Xiaoyan decoction inhibits tumor growth and improves the immunity of mouse with A549 lung carcinoma xenograft
Fan-Ming Kong, Yang Yao, Ren-Fen Deng, Tian-Qi Chen, Dong-Ying Liao, Xiao-Jiang Li, Ying-Jie Jia,*
Xiaoyan decoction inhibits tumor growth and improves the immunity of mouse with A549 lung carcinoma xenograft
Fan-Ming Kong1, #, Yang Yao1, #, Ren-Fen Deng1, Tian-Qi Chen1, Dong-Ying Liao1, Xiao-Jiang Li1, Ying-Jie Jia1,*
1Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China.
Previously, Xiaoyan decoction (XYD) has been found to have a potential anti-tumor effect in vitro. The aim of this study was to explore the effect of XYD in A549 lung carcinoma xenograft mouse model.: The A549 lung carcinoma xenograft mouse model were established and divided into control and experiment group. The dynamic growth of the xenograft tumors was observed and the immune cells such as T cell, regulatory Tcell (Treg) were explored by Fluorescence-activated cell sorter (FACS) analysis. In this study, network pharmacology was used to screen the anti-cancer monomers in XYD, and their effect targets were also predicted.: The result showed that the tumor growth was inhibited and the percent of Treg was down-regulated (<0.05). Ten drug monomers were screened to inhibit lung cancer cell proliferation and promote cell apoptosis through PI3K/Akt pathway.: Our results suggested that XYD inhibited tumor growth and improved the immunity of A549 lung carcinoma xenograft mouse model.
Non-small cell lung cancer, XYD antitumor activity, Xenograft
Introduction
Lung cancer is still the first cause of tumor-related death in both developing and developed countries [1, 2]. As a subgroup of lung cancer, non-small cell lung cancer (N-SCLC) accounts for approximately 85% of all lung cancer cases [3]. For the treatment, the NCCN guidelines recommend the surgery, radiotherapy, and platinum-based combination chemotherapy for N-SCLC [4]. Although the diagnosis and treatment for the N-SCLC have made good progress, and primary and clinical studies have been conducted in recent years, the long-term outcome remains poor [5]. Novel and practical approaches are still urgently required to improve the prognosis of N-SCLC.
There is currently increasing interest in traditional Chinese medicine (TCM) herbal mixtures, which have been used to treat cancer for thousands of years in China [6]. Unlike western medicine that generally uses purified compounds and aims to target a single molecule or pathway, TCM compositions usually comprise multiple herbs and components [7-10]. We have previously developed a TCM formulation of herbs, Xiaoyan decoction (XYD), that has been shown to have potential non-toxic therapeutic properties in clinically and improved the long-term prognosis in multiple solid tumors (data not published). However, as the mechanisms of anti-tumor for many TCM are undefined, the application of TCM for the treatment of cancer is limited. In the previous study, we observed the anti-tumor activity and investigated the antitumor mechanisms of XYD(the paper is under press).
In the present study, we aimed to explore the antitumor activity and the effect on the immunity of XYD inA549 lung carcinoma xenograft mouse model.
Materials and Methods
Ethics Statement
Our study protocol was approved by the Institutional Review Board of First Teaching Hospital of Tianjin University of TCM (No. TYLL2018K004), Tianjin, China.
Cell lines and animals
Human lung cancer cell line A549 was obtained from the American Type Culture Collection. Cells were cultured in RPMI-1640 (HyClone, South Logan, UT) supplemented with 10% fetal bovine serum, 100 unit/mL penicillin, and 100 μg/mL streptomycin (Sigma Aldrich Inc.). Cells were maintained in a humidified atmosphere of 5% CO2at 37 °C. Specific pathogen-free 6-8 weeks old male nude mice weighing 20 ± 2 g were purchased from Vital River Company (Beijing, China). All the mice were kept under a temperature and humidity-controlled animal facility with a 12 h light/dark cycle. Mice were had free access to feed pellets and tap water.
