Null links between vitamin B6 and B12 intake and occurrence risk of lung cancer: a meta-analysis
Li-Ping Shen, Rong Li, Ya-Juan Cao
Null links between vitamin B6 and B12 intake and occurrence risk of lung cancer: a meta-analysis
Li-Ping Shen1, Rong Li2, Ya-Juan Cao3,*
1Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200073, China.2Liuzhou Traditional Chinese Medicine Hospital, Guangxi 545000, China.3Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200071, China.
Vitamin B6 and B12 are involved in many biochemical reactions and might play a role in carcinogenesis. We summarized the evidences linking vitamin B6 and B12 to occurrence risk of lung cancer and conducted a meta-analysis of the relationship between vitamin B6 and B12 intake and the risk of lung cancer. Fixed-effect meta-analysis was used to calculate pooled relative risks (RRs) and their 95% confidence intervals (CIs). We identified four observational studies (participants, n = 206,290; cases, n = 1,134) and three randomized controlled trials (RCTs; participants, n = 97,569; cases, n = 952). Vitamin B6 and B12 supplementary was not statistically significantly associated with occurrence risk of lung cancer (124/11926 (1.04%) vs. 73/8497 (0.86%), RR = 1.06, 95% CI = 0.78-1.43,= 0.71). A consistent association was also observed, findings from RCTs did not support a protective effect of vitamin B6 and B12intake orally decrease the risk of lung cancer occurrence (HR = 1.13, 95% CI = 0.86-1.50,= 0.39). For the women, there was no confirmed evidence demonstrated vitamin B intake can decrease the occurrence risk of lung cancer across the subgroup analyses (HR = 0.96, 95% CI = 0.80-1.16,= 0.99). Dietary vitamin B6 and B12 intake also had no influence on occurrence risk of lung cancer in difference doses and intake duration. In conclusion, no confirmed links were found between vitamin B6 and B12 intake and risk of lung cancer occurrence.
Vitamin B, Lung cancer, Incidence, Vitamin B6, Vitamin B12
Introduction
Lung cancer was the top-ranked globally in both cancer incidence and mortality for decades, accounting for about 13% of total cancer diagnoses, especially in more developed countries. Lung cancer is one of the most preventable cancers, which may be avoided by eliminating smoking, reasonable diet habit and appropriate physical exercise [1, 2].
Vitamin B6 and vitamin B12 are present in many kinds of food, for example, nuts, grains, meat, fish, and some fruits and vegetables, and also can be supplemented by tablets [3, 4]. These soluble vitamins are coenzyme involved in the complex one-carbon metabolism pathway, which is considered play an essential role in the DNA integrity maintenance, gene expression regulation, supply of methyl groups, and the growth and repair of cells, and disruption of this process may promote carcinogenesis [5, 6].
However, results from previous studies, the relationship between vitamin B6 and B12 intake and the occurrence risk of lung cancer remains controversial. Further analysis to clarify an association vitamins B6 and B12 with occurrence risk of lung cancer is urgently needed. This study aimed to meta-analyze the evidence from observational studies and intervention trials.
Methods
Search strategy
PubMed, Ovid, Embase, Web of Science, and Cochrane Library databases for studies published until May 1, 2019, were systematically searched. The following search terms were used: (“Vitamin B6” OR “Pyridoxine” OR “Pyridoxine Hydrochloride”) OR (“Vitamin B12” OR “Cyanocobalamin” OR “Cobalamin”) AND (“Lung Neoplasms” OR “Lung carcinoma,” OR “Lung Cancer” OR “Pulmonary Neoplasms” OR “Pulmonary carcinoma” OR “Pulmonary Cancer”). Electronic searches were supplemented with manual searches of the reference lists of all retrieved review articles, primary studies, and abstracts from meetings to identify additional studies not found electronically. The literature was searched by two authors (LS and RL) independently.
Eligibility criteria
Two authors (LS and RL) independently selected the studies and discussed them with each other when inconsistencies were found. The articles that satisfied the following criteria were included: (1) case-control studies, cohort studies or randomized control trials (RCTs), (2) subjects 18 years of age or older, and (3) availability of the relative risk (RR) estimates and cancer risk of patients with lung cancer received vitamin B. Exclusion criteria were data published in abstract form only and lack of risk estimates (or data necessary to calculate them).
Data extraction and methodological quality assessment
Two researchers (YC and RL) independently read the full texts and extracted the following information: publication date, study design, sample size, population, patients’ ages, controlled variables and follow-up period. The Newcastle-Ottawa Scale (NOS) was used for assessing the methodological quality of all non- randomized control trial (RCT) publications selected in the final analysis. The scales allocate a maximum of 9 stars for the quality of selection, comparability, exposure, and outcome of the study participants [7]. Two authors (YC and RL) independently assessed the study quality, and any inconsistency was discussed with the review team.
Statistical analysis
RevMan software, version 5.3, was used for the data analysis. The effect measures of interest were the relative ratio (RR) or odds ratio (OR) and the corresponding 95% confidence intervals (CIs). RR and the corresponding 95% CI were transformed into log (Relative Ratio) and standard error (SE) with the Calculator tool in RevMan 5.3. The heterogeneity across studies was informally assessed by visually inspecting the Forest plots and formally estimated by Cochran’s Q test in which the chi-square distribution was used to make inferences regarding the null hypothesis of homogeneity (considered statistically significant at< 0.05). A simple guide to our interpretation of I2follows: 0% to 40% shows that heterogeneity may not be important; 30% to 60% indicates moderate heterogeneity; 50% to 90% indicates substantial heterogeneity; 75% to 100% indicates considerable heterogeneity [8, 9].
