Application Research of Earned Value Management in New Drug Research and Development Projects
Wang Wanting,Xing Hua
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To help investors assess and control the costs of new drug development and reduce the risks of new drug development projects.Methods Cost analysis and financial forecasting were carried out with the integrated approach of earned value management.According to the principle of earned value management deviation analysis,the basic process of the new drug research and development project was combined with the hypothesis method from the research of Tufts Drug Development Research Center.Results and Conclusion If the project progress check was carried out in the clinical trial,the project costs were found overspent,the efficiency was low,the project progress was faster,and the resource investment was ahead.It is recommended that the adjustment should be made to reduce the input of resources,and increase the efficient key personnel to take the place of some less efficient staff.
Keywords:new drug research and development;earned value management;cost analysis
1 Overview
The research and development(R&D)of new drugs is of great significance to China's pharmaceutical industry.It is not only the key to the competitive advantage of China's pharmaceutical industry,but also the core of the sustainable development of pharmaceutical enterprises.As we all know,new drug R&D is not only a high-tech,multi-disciplinary complex system,but also has the characteristics of a long cycle,high risk and huge investment[1].As the cornerstone of the survival and development of pharmaceutical enterprises,the successful development of a new drug can not only benefit the society,but also bring huge profits to the enterprise.
Since China's entry into WTO,our pharmaceutical industry has been facing great challenges.The main reason is that enterprises lack R&D capabilities of new drugs.Most enterprises mainly produce generic drugs in China.Although China has the biggest market for medicine,it is not a powerful medical country.The lack of innovation capacity is undoubtedly the weakness for China's pharmaceutical industry in international competition.A large number of China's pharmaceutical enterprises cannot afford the high risk of new drug R&D,so they will not become the mainstay of medical technology innovation.As a result,many pharmaceutical enterprises have insufficient R&D investment[2].The investment in new drug R&D in developed countries has reached 10% to 25% of their sales,while that has only 1% to 2% in China.Insufficient investment is also an important reason for the limited development of new drug research projects in China[3].
Therefore,it is particularly important to control the R&D investment of enterprises through effective cost management methods.Because the traditional project management method can only manage the progress,cost and scope separately,without effectively linking the three together,it cannot fully reflect the progress of the project and the existing problems[4].However,earned value management technology is an integrated method for the management of project cost and schedule,which solves the defects of traditional project management methods.This paper will adopt the relevant theory of earned value management to analyze the cost of new drug R&D projects to provide reference for relevant project managers.
2 Relevant theory of earned value management
2.1 The basic principle of earned value management
The essence of earned value management is actually to introduce an intermediate variable“budget cost of completed workload”to analyze the progress and cost of the project on the basis of deviation analysis.It is an integrated management method that is beneficial to managers to make a scientific prediction and judgment on the future development trend of the project cost and schedule[5].
There are three basic indicators of earned value management,namely,budgeted cost for work performed(BCWP),budgeted cost for work scheduled(BCWS),and actual cost for work performed(ACWP).There are also four deviation indicators,namely,cost variance(CV),schedule variance(SV),schedule performance index(SPI),cost performance index(CPI).Meanwhile,there are two prediction variables;one is forecasted cost at completion(FCAC)and the other is estimated completion date(ECD).
If we comprehensively analyze the three basic indicators,according to their absolute differences,we will get the following seven possible results:first,BCWP>ACWP>BCWS;second,BCWP>BCWS>ACWP;third,BCWS>BCWP>ACWP;fourth,BCWS>ACWP>BCWP;fifth,ACWP>BCWP>BCWS;sixth,ACWP>BCWS>BCWP;seventh,BCWS=BCWP=ACWP.However,there are many uncertain factors in the implementation of new drug development projects.So BCWS=BCWP=ACWP is an idealized result which is unlikely to happen in reality.In addition,we explain the other six cases according to the above serial numbers as follows.