Drugs and antibodies
XYD is made up of several herbs, including(Huang Qi),(Taizi Shen)(Xiaku Cao)(Jiang Huang)(Yu Jin)(Baihua Sheshe Cao). This decoction was prepared at the College of Pharmacy, Tianjin University of Traditional Chinese Medicine. Each herb was extracted in boiling water for 20 mins, and the solution was filtered. The residue was extracted with 75% ethanol, and the extract was filtered. Both the water extract and ethanolic extract were combined and concentrated by lyophilization. To obtain 100 mg of XYD required 459 mg of dried herbs. The lyophilized powder was suspended in PBS. The mixture was vortexed for 1 min and incubated at 80 °C for 30 min. The sample was cooled to room temperature and was then centrifuged at 2000 rpm for 10 min. The supernatant was collected at a final concentration to 80 mg/ml. XYD was then diluted and filtered through a 0.2 mm membrane for the experiment.
CD4-FITC monoclonal antibodies and CD25-PE monoclonal antibodies (Beckman Coulter, Brea, CA, USA), were produced by Beckman Coulter Company.
Flow cytometric analysis
The peripheral blood sample of 1 mL, with heparin for anticoagulation, was taken from each mouse, and treated with whole blood hemolysis and labeled by direct immunofluorescence, and tested for CD4+T cell, CD8+T cell, and CD4+CD25+Treg cells by flow cytometry. In detailed, a quantity of 20 μL of monoclonal antibody against CD4+CD25+Treg cells was added into the test tubes for flow cytometry, and 100 μL of blood sample treated with heparin added into the same test tube, followed by mixing. The tube stood for 30 min at dark at room temperature, and then the hemolysin was added twice each at 0.5 mL. The suspension was mixed and stood at dark at room temperature for 5 min before detection. The intensity of each fluoresce on the cell surface was detected via flow cytometry and saved as two-dimensional and scattering dot-plot in a computer. After automatic analysis of the number and percentage of positive cells of each monoclonal antibody, the percentages of CD4+CD25+Treg cells were calculated from the FITC-CD4/PE- CD25 two-parameter diagrams.
Studies with Xenografts
Cells were suspended in the matrigel basement membrane matrix (BD Biosciences, Billerica, MA) for implantation. Approximately 1 × 107A549 cells were injected subcutaneously into the right flank of mice. These mice were divided into the control group (received 12 g/kg/day water by gavage) and experiment group (received 12 g/kg/day XYD by gavage). Tumor xenografts were measured with calipers three times a week, and tumor volume was determined using the formula: (length × width2)/2. Results are presented as mean ± SEM.
Screening of Traditional Chinese Medicine Monomers
The BATMAN-TCM (http://bionet.ncpsb.org/batman- tcm/) was used to screen the monomers and predict potential targets. And then Gene Ontology (GO) term, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Online Mendelian Inheritance in Man/Therapeutic Target Database (OMIM/TTD) were applied to perform functional analyses on these targets. Cytoscape 3.5 Software (The Cytoscape Consortium, New York, NY) was used to draw the TCM ingredient-target-pathway/disease association network and biological pathway.
Statistical analysis
All results were expressed as mean and standard deviation. Measurement data were analyzed using a one-way analysis of variances (ANOVA, SPSS, Inc., Chicago, IL, USA). Rank data were analyzed with ridit. Groups were compared using ANOVA with Dunnett’s multiple comparison tests. Results with< 0.05 were considered to be statistically significant.
Results
XYD suppresses tumor growth of A549 lung cells in nude mice.
We examined the effect of XYD on the tumorigenicity and growth of lung cancer A549 cells in nude mice. And the growth curve was drawn based on the volume counted. As we found, all the mice were bear the tumors. Among these mice, the mice treated with XYD developed tumors more slowly than the control group (< 0.05; Figure 1). What’s more, the average tumor weight of XYD treated mice was significantly less than that of control (< 0.05; Figure 1)
XYD decreased the percent of Treg cells
To identify the effect of XYD on the immunity of A549 bearing mouse. We detected the T cells and Treg cells. And as Table 1 and Figure 2A shows, the CD3+CD8+T cell of the control group was not different from the experiment group (Treated with XYD), and a similar result was observed in the CD4+CD8+T and the ratio of CD4+and CD8+. However, mice treated with XYD decreased the Treg cells significantly in comparison with control (0.07 ± 0.01 vs 0.18 ± 0.01;< 0.05) (Table 1, Figure 2B).
