, N-cadherin expression is directly correlated with successful chondrogenic differentiation, because it mimics the mesenchymal condensation that occurs in embryos[85]by a process of self-assembly[86]. Decorin and extracellular matrix molecules such as tenascin, TSP-1 and COMP then interact with cell adhesion molecules to activate intracellular signaling pathways and trigger the maturation of chondroprogenitor cells into chondrocytes[84]. Furthermore, induced ASC differentiation upregulates a trio of SOX genes (SOX9,SOX5,SOX6), and this is followed by the downregulation of RUNX2, the master inducer of osteogenesis[87], and ALP, a gene involved in mineralization[88,89]. Although spheroids are capable of recapitulating chondrogenesis steps, in our model spheroid-based chondrogenesis does not progress to bone differentiation as seen in endochondral ossificationin vivo[35].
During endochondral ossification, mature hypertrophic chondrocytes express classic osteogenic markers, such as RUNX-2, osterix, collagen type I, osteocalcin and osteopontin[82,83,90]. Calcification starts in the cartilage template, when hypertrophic chondrocytes secrete vascular endothelial growth factor, leading to cartilage vascularization and enabling osteoblasts to replace the calcified cartilage by mineralized mature bone[91]. Various studies have attempted to harvest the high angiogenic potential of hypertrophic chondrocytes to improve bone repair, by mimicking the events of hypertrophyin vitroto engineer optimized bone-like tissue or to improve angiogenesis and bone repairin vivo[31]. Most studies were performed with cells surrounded by biomaterials, and obtained positive results[92-95]. Studies using spheroids as a template for ossification showed that spheroids present an elevated capacity to differentiate into bonein vitro[68], and to regenerate this tissuein vivo[73,96],which may be linked to their ability to form hypertrophic chondrocytes (Figure 2).
In conclusion, ASC spheroids can be used as a model to mimic the differentiation events of stable or hypertrophic cartilage, depending on inducers and oxygen conditions. Spheroid cells differentiate into chondrocytes mainly due to hypoxia, and are capable of maintaining a stable chondrocyte phenotype. Subsequent differentiation into bone tissue appears to rely on an intermediate state of chondrocyte hypertrophy, which recapitulates endochondral ossification.
SPHEROIDS AS BUILDING-BLOCKS
In the last decade, the major challenge in the field of tissue engineering has been thein vitromanufacture of tissues compatible in size to injury sites and with a high density of cells, similar to that observed in native tissues and organs[97]. These requirements were the driving force for the development of “bottom-up” tissue engineering[98],where tissues are created by assembling “building blocks” into higher ordered 3D structures. The building blocks are represented by engineered, scaffold-free, 3D constructs such as spheroids, which are assembled into higher order structures using different technologies, of which the most common is 3D bioprinting[98-100].
The success of “bottom-up” tissue engineering relies on the inherent capacity of building blocks to fuse to each other, resulting in larger tissue constructs[5]. Given the ability of spheroids to recapitulate the main morphogenetic events in tissue formation,including fusion, they represent an ideal choice for building blocks in bottom-up tissue engineering[2]. Improving our understanding of the cellular and molecular mechanisms that underlie spheroid fusion is essential for the biofabrication of complex tissues using spheroids[101].
SPHEROID FUSION
Tissue fusion is a spontaneous process in embryonic development and occurs by cellto-cell and cell-to-extracellular matrix interactions, involving complex molecular and biophysical processes[102]. When spheroids are used to mimic tissue fusion, the process is controlled by surface tension forces culminating into a single cohesive structure[102,103]. One advantage of spheroid fusion is that the kinetics and morphological changes can be easily quantified using high-throughput technology,mainly by time-lapse brightfield images and fluorescence microscopy[104]. Then, the images obtained can be analyzed with a customized image analysis script[104]running on the free NIH image analysis software ImageJ.
Different studies have investigated the fusion process of spheroidsin vitro. Fleminget al[105]fused uniluminal vascular spheroidsin vitro, in a process that closely resembles the formation of the descending aorta during embryonic development,in vivo. Lehmannet al[106]produced 3D cartilage-like single spheroids using dedifferentiated chondrocytes, and generated larger microtissues consisting of several spheroids fused together, as a scaffold-free strategy for reliable treatment of osteoarthritis and cartilage defects due to trauma. These authors observed that fused spheroids showed increased production of extracellular matrix and higher levels of collagen II compared with single spheroids. Susienkaet al[104]designed a highthroughput platform to quantify spheroid fusogenicity using two different assays:Initially, a “tack” assay is used to measure the minimum time taken by two spheroids to form a stable microtissue “doublet”, while a fusion assay tracks the morphological parameters of fusion. This method is useful to explore the mechanisms involved in spheroid fusion and can be applied to different cell types, to identify differences in fusion processes.
Our preliminary results using the fusogenicity assay described above showed that ASC spheroids, when placed in pairs, start fusing at 24 h, while the whole fusion process is finished by day 7. The cellular and molecular mechanisms that control spheroid fusion remain poorly described. According to our preliminary results, at day 4 of culture, a population of spheroid cells migrates from the spheroid periphery to the region of fusion, at the center of the spheroid. In agreement with these data,Fleminget al[105]showed that the fusion of uniluminal vascular spheroids is mediated by the ability of spheroid cells to reposition themselves, maximizing their interadhesive interactions and minimizing the free energy of the system as a whole.