A fixed-effects model was initially used for our meta-analyses. A random-effects model was subsequently used in the presence of heterogeneity. A descriptive analysis was performed when the quantitative data could not be pooled. A two-tailed< 0.05 was considered statistically significant.
Results
Study and patient characteristics
The study selection process was summarized in Figure 1. 2,227 abstracts were reviewed; among these articles, 142 were retrieved that are closely related to the current subject. Finally, 3 RCTs (10-12) and 4 cohort studies (13-16) were included in our analysis. The baseline characteristics of the included studies are described in Table 1.
Methodological Quality Assessment
The quality assessment of three RCTs was conducted in RevMan 5.3, as shown in Figure 2. All these three trials were randomized design, and participants in Ebbing’s study were from common community while the other two RCTs were performed in patients with recent stroke or transient ischemic attack or US female health professionals aged 42 years or older with preexisting cardiovascular disease or 3 or more coronary risk factors, which we considered high risk of selection bias in these two RCTs [10-12]. Two studies were blinded design for researchers, and one was double-blinded, we regarded as low risk of performance bias. Besides, the outcome assessment of the report by Hankey. was conducted by a blinded outcome and adverse events Adjudication Committee [11]. All these three trials were performed by ITT analysis, which we considered low risk of attrition bias. Other biases were unclear [10-12]. The quality assessment of the four cohorts were evaluated according to NOS, and the scores were presented in Table 1 [13-16].
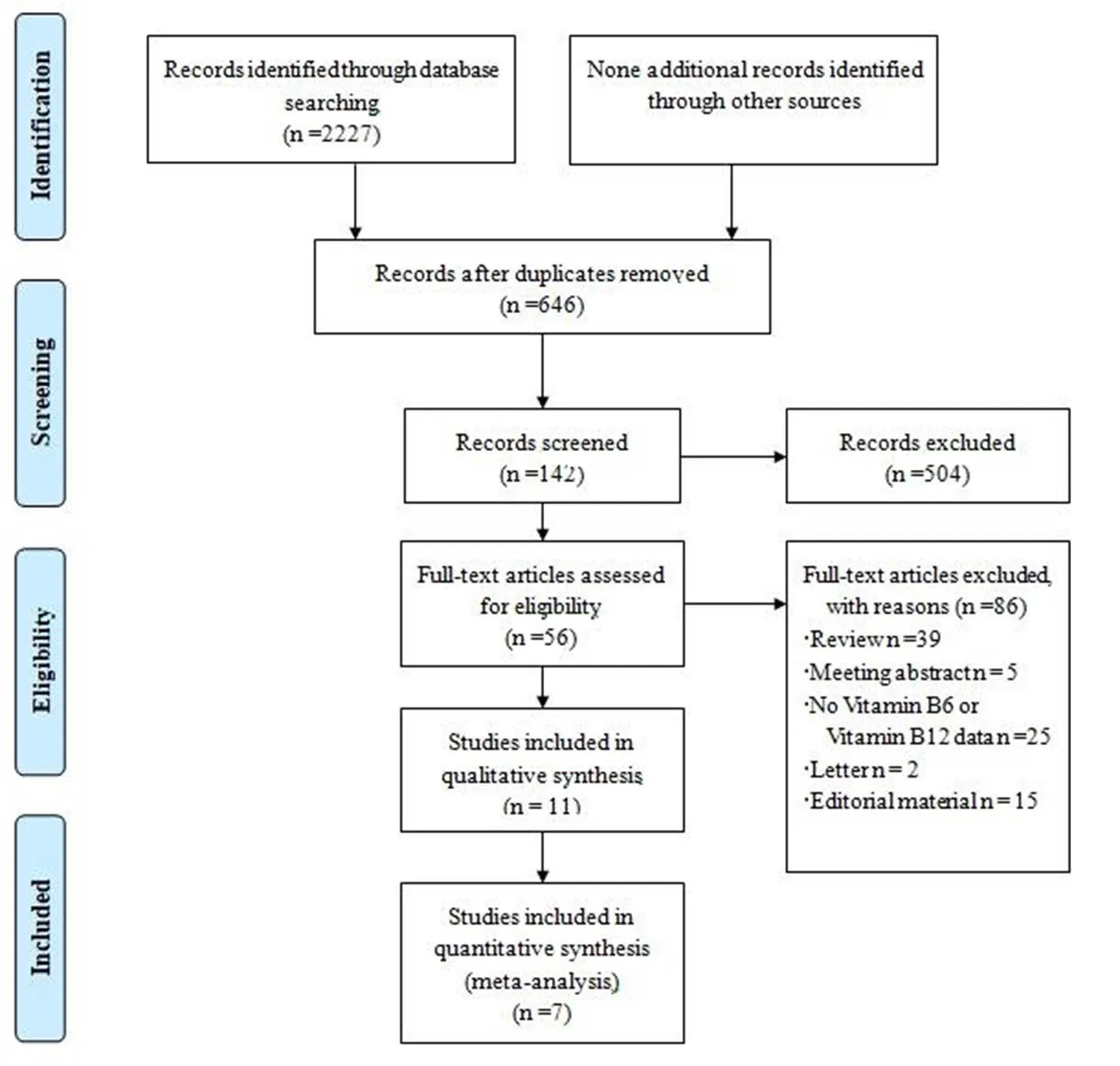
Figure 1 Study selection process
Vitamin B6/B12 and occurrence risk of lung cancer
No heterogeneities were found when evaluating the association between vitamin B6 and/or B12 intake orally and lung cancer incidence and risk (I2= 0%,= 0.78 and I2= 0%,= 0.54, respectively, Figure 3 and Figure 4). Meta-analysis with fixed-effect model of three studies demonstrated that patients received orally vitamin B6 and/or B12 had similar lung cancer incidence rate compared with those out of vitamin B6 and/or B12 (124/11926 (1.04%) vs. 73/8497 (0.86%), RR = 1.06, 95% CI = 0.78-1.43,= 0.71, Figure 3) [10-12]. Additionally, meta-analysis of these three studies with fixed-effect model showed that vitamin B6 and/or B12 intake orally couldn’t decrease the occurrence risk of lung cancer (HR = 1.13, 95% CI = 0.86-1.50,= 0.39, Figure 4) [10-12].
Vitamin B6 and occurrence risk of lung cancer
We conducted a subgroup analysis of relationship between vitamin B6 intake and occurrence risk of lung cancer. Still no heterogeneities were found in this comparison (I2= 33%,= 0.22, Figure 5). As shown in Figure 5, no confirmed link was found between vitamin B6 intake and occurrence risk of lung cancer by meta-analysis with fixed-effect model (HR = 1.17, 95% CI = 0.96-1.43,= 0.12).
Vitamin B6/B12 intake and occurrence risk of lung cancer in female
No heterogeneity was found when we compared vitamin B6 and/or B12 intake and occurrence risk of lung cancer in females (I2= 0%,= 0.99, Figure 6). Meta-analysis of these two studies [12, 13] with fixed-effect model indicated that vitamin B6 and/or B12 intake did not influence the occurrence risk of lung cancer (HR = 0.96, 95% CI = 0.80-1.16,= 0.68, Figure 6). Consistent with this result, no difference of lung cancer incidence rate was found between vitamin B6 and/or B12 group and control group in female (HR = 0.91, 95% CI = 0.78-1.06,= 0.22, Figure 7)
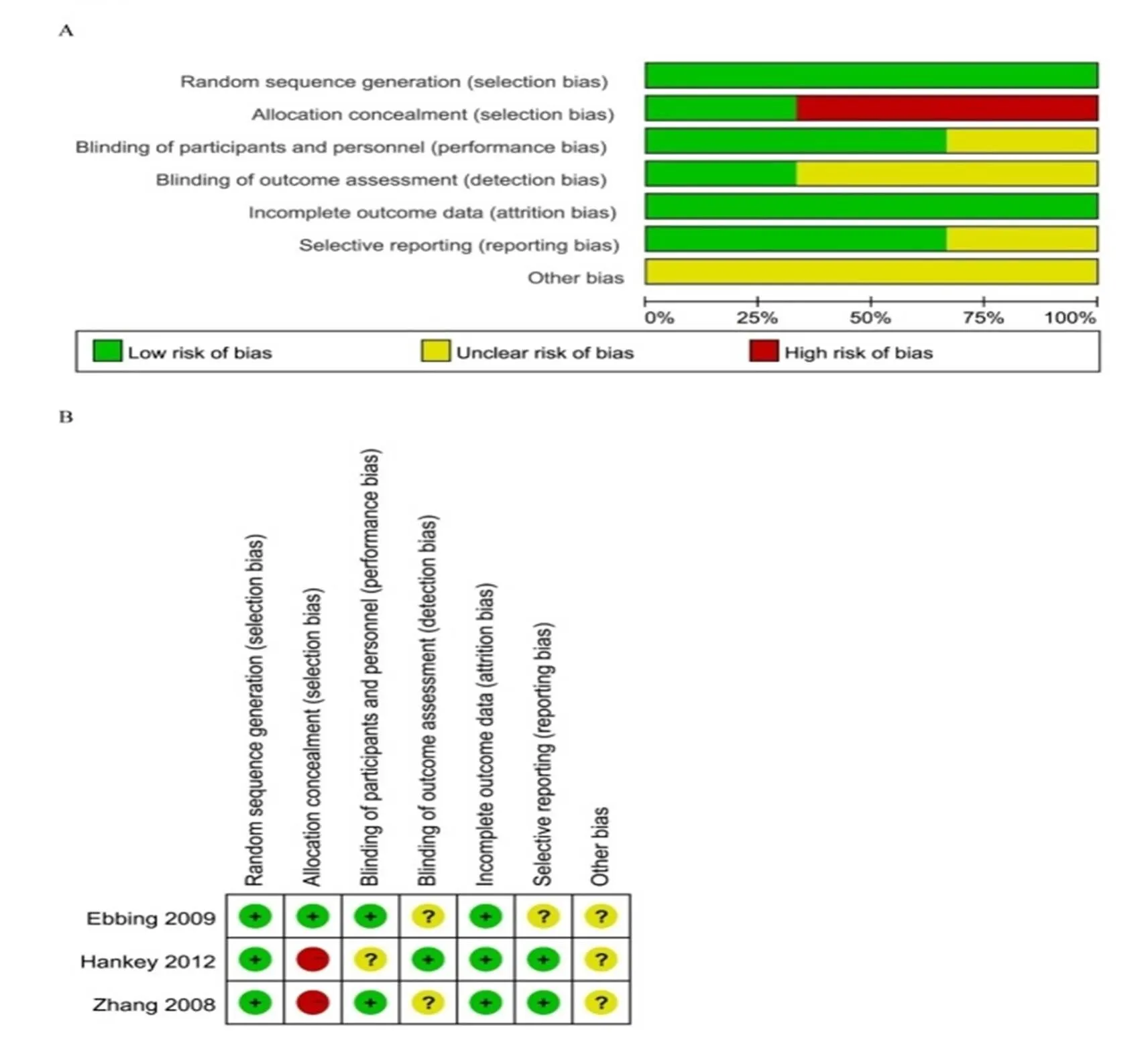
Figure 2 Risk of bias graph and risk of bias summary
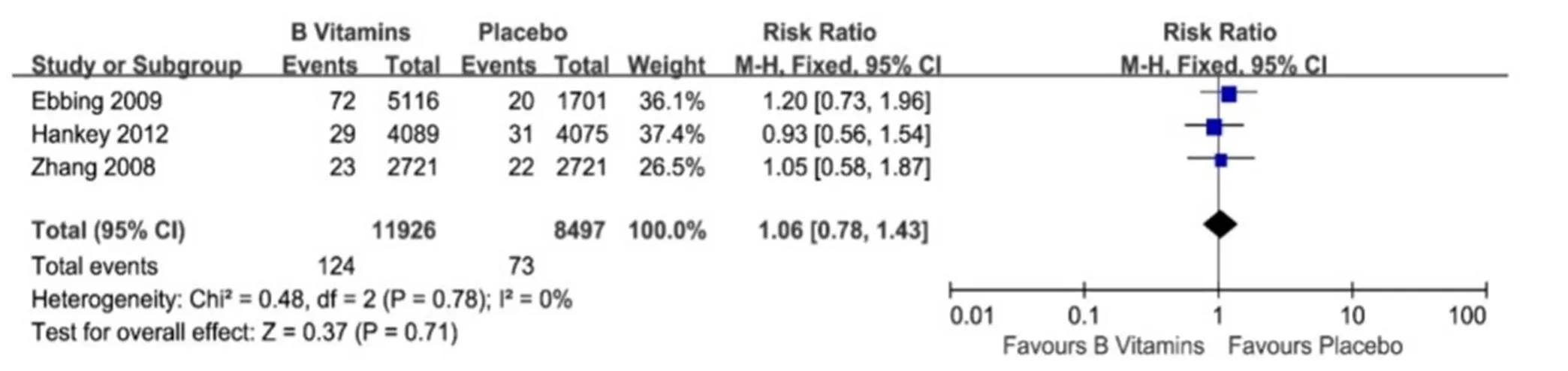
Figure 3 Vitamin B for lung cancer incidence
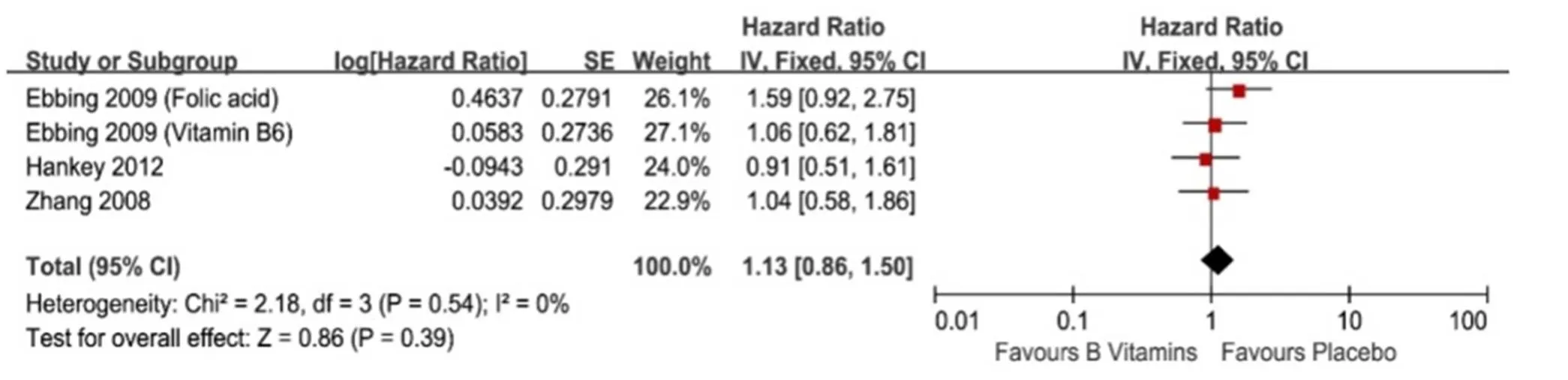
Figure 4 The lung cancer incidence affected by vitamin B intake orally
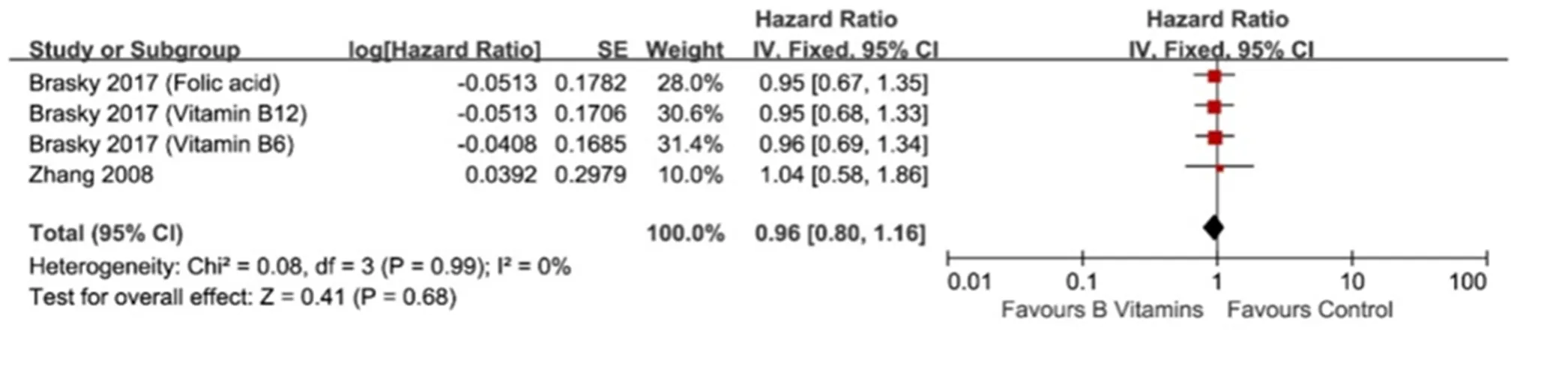
Figure 5 Subgroup analysis of relationship between vitamin B6 intake and occurrence risk of lung cancer

Figure 6 Vitamin B6 and/or B12 intake and occurrence risk of lung cancer in female
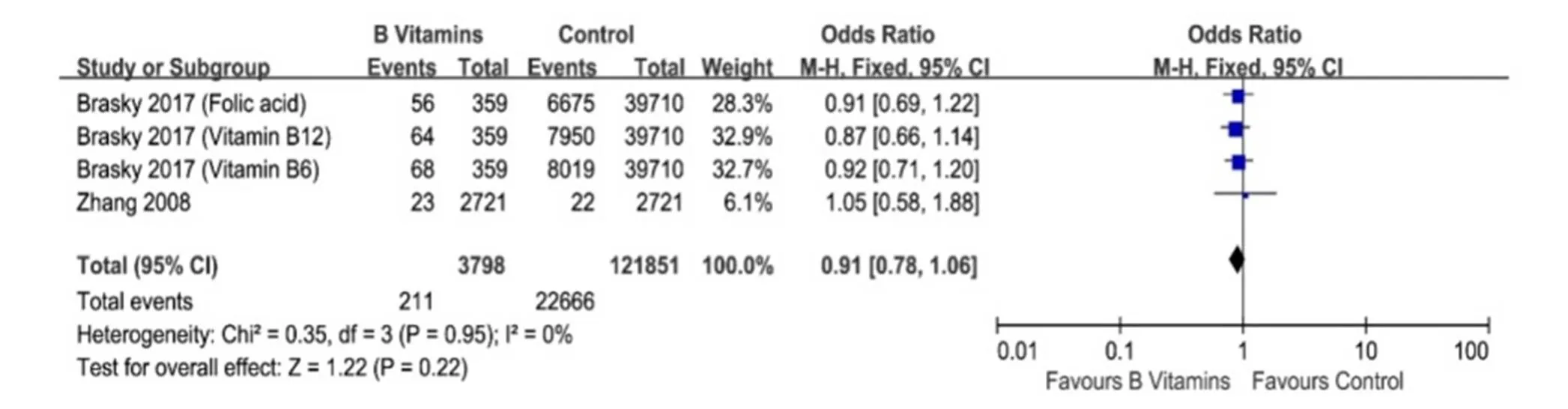
Figure 7 Lung cancer incidences between vitamin B6 and/or B12 group and control group in female
Dietary vitamin B and occurrence risk of lung cancer
Except for vitamin B intake orally, dietary vitamin B also of great importance for cancer prevention. Three studies presented the data of dietary vitamin B and the occurrence risk of lung cancer [14-16]. All of these studies drew the same conclusion that dietary vitamin B intake had no influence on occurrence risk of lung cancer in different doses and intake durations. The detailed data were summarized in Table 2.
Discussion
More recently, considerable attention has been attracted by the possibility that vitamin B intake may influence the overall risk of developing cancer. This hypothesis has been investigated in previous observational studies. High vitamin B intake may reduce the risk of bladder cancer [17], esophageal adenocarcinoma [18], gastric cancer [18, 19], head and neck cancer [20] and oral squamous cell carcinoma [18, 21], and no significant benefits on the risk of pancreatic cancer [22, 23]. Moreover, the relationship between vitamin B and the risk of breast cancer [24-26], non-Hodgkin’s lymphoma [27, 28], ovarian cancer [29, 30], prostate cancer [31, 32], colorectal cancer [33-35] and lung cancer remains controversial.
A meta-analyze demonstrated vitamin B supplementation had little or no effect on the incidence of cancer and total mortality. Unfortunately, there was no definite evidence confirms the link vitamin B intake with the risk of lung cancer [36]. Three systematic reviews and meta-analyses have evaluated the impact of folic acid (vitamin B9) supplementation on the risk of cancer and have found no evidence to support a significant effect [37-39]. Three meta-analyses provide evidence of a no considerable decrease in colorectal cancer and breast cancer risk associated with the high level of vitamin B6 and B12 intake [40-42]. A meta-analyze showed that vitamin B12 intake is associated with an increased prostate cancer risk [43]. However, the effect of vitamin B6 and B12 supplementation on the risk of lung cancer has not been confirmed. To this aim, we included both observational and intervention studies, gathering together the largest collection of data ever reported on this subject. The results of RCTs data don’t support a strong association between both vitamin B6 and B12 intake and the risk of lung cancer, the results of cohort data indicate the same conclusion between vitamin B6 and B12 dietary intake in different doses and intake duration and the risk of lung cancer. There was no definite evidence demonstrates vitamin B intake can decrease the risk of lung cancer in females. One meta-analyze appears to be in line with these findings when food and supplements intake were considered or dietary intake only, the associations between vitamin B6 and cancer risk was weak [44].
Limitation of the present results must be pointed out: (1) the confounding factors potentially influencing the results, particularly in the case of dietary vitamin intake. Food, such as fruit, vegetables, and meat, contains other kinds of potential anticancer nutrients except for vitamin B6 and B12. The real effect of vitamin B6 and B12 remain very difficult to examine separately from other nutrients. (2) available evidence is scarce on the interaction of vitamin B6 and B12 and other unveiled factors, such as smoking, alcohol. (3) the underlying disease among participants taking vitamin B might have impaired ability to identify the treatment effect.
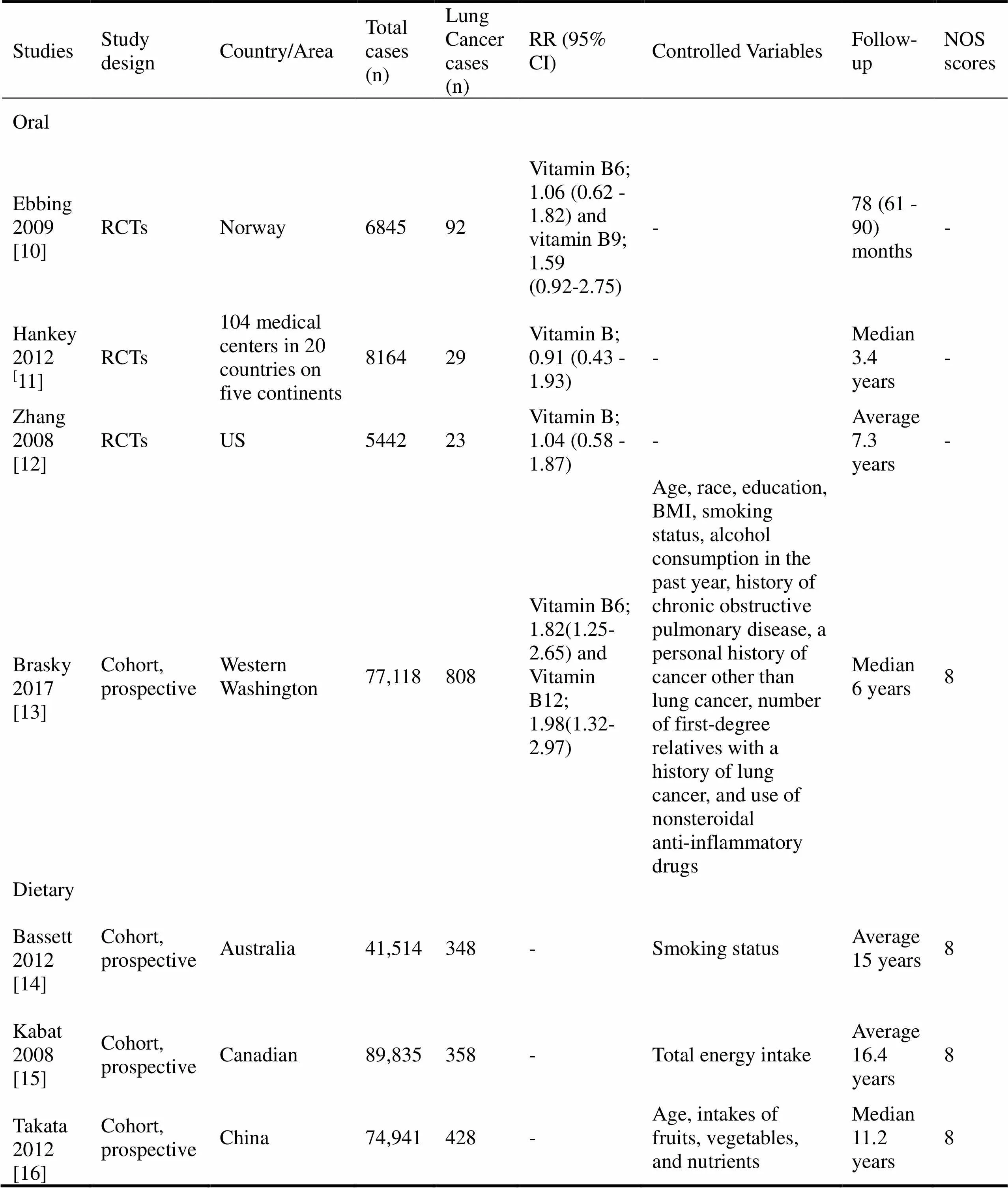
Table 1 Baseline characteristics of included studies
Note: “-” means not available.