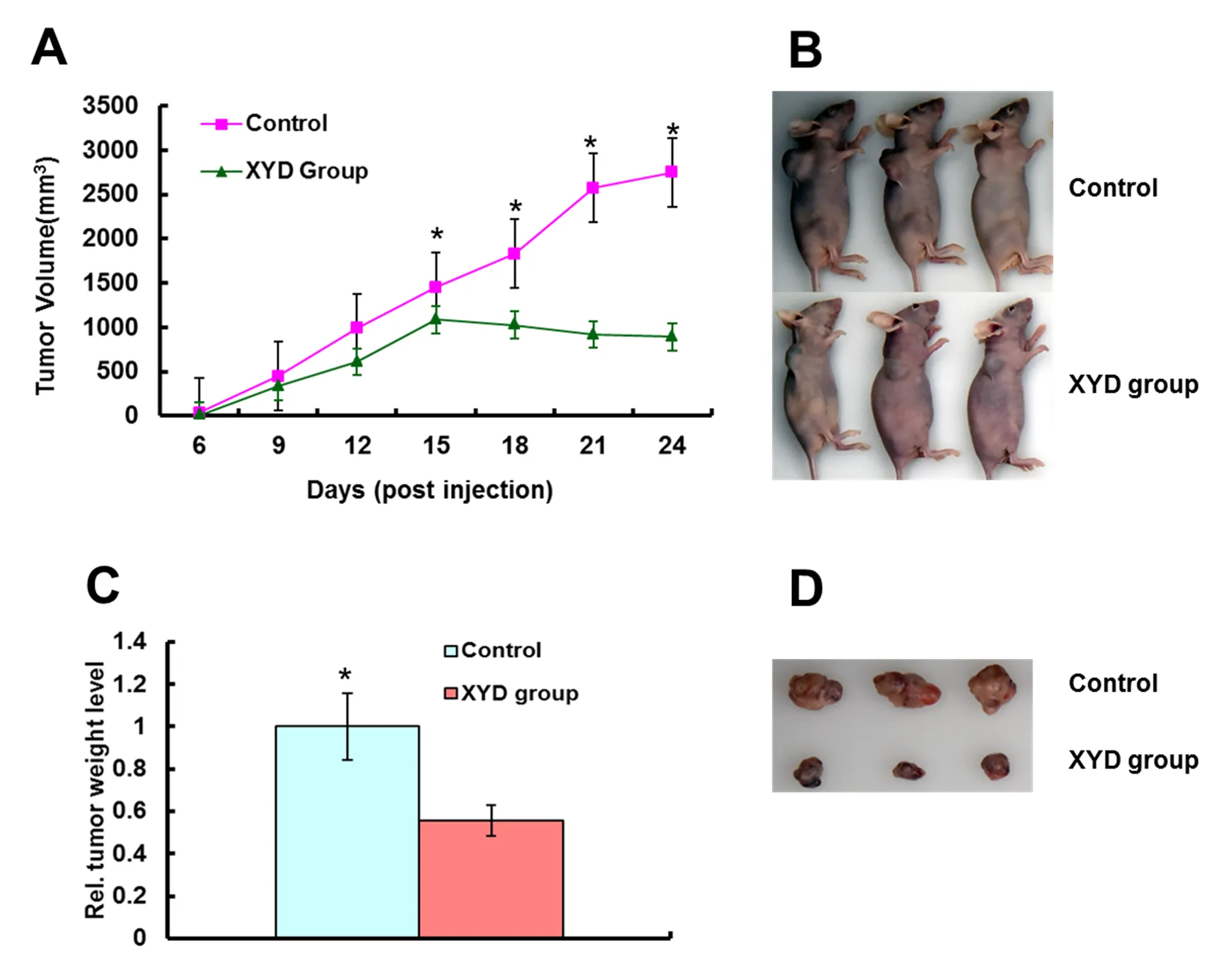
Figure 1 XYD suppresses tumor growth of A549 lung cells in nude mice
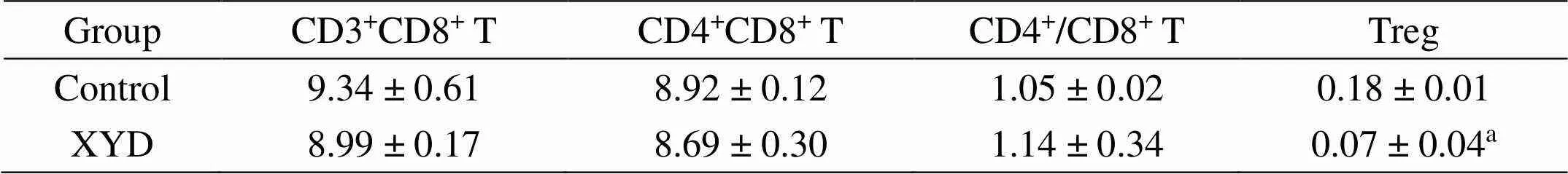
Table 1 The effect of XYD on the T cells
a:< 0.05
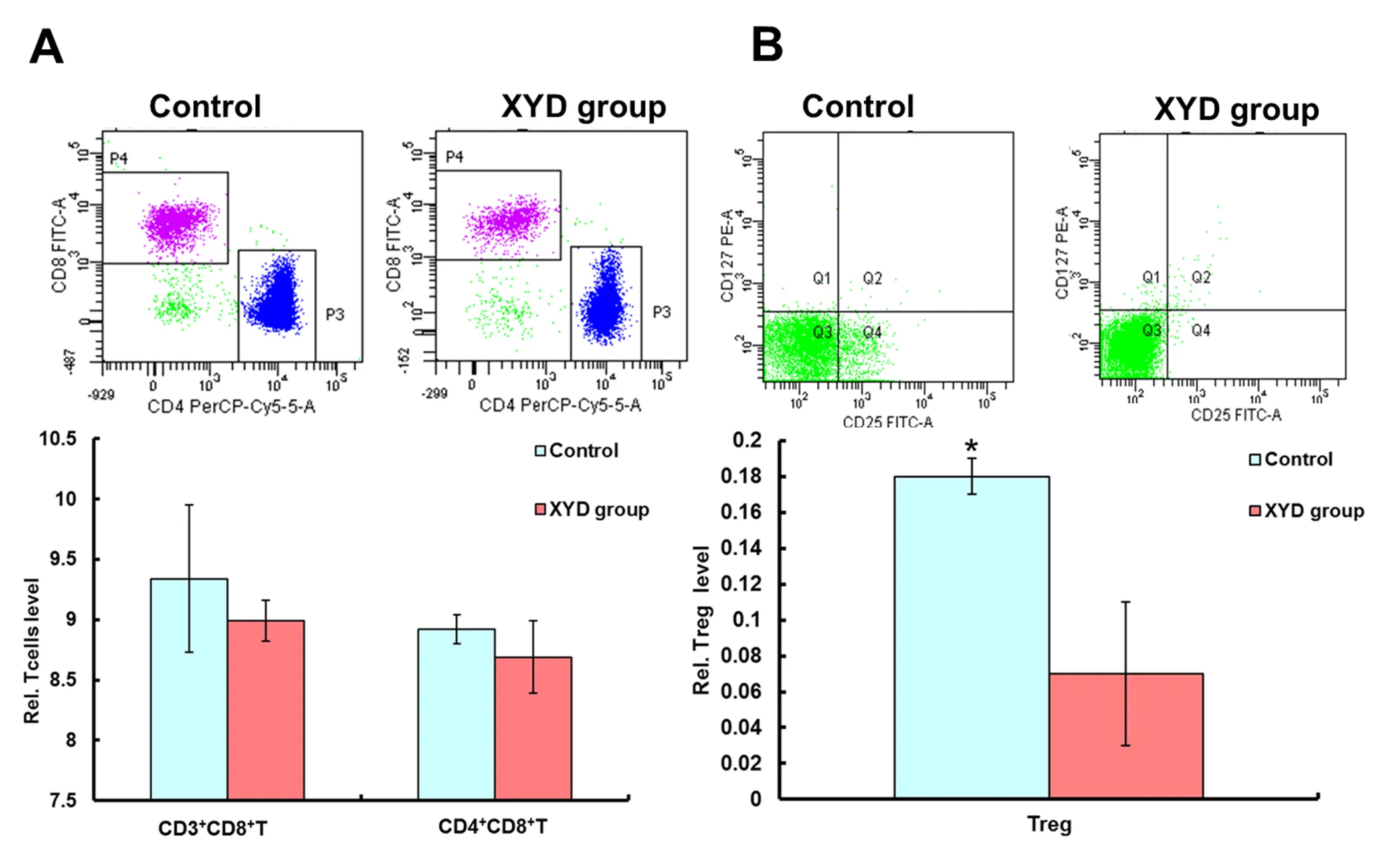
Figure 2 The effect of XYD on the immunity of A549 bearing mouse
Monomers and the genes they work on
Accordingly, we screened drug monomers through the network pharmacology