First,the cost is saved,the efficiency is high,the progress of the project is advanced,and the input of resources is ahead of schedule.Therefore,some resources can be extracted to slow down the progress of the project.Second,the cost is saved with high efficiency,and the project progress is ahead,but the input of resources is delayed.If the progress of the project does not deviate from the plan,the status quo can be maintained.Third,the cost is saved,the efficiency is high,but the progress of the project is slow,and the resource investment is delayed.Therefore,we should increase investment quickly and speed up the project to catch up with the project plan.Fourth,the cost is overrun,the efficiency,the progress of the project and the inputs of resources are slow.Therefore,highly efficient personnel should be added to improve work efficiency and speed up project progress.Fifth,the cost is overrun,the efficiency is low,but the progress of the project is faster,and the resources are invested ahead.Therefore,some people should be drawn out and the resources should be cut,but high-efficiency key personnel are needed.Sixth,the cost is overrun,the efficiency is low,the progress of the project is slow,but the investment in resources is ahead.Correction can be done by adding highefficiency personnel[6].
2.2 The advantages of earned value management
Earned value management can display cost and schedule deviations clearly.The earned value curve can be used to comprehensively analyze costs and schedules.However,these advantages are not available in traditional cost control methods.The cost deviations found in traditional cost control methods can only clarify the difference between the actual cost and the budgeted cost,but the impact on the cost due to the change in schedule is difficult to determine.When the project progress is ahead or behind,the project workload adjustment will also lead to cost deviation.Therefore,if the traditional method is used to supervise and analyze the progress of the project,it is difficult to determine the progress deviation,and it is hard to know the impact of such schedule deviation on the cost.However,earned value management can effectively reduce project risks and strengthen project management and control by understanding the existing cost status through an analysis system of three basic indicators,four deviation indicators,and two predictive variable to predict the future state of cost and progress.Earned value management can disperse the project work into all stages of the project,which helps to form an all-round responsibility system.Earned value management can effectively improve the management and cost control.The value management method comprehensively reflects the project progress status and cost deviation through three-valued disaggregation,which is convenient for project cost control personnel to understand and apply[7].
2.3 The practical significance of earned value management applied to new drug R&D projects
The development of medicine needs innovation and promotion.With the changes in economic development and people's lifestyles,China's disease map has also undergone major changes.Complex diseases such as tumors and metabolic diseases seriously endanger people's health.But there are differences in the causes of death among the same types of diseases.For example,high blood pressure patients in China mostly die of stroke,but most American patients are myocardial infarction,which means that the treatment methods and medicines used in China are not exactly the same as those in the United States.Therefore,it is imperative to strengthen the R&D of independent new drugs in China.The problems faced by China's new drug R&D are not only technology,but also many other problems.Among them,the most important issue is the introduction of capital.Sang Guowei,an academician of the Chinese Academy of Engineering,once said:“What we lack most now is venture capital and personal investment.”The reason why investors are uncertain about the investment decisions of new drug R&D projects is that both the investment and the risks are too high.Therefore,it is vital to assess and control the cost of new drug R&D and reduce the research risk,thus reducing the risk of investors.
The requirement for new drugs to be marketed is safety and effectiveness,but we know that even if the safety and effectiveness are met,there is no guarantee that the market prospect of the drug will be good.If the input cost is too large,it is likely that the economy of the drug deteriorates and causes losses.Therefore,it is necessary to carry out cost analysis and control at each stage of the process of drug R&D[8].The safety,effectiveness and economy of the drug determine whether to proceed to the next phase of the research.
The cost of new drug R&D cannot rely on one factor only,quality and environment must be taken into consideration.The control of R&D cost is not a single cost reduction.It is beneficial for enterprise to improve efficiency and capital utilization.Focusing on the cost economy of the entire product life cycle and optimizing the resource allocation in the entire R&D process is the key to our new drug R&D cost control.So far,some traditional methods such as cost analysis,market analysis,cash flow discounting,and real options can be used to evaluate the cost of new drug R&D projects[9].However,due to many variables in the development of new drugs,traditional cost analysis methods have many drawbacks for new drug R&D.For example,the research scope is narrow and it lacks innovation awareness,which is shown in the fact that enterprises pay more attention to the product cost in production field while neglecting the cost of other links.Because output is considered as the only reason to explain the change of product costs[10].
Applying earned value management analysis in new drug R&D projects can measure and analyze various indicators in cost control.Therefore,project cost is always under monitoring state,which is convenient for enterprises to timely and accurately grasp relevant cost information and understand the progress trend of the project.In this process,corresponding measures are taken to keep it within the normal range.Practices in many industries have proven that applying earned value management to cost control of projects is effective and feasible.It can make the project management more efficient and orderly with clearer work objectives and true results.Thus,we have reason to believe that in the new drug R&D project,the earned value management method can still play its role in bringing us a more scientific and effective financial forecasting than traditional cost management.
