Compulsory Licensing of Pharmaceutical Patents System from the Perspective of Public Interest-Public Health in China
Zhang Yubo,Yuan Hongmei
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To study the types and acquisition paths of drug data in the administrative act of compulsory licensing of pharmaceutical patents so as to provide data support for the implementation of compulsory licensing of pharmaceutical patents from the perspective of public interest-public health in China.Methods Situation of compulsory licensing of pharmaceutical patents in the context of public interest-public health in China was selected,combined with international conventions and the principle provisions of relevant laws,and cases of compulsory licensing of pharmaceutical patents in foreign countries were reviewed to extract drug-related data of compulsory licensing of pharmaceutical patents.Results and Conclusion According to the analysis of China's compulsory licensing of pharmaceutical patents theory and legal provisions,the data could be divided into five categories for compulsory licensing of pharmaceutical patents in China:diseases,drugs,patents,generic drug enterprises,and licensing fees.Although,some data are traceable.There were also problems in obtaining data or inconsistent standards in five levels of compulsory licensing of pharmaceutical patents in the context of public interest-public health.It was not conducive to the implementation of compulsory licensing of pharmaceutical patents in China.Thus,it should be further established a unified data support system at the national level,specifying the standard of licensing fee,implementing relevant laws and regulations.
Keywords:compulsory license;public interest;public health;data acquisition
The compulsory licensing of pharmaceutical patents system has been recognized by the international community due to its incorporation into TRIPS.Some countries,such as the United States,India,and Thailand have used this system in practice and obtained good social benefits.This system is not strange in China,but there is no precedent of using this system in practice.Nowadays,the conditions for implementing the compulsory licensing of pharmaceutical patents system are mature.However,the implementation of this system involves many important and sensitive issues,such as the complex drug data types with specialty.Thus,in order to ensure the legality,science and order,accurate research and demonstration on the types of drugs involving data and acquisition paths become one of the necessary procedures to launch the preliminary preparation of compulsory licensing of pharmaceutical patents in China.Based on the research of theory and system,some drug data types and access path were studied to provide support for realizing the drug patent compulsory license under the condition of public interest-public health.Thus it can promote the implementation of the compulsory license,drug accessibility,and improve the protection of public health in China.
1 The public interest-public health and compulsory licensing of pharmaceutical patents
As an important part of the intellectual property system,TRIPS agreement stipulates the principles for the compulsory licensing of pharmaceutical patents[1],giving each country the right of free decision-making and it can design a compulsory licensing system of pharmaceutical patents according to its own national conditions.In practice,many countries explore the system design suitable for their own national conditions,and have achieved significant results.In view of the different situations of compulsory licensing of pharmaceutical patent and the particularity of each situation,it is believed that selecting a specific situation for research based on China's national conditions is necessary.After repeated deliberation,we choose public interest-public health as the research entry point,and the reasons are the followings.
First of all,the essential attribute of compulsory licensing strictly limits the start-up situation.Compulsory licensing is to deprive citizens' legal private rights through administrative power.Thus,both TRIPS agreement and the laws of all countries in the world have made extremely strict regulations on the starting situation of this administrative act[2].They require that the different situation should be dealt with respectively.Therefore,a more broad and independent research horizon—the public interest-public health is selected as the research situation.
The second is the international convention system design requirements.In 2001,all the member countries signed the Declaration on TRIPS and Public Health in Doha[3],which recognized that public health problems seriously affected many developing and the poorest countries,especially those suffering from AIDS,tuberculosis,malaria and other infectious diseases.In 2005,the WTO General Council adopted the Revised TRIPS Protocol,which elevated the contents made in Doha Health Declaration into a formal provision of TRIPS agreement in the form of a treaty,marking that public health,an integral part of public interest,became the legal basis for members to issue compulsory licensing of pharmaceutical patent.
Then,the specific background of compulsory licensing in China was taken into consideration.According to China's patent law,China's patents compulsory licensing was divided into six situations,as shown in Table 1.