methods, and our results showed that 101monmers and 1808 potential targets were screened. And then, we performed functional analyses on these targets including Gene Ontology (GO) term, KEGG pathway, and OMIM/TTD disease enrichment analyses. At last, 10 drug monomers () were screened to inhibit lung cancer cell proliferation and promote cell apoptosis through the PI3K/Akt pathway (Table 2).
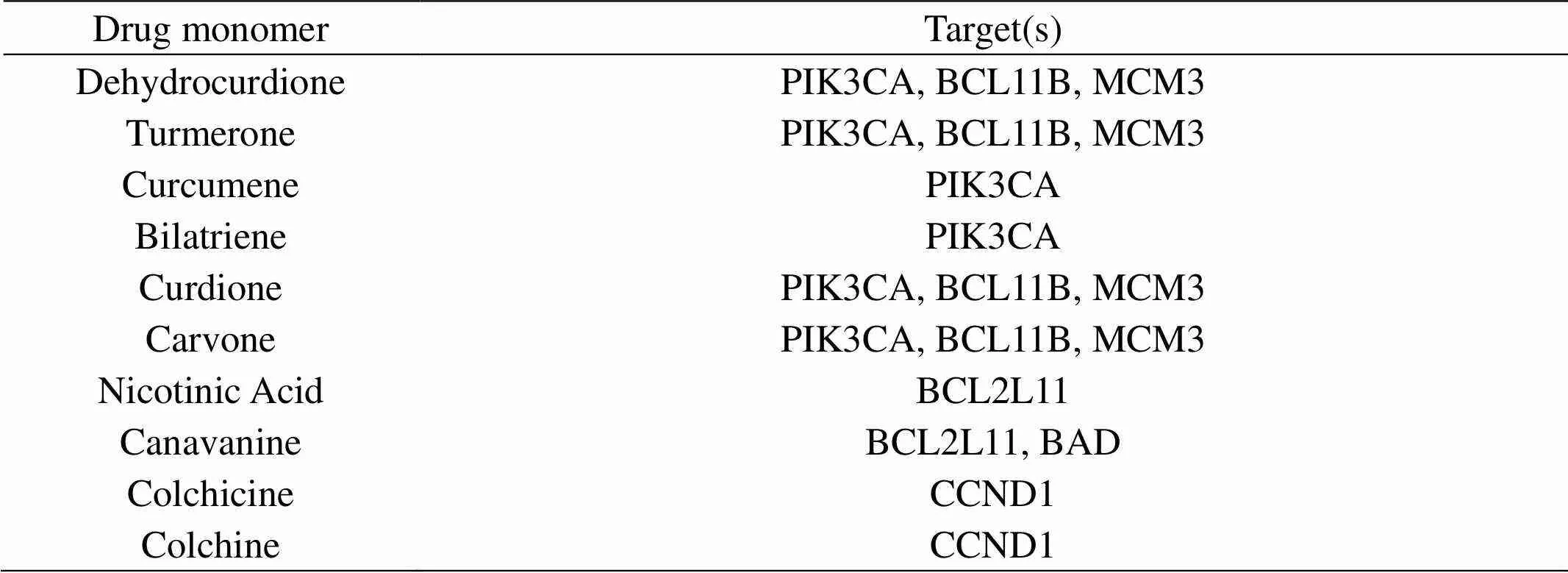
Table 2 the effect of XYD Monomers on the potential genes
Discussion
The present study is the first study to investigate the antitumor effect of XYD in vivo. In this study, we demonstrated that XYD might inhibit the tumor and improve the immunity in A549 lung carcinoma xenograft mouse model. These results suggested that XYD could be considered in the clinical treatment of lung cancer patients.