In ASC spheroids, we also observed a resistance to fusion directly related to osteogenic differentiation (manuscript in preparation). Similarly, Ahmadet al[75]showed that, when subjected to a protocol for mineralizationin vitro, ASCs spheroids only fused after seven days in culture; in this period, the cells remained viable and stained for Alizarin red O, indicating the presence of calcium deposits.
With regard to cartilage engineering, a study by Lehmannet al[106]showed that chondrogenesis increases when spheroids undergo fusion. Fused spheroids presented some similarities to native hyaline cartilage and were highly positive for collagen type II and proteoglycans, which are typical of cartilage extracellular matrix. Our group has shown that fusion is not impaired in ASC spheroids induced to undergo chondrogenesis[35]. Furthermore, in a different spheroid model, using CPCs, we observed that spheroids undergo fusion at day 7[58]; however, the contact area in fused CPC spheroids is reduced compared with that observed in ASC spheroids induced to undergo chondrogenesis.
In conclusion, spheroid fusion is the event that allows bottom-up tissue engineering to form larger tissue constructs. Ours and other research groups have shown that spheroid fusion is a fast, efficient and scalable process. However, further molecular and cellular studies are necessary to understand the mechanisms involved in fusion,in order to produce stable tissue constructs that recapitulate tissue morphogenesis and exhibit the desired functionality.
SPHEROID BIOPRINTING
The 3D bioprinting of tissue constructs is considered one of the latest technologies in tissue engineering and regenerative medicine, promising to facilitate the development of complex tissues and organ constructs[107]. Bioprinting evolved from 3D scaffold printing, a technique developed by Hull[108]in the 80s, and initially applied to improve scaffold properties[109,110].
Currently, 3D bioprinting techniques are an attractive strategy for bottom-up tissue engineering due to the possibility of engineering with precision larger and complex tissue constructs with suitable mechanical properties and desirable biological functions. In 3D bioprinting, biomaterials containing bioactive molecules and encapsulated cells, referred to as the “bioink,” are added layer-by-layer to form previously designed patterns[109]. The state-of-the-art is to distribute cells or spheroids and bioactive agents with precision to form a 3D structure,viathe controlled extrusion activity of a bioprinter[111].
Viscontiet al[103]discussed the importance of spheroid fusion to form an intra-organ vascular tree by 3D bioprinting. Vascular spheroid fusion resulted in a functional and physiologically relevant 3D structure similar to a blood vessel, showing both vasodilatory and contractile responses[112]. Importantly, the fabrication of a vascular structure is an essential initial step to successfully engineer large tissue constructs due to the need for vascularization in native organs. The biofabrication of larger constructs requires spheroids to be homogeneous in the desired size and shape[113], and our research group has shown that this homogeneity can be obtained by the use of molded non-adherent hydrogel[35].
One interesting strategy, developed by Yuet al[114]used a scalable bioink referred to as “tissue strands” in scaffold-free bioprinting, to facilitate the accurate biofabrication of biomimetically developed tissues. The model was based on chondrocyte spheroid fusion to produce the tissue strands, which were then bioprinted into a more complex cartilage construct without the use of hydrogels. The authors successfully produced bovine articular cartilage tissues with morphological, biochemical and mechanical properties close to those of native cartilage.
In a recent outstanding study, Dalyet al[115]used inkjet bioprinting to deposit a cell suspension of MSCs and chondrocytes into 3D printed microchambers, to form highly organized arrays of spheroids. The morphological composition and the biomechanical properties of the bioprinted cartilage-like tissue construct were similar to those of native cartilage foundin vivo.
Despite different efforts and advances in spheroid 3D bioprinting, many challenges must still be overcome to allow this technique to reach its full potential. These challenges include the incorporation of blood vessels and nerve fibers in tissue constructs[116], and the production of large and uniform constructs suitable for future clinical applications[117].
CONCLUSION AND PERSPECTIVES
Recent advances in the developmental engineering area, which aims to recapitulate the cell and molecular stages of embryonic development to form a desired tissue[76,77],have allowed the establishment of spheroid-basedin vitromodels that mimic more closely embryonic processes, including endochondral ossification and mesenchymal condensation, which represent stages of bone and cartilage formation, respectively.Spheroids are ideal building blocks for bottom-up tissue engineering mainly due to their high fusion capacity. Further studies on the spheroid fusion process and the refinement ofin vitrotissue biofabrication technologies such as 3D bioprinting are now essential for the production of higher order tissuesin vitro. In conclusion, 3D bioprinting using ASC spheroids as cartilage and bone building blocks is a promising technology for future development of tissue constructs for clinical use, by bottom-up tissue engineering.
ACKNOWLEDGEMENTS
We would like to thank Ana Claudia O Carreira, PhD and Professor Mari Cleide Sogayar, PhD for human recombinant BMP-7 production.
 World Journal of Stem Cells2020年2期
World Journal of Stem Cells2020年2期
- World Journal of Stem Cells的其它文章
- Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective
- Clonal isolation of endothelial colony-forming cells from early gestation chorionic villi of human placenta for fetal tissue regeneration
- Comparison between the therapeutic effects of differentiated and undifferentiated Wharton's jelly mesenchymal stem cells in rats with streptozotocin-induced diabetes
- C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization