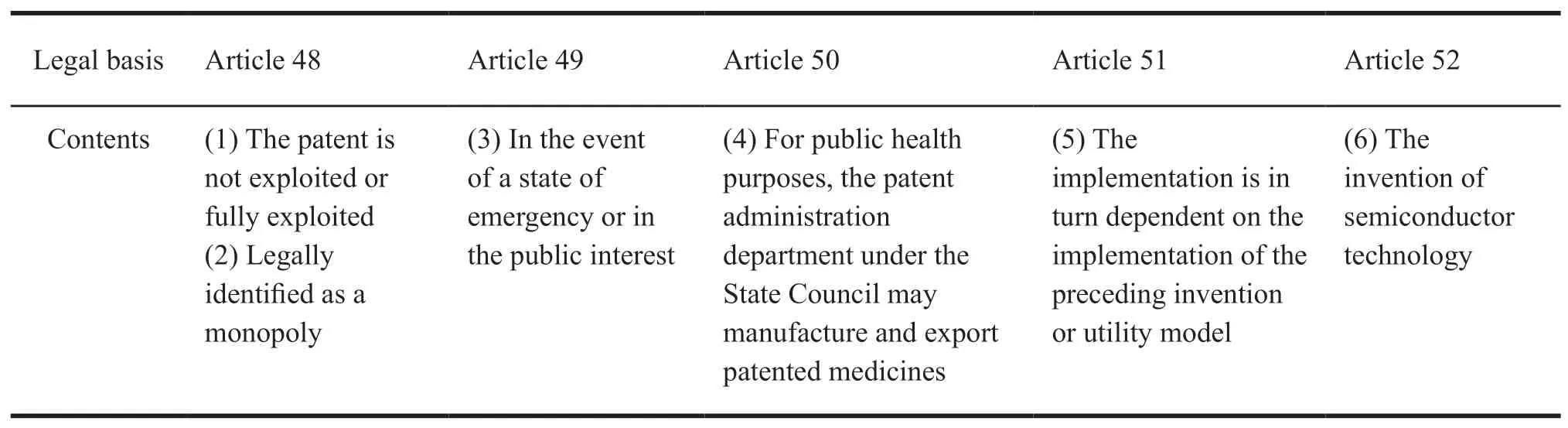
Table 1 China's patent law on patent licensing situations
Firstly,the domestic patent compulsory licensing of the basic issues of theory and application should be clarified.Then,export dimensions should be clarified too.Based on the above considerations,this article selected(3)as the research situation.Due to the rare national emergency,the public interests are taken as the research key.In view of the fact that public interests in the world are within the scope of public health category of compulsory licensing of pharmaceutical patents,this paper focuses on public health in order to be more targeted and operable.Therefore,the chosen situation is public interest-public health.
2 Elements of compulsory licensing of pharmaceutical patents from the perspective of Public interest-public health
2.1 Drug factors
Base on the trade-related intellectual property agreement amendment protocol,drug is defined as a patented product of pharmaceutical industry or a product produced by a patented process for public health.The active ingredients of the products and the diagnostic kits are also included[4].
According to the Drug Administration Law of the People's Republic of China in 2019,drugs refer to substances used for the prevention,treatment and diagnosis of human diseases,for the purpose of regulating human physiological functions and for prescribing indications,functions,usages and dosage,including traditional Chinese medicine,chemical drugs and biological products.Meanwhile,the detailed rules for the implementation of the patent law in article 73 pointed out that the patent drugs refers to any patented products used for public health or direct access to products in accordance with the patent method,including the patent right of manufacture for active ingredients of the products and diagnostic products.
It can be found from the cases of compulsory licensing of pharmaceutical patents in India,Brazil,Thailand and other developing countries that the selection of target drugs for compulsory licensing should meet the following conditions,shown in Table 2.