Lung cancer is the most common cause of tumor death in the United States. Over 200,000 new cases of lung and bronchial cancer will be diagnosed in 2015, and 150,000 deaths are estimated to occur because of cancer [1]. According to tumor biology, therapy, and prognosis, lung cancer was divided into NSCLC and small cell lung cancer (SCLC) [11]. NSCLC accounts for more than 85% of all lung cancer cases, and it includes non-squamous carcinoma and squamous cell carcinoma [5, 12]. Many studies and systematic empirical research specific on NSCLC have identified certain prognostic factors and several biomarkers for NSCLC [13-16]. These findings improved the outcome of the NSCLC. However, the 5-year survival rate for the NSCLC is only 16% [17]. It is crucial to explore the novel and reliable treatment to improve the prognosis of NSCLC. Traditional Chinese medicine provides a rich source of potential chemopreventive and therapeutic agents for the treatment of cancer. The XYD used in this study followed TCM treatment principles based on syndrome differentiation. TCM theory proposes that lung cancer is mainly related to the deficiency of both Qi and Yin, as well as pathological changes of Qi stagnation, blood stasis, and accumulation of phlegm. Therefore, the treatment principle is to resolve phlegm, activate blood and Qi circulation, remove stasis, and nourish Yin.
In our previous study, we found chemotherapy used with XYD could improve the long-term prognosis of advanced NSCLC (data not published). Also XYD can inhibit A549 lung cancer cell growth in vitro (under press). Inconsistent with these results, we found XYD also could inhibit the growth of A549 lung cell bearing tumors (Figure 1).
It was found in immunological studies that the Treg cells are closely related to the body’s immune functions. Numerous studies found that one of the reasons for lower cellular immune function in cancer patients was the increase in the proportion of CD4+CD25+Treg cells [18, 19]. Besides, any changes in the percentage of these lymphocytes have a significant effect on these cancer patients. To date, many studies have confirmed CD4+CD25+Treg cells were significantly increased in kinds of solid tumors, and its expression was associated with poor long-term prognosis [20, 21]. Recently, Ju and his colleagues reported a positive correlation between the CD4+CD25+Treg cells and the clinical stage of NSCLC [22]. The mechanism was complicated, the partial reason the increased proportion CD4+CD25+Treg cells could induce the chemokines (TGF-β, COX-2, CD70, Galectin-1, and IDO) invaded from the tumor microenvironment into local cancer, which could promote the tumor progression. In the present study, we also found XYD decreased the Treg and improved the immunity in the xenograft mouse model (Table 1). These results showed that our XYD had antitumor activity, could be considered using clinically.
The therapeutic mechanism of TCM formula must be very complicated as a result of its complex composition. We further screen drug monomers through network pharmacology methods, and our results showed that 101 monomers and 1808 potential targets were screened (Not shown in the article). And then, we performed functional analyses on these targets, including Gene Ontology (GO) term, KEGG pathway, and OMIM/TTD disease enrichment analyses. At last, 10 drug monomers were screened to inhibit lung cancer cell proliferation and promote cell apoptosis through the PI3K/AKT pathway (Table 2), which could explain that after applying Xiaoyan decoction, the tumor size of the experimental group began to shrink after 15 days (Figure 1A). In the future, a series of studies in cells and mouse models will be conducted to verify the pharmacological effect of XYD and the verification of pharmacological targets.