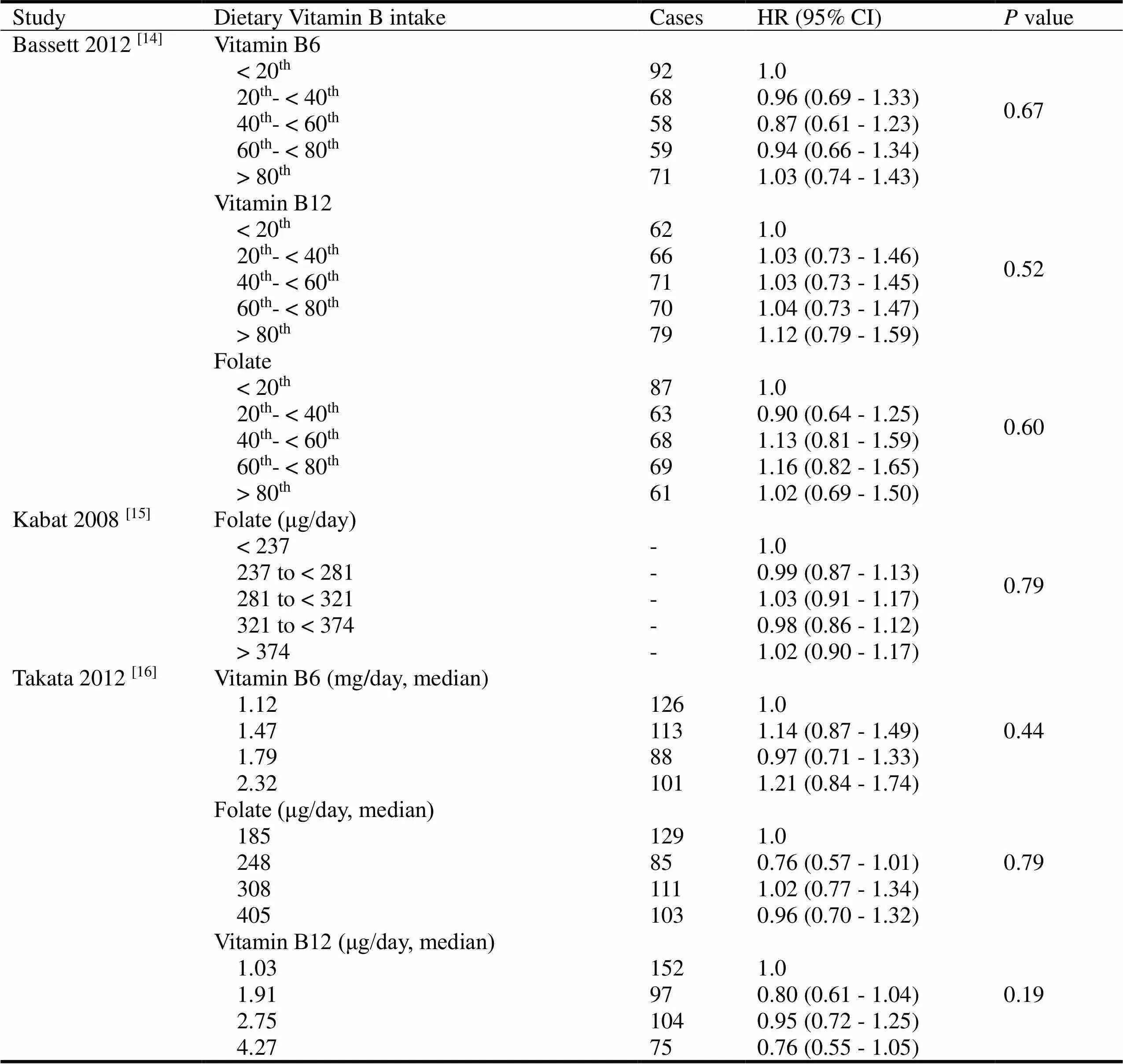
Table 2 Dietary vitamin B intake and occurrence risk of lung cancer
Note: “-” means not available.
Conclusion
In summary, our work provides both clinicians and researchers with evidence that vitamin B6 and B12 intake (supplement and food) have no significant effect on the occurrence risk of lung cancer, even in female population. Further randomized trials are needed to clarify the association between vitamin B intake and occurrence risk of lung cancer.
1. Bray F, FerlayJ, Soerjomataram I,. Global Cancer Statistics 2018. CA Cancer J Clin 2018: 1-31.
2. Ferlay J, Shin HR, Bray F,. Estimates of worldwide burden of cancer in 2008. Int J Cancer 2010, 127: 2893-2917.
3. Fedde KN, Whyte MP. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-50-phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. Am J Hum Genet 1990, 47: 767-775.
4. Roth-Maier DA, Kettler SI, Kirchgessner M. Availability of vitamin B6 from different food sources. Int J Food Sci Nutr 2002, 53: 171-179.
5. Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr 2008, 87: 517-533.
6. Kim YI. Folate and DNA methylation: A mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev 2004, 13: 511-519.
7. Wells GA, Shea B, O'Connell D,. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
8. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration. http://www.cochrane-handbook.org.
9. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002, 21:1539-1558.
10. Ebbing M, B?naa KH, Nyg?rd O,. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009, 302: 2119-2126.
11. Hankey GJ, Eikelboom JW, Yi Q,. Treatment with B vitamins and incidence of cancer in patients with previous stroke or transient ischemic attack: results of a randomized placebo-controlled trial. Stroke 2012, 43: 1572-1577.
12. Zhang SM, Cook NR, Albert CM,. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA 2008, 300: 2012-2021.
13. Brasky TM, White E, Chen CL. Long-Term, Supplemental, One-Carbon Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J Clin Oncol 2017, 35: 3440-3448.
14. Bassett JK, Hodge AM, English DR,. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr 2012, 66: 182-187.
15. Kabat GC, Miller AB, Jain M,. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer 2008, 99: 816-821.
16. Takata Y, Cai Q, Beeghly-Fadiel A,. Dietary B vitamin and methionine intakes and lung cancer risk occurrence risk of lung cancer among female never smokers in China. Cancer Causes Control 2012, 23: 1965-1975.
17. García-Closas R, García-Closas M, Kogevinas M,. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer 2007, 43: 1731-1740.
18. Mayne ST, Risch HA, Dubrow R,.Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 2001, 10: 1055-1062.
19. Kaaks R, Tuyns AJ, Haelterman M,.Nutrient intake patterns and gastric cancer risk: a case-control study in Belgium. Int J Cancer 1998, 78: 415-420.