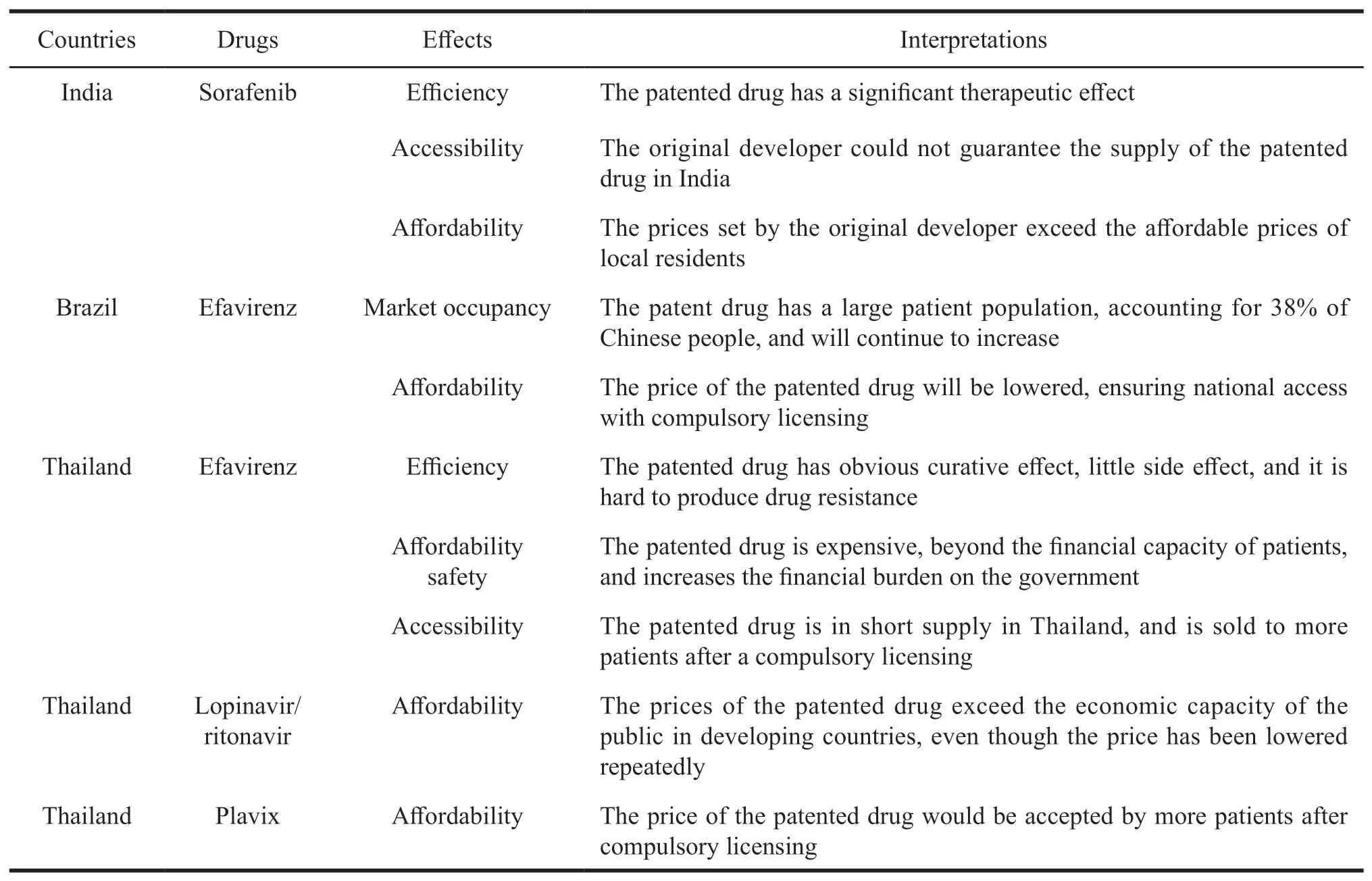
Table 2 Conditions of compulsory licensing of pharmaceutical patents in various countries
According to the existing practice examples of compulsory licensing in foreign countries,it is believed that the patented drugs suitable for compulsory licensing should meet the following conditions.First,the substitutability of patented drugs is low.When the public are faced with health problems,the irreplaceable and important role of patented drugs is the first requirement for compulsory license of the target drugs.Second,the accessibility of the patented drug is poor.When the public are faced with health problems,they have a greater demand for patented drugs,so patented drugs must have adequate access.Third,the affordability of the patented drug is low.If the price of patented drugs is far beyond the public's expectation,many patients will not receive good treatment,which seriously threatens the national health and quality of life.
2.2 Patent elements
In the term of patent protection,the following stipulations were made in article 28 of the TRIPS agreement.A patentee should have the following exclusive rights.For the product patent,the patentee has the right to prohibit the third party from engaging in these acts:manufacturing,using,and providing for the sale,selling,or importing the product for the above purposes without his consent.For the method patent,the patentee has the right to prohibit third parties from using the method without his consent as well as selling or importing products directly obtained by the method.The patentee shall have the right to assign or transfer patent through inheritance,and to conclude a license contract.
Invention means creation,utility model and designs according to the patent law of China.It refers to a new technical proposal for a product,a method or an improvement.The method invention includes a technical proposal for an operation method,a manufacturing method and a technological process,etc.The term utility model refers to a new technical scheme for the shape,construction or combination of a product that is fit for use.Meanwhile,appearance design refers to the new design made on the shape,pattern or their combination.Besides,it is the combination of color,shape and pattern,which is rich in aesthetic feeling and suitable for industrial application.Therefore,it is believed that the patent of a drug suitable for compulsory licensing should have such condition that the patent related to the drug must be authorized in China and it is in an effective state.
2.3 Definition of generic drug enterprise qualification
The appendix of the revised protocol to the TRIPS agreement stipulated the criteria and methods for assessing the lack of productive capacity of importing countries[5].The production capacity of the pharmaceutical industry is as follows.
The least developed member states are considered to have no or insufficient manufacturing capacity in the pharmaceutical industry.For other member states that are eligible for import,it can be determined by one of the following ways that they do not have or lack the production capacity for the relevant drugs.The member state has proved that it has no production capacity in the pharmaceutical industry,but it has some production capacity in the industry.The member state has been investigated that capacity is currently insufficient to meet its own needs except for the production capacity owned or controlled by the patent owner.The system may no longer apply to this member state when it is proved that the productive capacity is sufficient to meet its needs[6].
Article 48 of the Patent Law in China provides that under any of the following circumstances,the patent administration department under the State Council may grant a compulsory licensing to exploit the patent for invention or utility model upon the application of any qualified entity or individual.The above having the conditions for implementation indicates the qualifications of generic drug enterprises in compulsory licensing of pharmaceutical patents.Firstly,it should have the technical conditions to manufacture patented products with patented methods.Secondly,it has the capital and assets conditions.Thirdly,it should meet the market supervision requirements for manufacturing patented products with patented methods,and have the market access qualification.If the exploitation of the patent by the licensee under compulsory licensing belongs to the licensee's import of the patented product,the conditions for implementation mentioned in article 48 should be interpreted as follows.First,the import and export of the patented technology and product should comply with the requirements of the import and export control regulations of the relevant country.Second,it must have proof of funds.
2.4 Definition of license fee
In 2012,Indian Patent Office issued a compulsory license for the anti-cancer drug Nexamei,and Indian generic drug company Natco had to pay Bayer a patent fee of 6% of sales[7].The Thai government issued a compulsory license for Efavirenz.The government recommended a license fee of 0.5% of the generic drug price.The amount of the generic drug price was determined by the additional negotiation between the licensee and the patentee.In 2003,in order to import the generic drug of the patented drug ARV from the Indian company CIPLA,Malaysia issued a compulsory license for the import of Zidovudine,Didanosine and Zidoramide Difudine from India by the Ministry of Domestic Trade and Consumer Affairs within 2 years.As to compensation,the Ministry of Health proposed to give the patentee 4% of the actual shipment value per year,but the patent holder did not accept this compensation[8].By summarizing the calculation of the compulsory license fee in various countries,four modes were sorted out[9-10].They are UNDP mode,Canadian mode,fixed ratio method,and tort compensation method.