3 Specific application of earned value management in new drug R&D projects
According to the research results obtained by the Tufts Drug Development Research Center in 2014,we can get some mean data.In the discovery and screening stage,the researchers of pharmaceutical enterprises discovered new drugs through in-depth study of the disease process,enabling them to design products that can reverse or inhibit the spread of disease.Normally this phase will cost $ 196 million in 1.5 years.In the preclinical phase,the researchers conduct research to provide detailed information on toxicity levels and optimal doses,which is regulated strictly by FDA,with an average cost of $ 122 million in 1.5 years.The initial phase of clinical trial design,investigational new drug application,and FDA approval is relatively low in cost,which has no specific numerical estimation.Clinical research phase consists of three parts to test the safety and efficacy of the drugs on humans and their optimal doses.In the first part,drugs are tested in 20 to 100 volunteers with disease,and the goal is to determine the optimal dose and ensure patient safety.This part runs an average of 1.6 years at a cost about 169 million US dollars.In the second part,drugs are tested in hundreds of volunteers who suffer from the disease to get the efficacy and possible side effects of the drug,with an average cost of $ 252 million and an average duration of 2.5 years.In the third part,the test will be carried out in 300 to 3 000 volunteers,which is to further test the efficacy of the drug,and to monitor adverse reactions or side effects after taking the drug for 1-4 years.It will take an average of 2.5 years and cost about $ 536 million.As an additional safety program,sometimes thousands of volunteers will be given a fourth part to test the efficacy and safety of the drug.But,in summary,the clinical research phase can cost an average of $ 1.1 billion over 6.6 years.
In order to apply the method of earned value management to practice,we use the hypothesis method and the above data as a reference to combine some other basic data to explain the management of earned value in new drug R&D projects for reference without considering the time value of funds.
Suppose a new drug development cycle takes 11 years,costing $ 1.1 billion,and planned costs are evenly distributed over time.In the discovery and screening phase,the plan takes 1.5 years and costs $100 million per year.But the actual time is 1.6 years,which costs $ 122.5 million per year.In the preclinical research phase,the plan takes 1.5 years and costs $100 million per year.But the actual time is 1.4 years,which costs $ 87 million per year.In the first part of clinical trial phase,the plan takes 1.6 years and costs$ 100 million per year.But it takes 2 years and spends$ 84.5 million per year.In the second part of clinical trial phase,it takes 2.5 years and costs $ 100 million a year.Actually it takes 2.3 years and costs $ 109.6 million annually.