20. Negri E, Franceschi S, Bosetti C,.Selected micronutrients and oral and pharyngeal cancer. Int J Cancer 2000, 86: 122-127.
21. Galeone C, Pelucchi C, Levi F,. Folate intake and squamous-cell carcinoma of the oesophagus in Italian and Swiss men. Ann Oncol 2006, 17: 521-525.
22. Schernhammer E, Wolpin B, Rifai N. Plasma folate, vitamin B6, vitamin B12, and homocysteine and pancreatic cancer risk in four large cohorts. Cancer Res 2007, 67: 5553-5360.
23. Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control 2009, 20: 1317-1325.
24. Zhang SM, Willett WC, Selhub J,. Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst 2003, 95: 373-380.
25. Lajous M, Lazcano-Ponce E, Hernandez-Avila M,. Folate, vitamin B(6), and vitamin B(12) intake and the risk of breast cancer among Mexican women. Cancer Epidemiol Biomarkers Prev 2006, 15: 443-438.
26. Zhang CX, Ho SC, Chen YM,. Dietary folate, vitamin B6, vitamin B12 and methionine intake and the risk of breast cancer by oestrogen and progesterone receptor status. Br J Nutr 2011, 106: 936-943.
27. Lim U, Schenk M, Kelemen LE,. Dietary determinants of one-carbon metabolism and the risk of non-Hodgkin's lymphoma: NCI-SEER case-control study, 1998-2000. Am J Epidemiol 2005, 162: 953-964.
28. Lim U, Weinstein S, Albanes D,. Dietary factors of one-carbon metabolism in relation to non-Hodgkin lymphoma and multiple myeloma in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev 2006, 15: 1109-1114.
29. Kotsopoulos J, Hecht JL, Marotti JD,. Relationship between dietary and supplemental intake of folate, methionine, vitamin B6 and folate receptor alpha expression in ovarian tumors. Int J Cancer 2010, 126: 2191-2198.
30. Harris HR, Cramer DW, Vitonis AF,. Folate, vitamin B6, vitamin B12 , methionine and alcohol intake in relation to ovarian cancer risk. Int J Cancer 2012, 131: E518-E529.
31. Key TJ, Silcocks PB, Davey GK,. A case-control study of diet and prostate cancer.Br J Cancer 1997, 76: 678-687.
32. Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R,. Null association between prostate cancer and serum folate, vitamin B6, vitamin B12, and homocysteine. Cancer Epidemiol Biomarkers Prev 2012, 12: 1271-1272.
33. Harnack L, Jacobs DR Jr, Nicodemus K,.Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer 2002, 43:152-158.
34. Zhang SM, Moore SC, Lin J,. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol 2006, 163: 108-115.
35. Sharp L, Little J, Brockton NT,. Polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene, intakes of folate and related B vitamins and colorectal cancer: a case-control study in a population with relatively low folate intake. Br J Nutr 2008, 99: 379-389.
36. Zhang SL, Chen TS, Ma CY,. Effect of vitamin B supplementation on cancer incidence, death due to cancer, and total mortality: A PRISMA-compliant cumulative meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016, 95: e3485.
37. Clarke R, Halsey J, Lewington S,. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals.Arch Intern Med 2010, 170: 1622-1631.
38. Vollset SE, Clarke R, Lewington S,. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013, 381: 1029-1036.
39. Qin X, Cui Y, Shen L,. Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials. Int J Cancer 2013, 133: 1033-1041.
40. Jia K, Wang R1, Tian J. Vitamin B6 Intake and the Risk of Colorectal Cancer: A Meta-Analysis of Prospective Cohort Studies. Nutr Cancer 2017, 69: 723-731.
41. Sun NH, Huang XZ, Wang SB,. A dose-response meta-analysis reveals an association between vitamin B12 and colorectal cancer risk. Public Health Nutr 2016, 19: 1446-1456.
42. Wu W, Kang S, Zhang D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: a dose-response meta-analysis. Br J Cancer 2013, 109: 1926-1944.
43. Collin SM, Metcalfe C, Refsum H,. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2010, 19: 1632-1642.
44. Mocellin S, Briarava M, Pilati P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J Natl Cancer Inst 2017, 109: 1-9.
Vitamin B6 and vitamin B12 are coenzyme involved in the complex one-carbon metabolism pathway.
The relationship between vitamin B6 and B12 intake and occurrence risk of lung cancer remains controversial.
Vitamin B6 and vitamin B12 are considered play an important role in the DNA integrity maintenance, gene expression regulation, supply of methyl groups, and the growth and repair of cells, and disruption of this process may promote carcinogenesis, which may play an essential role in tumor progression.
Submitted: 06 March 2019,
13 June 2019,
Online: 15 August 2019.
Competing interests:Authors declare that they have no competing interests.
Funding: This work was sponsored by the Natural Science Foundation of China (81603590); National Traditional Chinese Medicine Clinical Research Base Long-Yi-Xue-Zhe (nurturing plan, LYTD-81).
Copyright:?2019 TMR Publishing Group Limited. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License.
*Correspondence: Ya-Juan Cao, Shanghai Municipal Hospital of Traditional Chinese Medicine, No. 274, Zhijiang Middle Road, Shanghai University of Traditional Chinese Medicine. Email: dr_caoy@aliyun.com.
- Cancer Advances的其它文章
- Narrative nursing for cancer patients: a meta-analysis
- Xiaoyan decoction inhibits tumor growth and improves the immunity of mouse with A549 lung carcinoma xenograft
- Miao medicine may serve as an essential adjuvant therapy in cancer treatment
- The role of Fuzheng Peiben in clinical oncology