2.4.1 UNDP model
The UNDP Human Development Report recommends a simple system of patent royalty guidelines,which is primarily for developing countries with the aim of ensuring the transparency and predictability of compulsory licenses.The basic compensation is 4% of the generic drug price,which can be increased or decreased by 2% depending on the degree of innovation of the drug or the government's investment in research and development.Therefore,the compensation fee is 2% to 6% of the generic drug price.
2.4.2 Canadian model
The Canadian government has developed a guideline on royalty for compulsory licensing of pharmaceutical patents imported by countries that lack pharmaceutical capabilities.According to the national ranking in the UNDP Human Development Index(HDI),the compensation ratio is 0.02% to 4%.For most developing countries,the ratio is less than 3%,and for most African countries,the ratio is less than 1%(Compulsory license fee ratio=0.04 *(178-Drug importing country HID ranking)/177).
2.4.3 Fixed ratio method
It refers to the determination of the compensation fee for compulsory license according to a certain proportion of the sales of patented products.There are two ways.One is to determine the proportion of patent royalties by the discretionary authority to exercise discretion in specific cases.The other is to determine the proportion of royalties through legislation or lawsuit.
2.4.4 Tort compensation method
It is originated from the Diddell v.Vickers case in the United Kingdom.According to this method,the compensation for patent compulsory licenses should be calculated based on the amount of compensation that should be paid for infringement of patent rights.
At present,Patent Law of China,Patent Law Implementation Rules and Patent Implementation Compulsory License Measures have not clearly explained the reasonable use fee.Besides,there is no compulsory license fee measurement example.So when the license is used to carry out the enforcement in the future,the measurement of the license fee can be drawn from the above four models in foreign countries.In the case of simulation,this paper also studied the data points involved in the four calculation methods of license fee to provide reference for the future compulsory license in China.
3 Public interest-public health compulsory license program data design
The starting point of this study is compulsory licensing of pharmaceutical patents in the public interest-public health context.The types of data needed by compulsory licensing of pharmaceutical patents and the acquisition path in the public interestpublic health context are chosen according to the relevant legal provisions mentioned references.After the previous analysis of the legal provisions and examples of compulsory licenses,the data are finally classified into five categories,which include disease data,drug data,patent data,generic drug enterprise data and license fee data.
3.1 Selecting disease data
In terms of diseases,data on seven aspects are selected,including macroscopic statistics of diseases,chronic non-communicable diseases,sexually transmitted diseases,AIDS,viral diseases,tuberculosis,parasitic diseases and infectious diseases.Among them,the Yearbook of National Health and Family Planning Commission Statistical provided macroscopic statistics on 28 infectious diseases and deaths from the reports of Class A and B.The number of incidence,mortality and death of 28 infectious diseases are shown in each year or province.The 28 infectious diseases are classified into different age groups,genders,and statistics on the mortality of urban and rural areas.At the Center for Disease Control and Prevention of China,the status of chronic non-communicable diseases,sexually transmitted diseases and AIDS can be checked.In addition,monitoring dynamic information on viral diseases,tuberculosis and parasitic diseases can be provided as well.Last,the Emergency Command Center for Public Health can also provide an epidemic report when an infectious disease occurs.
Thus,it is not difficult to observe disease conditions from various channels and comprehensive information to support the data acquisition and analysis of the compulsory licensing program.