Based on the above assumptions,the earned value method is used to analyze the project till clinical phase II,and the following analysis process is shown in Table 1 and Table 2.
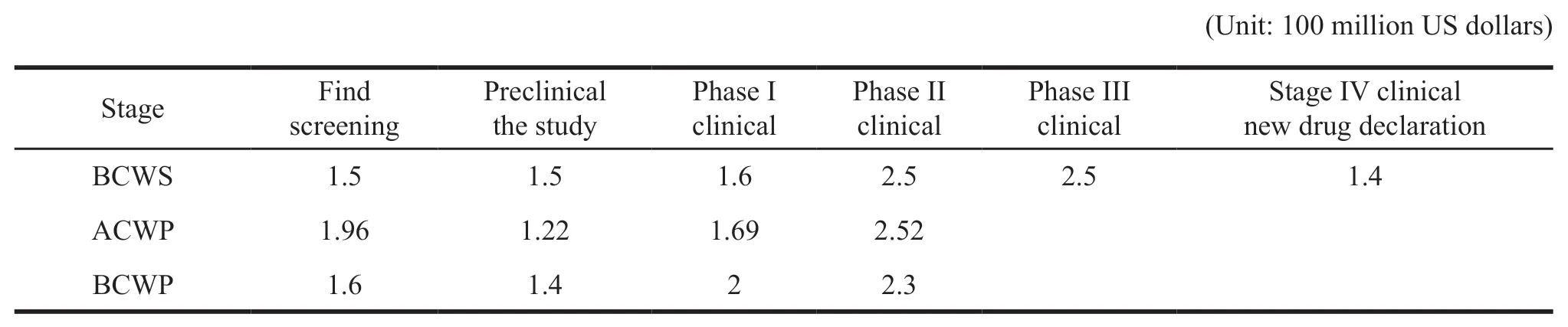
Table 1 Basic parameters of the project
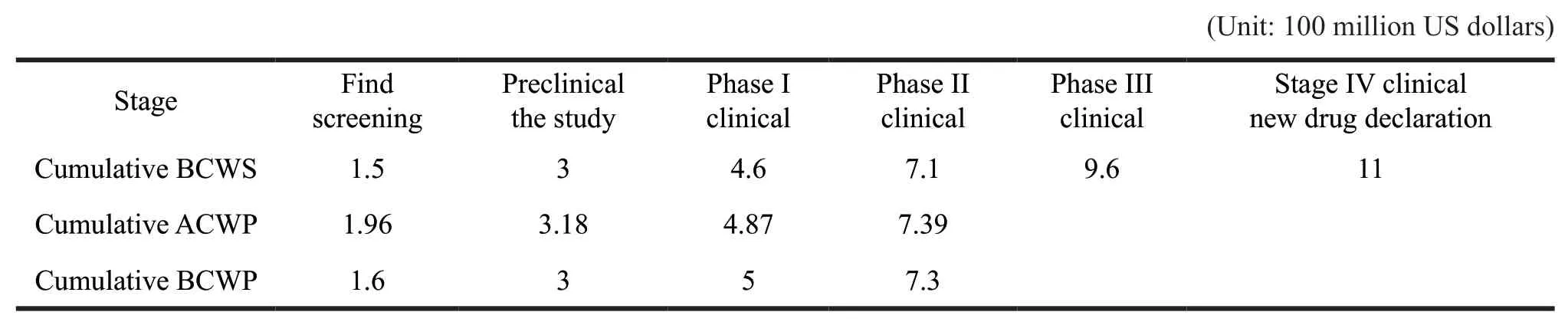
Table 2 Cumulative values of basic parameters of the project
First,the calculation of evaluation indicators:CPI=BCWP ÷ ACWP=7.3 ÷ 7.39=0.988;
SPI=BCWP ÷ BCWS=7.3 ÷ 7.1=1.028;
CV=BCWP-ACWP=-0.09;
SV=BCWP-BCWS=0.2
Second,the estimated completion cost:
(a)Assume that unfinished projects can work at current efficiency:
FCAC=TBC ÷ CPI=11 ÷ 0.988=11.13
(b)Assume that unfinished projects are proceeding according to planned efficiency:
FCAC=ACWP+(TBC-BCWP)=11.09
Third,the project duration is expected:
Assume that unfinished projects can work at current efficiency:
ECD=OPD ÷ SPI=11 ÷ 1.028=10.7
4 Conclusion
Referring to the above case,if the cumulative progress of BCWS,ACWP,BCWP,and the phenomenon of CV<0,SV>0 are compared in the phase II clinical project check,it can be determined that the project belongs to the absolute difference analysis.It belongs to the fifth case,the cost is overrun,the efficiency is low,the progress of the project is fast,and the resources are invested ahead.At the same time,combined with the situation of CPI<1,SPI>1,the progress of the project is faster,ahead of schedule,but its cost is overrun and the efficiency is low.We can reduce the input of resources and replace some low efficient personnel with the high efficient ones to adjust.According to current efficiency estimation,the completion of the project is expected to use 10.7 years and spend $ 1.113 billion.
Due to the complexity of the earned value analysis and cost control of the new drug R&D project,the six situations of earned value management in the actual analysis cannot be explained one by one.Only one of them is explained to clarify the basic principles and the actual application.Though the specific implementation situation may be very complicated,the principles are consistent for enterprises to control the cost and efficiency of new drug R&D projects quantitatively.In this paper,the research on the use of earned value management in the cost control of new drug R&D projects is not profound.It only provides a reference and method for the relevant managers of new drug R&D projects.In order to improve the cost control system of new drug R&D projects in China,further research and discussion are still needed.
 Asian Journal of Pharmacentical Sciences2020年1期
Asian Journal of Pharmacentical Sciences2020年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Information for Authors
- The Analysis of Marketing Strategy of Plendil
- Comparative Study on the Development of Urban Basic Medical Insurance in 14 Cities of Liaoning Province
- Research on the Transformation of Mapping Method for Cancer Patients' Health Utility in the Asia-Pacific Region
- Pharmacoeconomic Evaluation of Rituximab(Hanlikang)for Patients with Rheumatoid Arthritis
- Compulsory Licensing of Pharmaceutical Patents System from the Perspective of Public Interest-Public Health in China