3.2 Selecting drug data
In terms of drugs,the substitutability,accessibility,and affordability are the key factors to determine whether a patented drug could become a public interest-public health target.As to substitutability,it should include three levels of data such as technical information,safety information and market information.The Drug Evaluation Center of the State Drug Administration is responsible for conducting technical reviews of drug registration applications and providing technical information services.The National Adverse Drug Reaction Monitoring Center can get the reports about adverse drug reaction.The health administrative department can obtain the information of market occupancy through public hospitals.In addition,the information on the supply of medicines in public hospitals and retail pharmacies in key cities can also be obtained through the intranet and China Medical Industry Information Center.For accessibility,it can be considered from the four levels of domestic production capacity,production,import volume and reserves.Local Drug Regulatory Departments master production capacity information of pharmaceutical personnel and equipment.Meanwhile,the production information is reported by enterprises and the import quantity information is supervised by the Port Drug Supervision Bureau which allows the import.The State Material Reserve Bureau,a subordinate unit of the National Development and Reform Commission,prepares a catalogue of national strategic material reserves,and supervises the quantity,quality and storage safety of the reserve strategic materials so that it can grasp the information on the amount of relevant medicines.In terms of affordability,it can be considered from the three levels of sales price,patient payment ability,and medical insurance reimbursement.The drug sales price data mainly includes the drug bidding price of the health administrative department and the recruitment platform.Patients' capability of paying mainly includes the total health expenditure per capita,health care expenditure per capita and proportion in all provinces provided by the Health and Health Commission,as well as GDP per capita,income per capita and consumption expenditure provided by the National Bureau of Statistics.The medical insurance reimbursement data mainly includes the medical insurance reimbursement information of the National Basic Medical Insurance,the Work Injury Insurance and Maternity Insurance Drug List provided by the Ministry of Human Resources and Social Security,and the annual medical insurance expenditure per capita.In addition,personal payment ratio data of the B medicines are provided by the municipal social security bureau.
Through the above analysis,it is not difficult to find that the situation of drugs can be observed from a variety of channels and information to provide support for data acquisition and analysis of compulsory licensing procedures.
3.3 Selecting patent data
In the aspect of patents,we need to know the information of drugs,the basic information of patents,the technical information of patents,and the legal status of patents.
Pharmaceutical patent information can be obtained from the publication of patents related to drug registration of the State Drug Administration and the collection of listed drug catalogues in China.Information on the basic technology and legal status of patents can be obtained from the patent retrieval module of the website of the State Intellectual Property Office of China and IncoPat Patent Database.Information on patent challenges can be retrieved from the intellectual property module of the Judgment Document of China's Supreme People's Court's.
Through the above analysis,it is not difficult to find that patents can be observed from a variety of channels and information to provide support for data acquisition and analysis of compulsory licensing procedures.
3.4 Data selection of generic pharmaceutical enterprises
As to generic pharmaceutical enterprises,their qualification,copying ability,and cost accounting of generic drugs are essential elements for them to be the candidates for compulsory license of pharmaceutical patents.Qualifications of generic pharmaceutical enterprises and their business license information can be obtained from the State Administration of Market Supervision-State Enterprise Credit Information Publicity System.But the conclusion document of on-the-spot inspection of copying ability and the production compatible with the production of drugs can be consulted with the local drug regulatory authorities,or through the Shenzhen Stock Exchange and Shanghai Securities Exchange.Information acquired by exchanges can be used to measure the imitation ability of enterprises,and the information of enterprises applying for production registration can also be known in the information disclosure module of the drug evaluation center.
In addition,the administrative matters acceptance service module can inquire about the progress of drug registration according to the drug acceptance number.As for the cost accounting of generic drugs,it is often necessary to check the progress of drug registration according to the drug acceptance number.It takes selfreport to get it.Through the above analysis,it is easy to find that the situation of generic pharmaceutical enterprises can be observed from a variety of channels and information for data acquisition and analysis of compulsory licensing procedures.
3.5 Selecting data of license fee
In terms of licensing fee,because there is no precedent in China,we can only learn from some models abroad,mainly the above four models:UNDP model,Canadian model,fixed proportion law,tort damage compensation law.The formula of UNDP model means Licensing fee=Generic drug price *(2%-6%).The formula of Canadian model is Licensing fee=Generic drug price * Licensing fee ratio.Licensing fee ratio=0.04 *(178-HID ranking of drug importing countries)/177.Its formula of fixed proportion method is Licensing fee=Sales volume * A certain proportion(case specific analysis,recommended 4%-6%).Tort compensation method includes the following ways,such as calculating licensing fee based on the actual loss of the patent holder,Licensing fee=Reasonable profit of the unit patented product * Sales volume of the infringed product.The second is based on the profits obtained from the infringement,Licensing fee=Operating profit of the unit infringed product * Sales volume of infringed products.The third is calculated by the people's court with fixed compensation,Licensing fee=More than 10 000 yuan,less than 1 million yuan.The calculation is based on the cost recovery of original research and development of drugs.The specific data acquisition methods of each formula have been described before.The formula can be obtained from various channels and information to calculate the required data.Therefore,the licensing fees of different models can be calculated to support the data acquisition and analysis of compulsory licensing procedures.
4 Conclusions and suggestions
The design of the international drug patent compulsory licensing system is analyzed and combined with our own national conditions.And then the situation of public interest-public health compulsory licensing is chosen as the starting point.According to the international conventions and the relevant laws and regulations of China,the connotation of public interest-public health in the context of our country was discussed,and the cases of compulsory licensing of pharmaceutical patents in India,Brazil and Thailand were analyzed to design the implementation of compulsory licensing of pharmaceutical patents.Data acquisition path can provide data support to promote the follow-up implementation of compulsory license of pharmaceutical patents in China.But it can be seen from the article that although some data are traceable,there are still some data without uniform provisions.So some suggestions are put forward.
4.1 Establishing a unified data support system
It can be seen from the fourth part of this article that drug data of patent compulsory licensing is complex,involving the official data of National health commission,food and drug administration,the state intellectual property office,the national bureau of statistics,ministry of industry,the national development and reform commission,the general administration of customs as well as other administrative agencies.It also involve in other technical data such as epidemic,pharmaceutical production consistency,generic drugs registration and drug patents,medical insurance as well as market prices and raw material supply.Corresponding data support system should be established by the government to improve the feasibility of compulsory licensing of pharmaceutical patents.
4.2 Specifying the license fee standard
The reasonable royalty in the patent law needs clear and operable standards.TRIPS agreement stipulates that adequate compensation should be given on the premise of the economic value factors of the authorization,but it does not define the method or standard.This article has mentioned that India,Thailand,Canada and Malaysia have their own compulsory licensing fees for drugs.The above information can be used as the preparation for China to determine the compulsory licensing fees for drugs.
4.3 Realizing the systematic and scientific compulsory license law
The fourth revision of the patent law can be taken as an opportunity to integrate and improve the regulatory system of compulsory licensing of pharmaceutical patents.In setting up the legal standard system,provisions of the legislation law on the scope of legislative power should be strictly followed,and the types,causes,conditions and main procedures of the compulsory licensing of pharmaceutical patents system must be further clarified.According to the needs,the administrative regulations of compulsory license or compulsory licensing of pharmaceutical patents should be formulated to refine procedural and operational regulations[3].A regulatory system of compulsory licensing of pharmaceutical patents should be formed,which is clear in legal effect,concise in expression,and easy to operate.
 Asian Journal of Pharmacentical Sciences2020年1期
Asian Journal of Pharmacentical Sciences2020年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Information for Authors
- The Analysis of Marketing Strategy of Plendil
- Comparative Study on the Development of Urban Basic Medical Insurance in 14 Cities of Liaoning Province
- Research on the Transformation of Mapping Method for Cancer Patients' Health Utility in the Asia-Pacific Region
- Pharmacoeconomic Evaluation of Rituximab(Hanlikang)for Patients with Rheumatoid Arthritis
- Application Research of Earned Value Management in New Drug Research and Development Projects