This study has several limitations as well as notable strengths. The limitations of our research include, (1) the measurement of the tumor is not a fluorescence imaging system, and (2) we cannot observe the dynamic T cells and Treg cells. Nevertheless, this study had a rigorous data collection. So we believe the result of the present study was convincing, though further validation studies are needed.
Conclusions
In conclusion, the findings of our study indicate that XYD can inhibit the tumor and improve the immunity in A549 lung carcinoma xenograft mouse model. These results suggest that XYD could be considered in the clinical treatment of lung cancer patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015, 65: 5-29.
2. Torre LA, Bray F, Siegel RL,Global cancer statistics. CA Cancer J Clin 2015, 65: 87-108.
3. Buyukcelik A, Yalcin B, Utkan G. Multidisciplinary management of lung cancer. N Engl J Med 2004, 350: 2008-2010.
4. Ettinger DS, Akerley W, Borghaei H,Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013, 11: 645-653.
5. De Ruysscher D, Faivre-Finn C, Le Pechoux C,High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol 2014, 15: e620-624.
6. Hu M, Zhao M, An C,Real-time imaging of apoptosis induction of human breast cancer cells by the traditional Chinese medicinal herb tubeimu. Anticancer Res 2012, 32: 2509-2514.
7. Zhang L, Wu C, Zhang Y,Efficacy comparison of traditional Chinese medicine LQ versus gemcitabine in a mouse model of pancreatic cancer. J Cell Biochem 2013, 114: 2131-2137.
8. Chen Y, Zhu J, Zhang W. Antitumor effect of traditional Chinese herbal medicines against lung cancer. Anticancer Drugs 2014, 25: 983-991.
9. Salaga M, Zatorski H, Sobczak M,Chinese herbal medicines in the treatment of IBD and colorectal cancer: a review. Curr Treat Options Oncol 2014, 15: 405-420.
10. Li C, Sun BQ, Gai XD. Compounds from Chinese herbal medicines as reversal agents for P-glycoprotein-mediated multidrug resistance in tumors. Clin Transl Oncol 2014, 16: 593-598.
11. Remmelink M, Sokolow Y Leduc D. Techniques and strategy of pathological sampling in the diagnostic and therapeutic management of lung cancer. Rev Mal Respir 2015, 32: 381-393.
12. Zer A, Leighl N. Promising Targets and Current Clinical Trials in Metastatic Non-Squamous NSCLC. Front Oncol 2014, 4: 329.
13. Kasapoglu US, Arinc S, Gungor S,Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J 2016, 10:791-799.
14. Berghmans T, Ameye L, Lafitte JJ,Prospective Validation Obtained in a Similar Group of Patients and with Similar High Throughput Biological Tests Failed to Confirm Signatures for Prediction of Response to Chemotherapy and Survival in Advanced NSCLC: A Prospective Study from the European Lung Cancer Working Party. Front Oncol 2014, 4: 386.
15. Liang W, Zhang L, Jiang G,Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015, 33: 861-869.
16. Spigel DR, Patel JD, Reynolds CH,Quality of life analyses from the randomized, open-label, phase III PointBreak study of pemetrexed- carboplatin-bevacizumab followed by maintenance pemetrexed-bevacizumab versus paclitaxel- carboplatin-bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Thorac Oncol 2015, 10: 353-359.
17. Zhu L, Yu H, Liu SY,Prognostic value of tissue inhibitor of metalloproteinase-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2015, 10: e0124230.
18. Wang J, Zhao F, Dou J,Immunotherapy of melanoma by GPI-anchored IL-21 tumour vaccine involves down-regulating regulatory T cells in mouse model. Int J Immunogenet 2011, 38: 21-29.
19. He LY, Li L, Guo ML,Relationship between CD4+CD25+Treg and expression of HIF-1alpha and Ki-67 in NSCLC patients. Eur Rev Med Pharmacol Sci 2015, 19: 1351-1355.
20. Zhang R, Li F, Li H,The clinical significance of memory T cells and its subsets in gastric cancer. Clin Transl Oncol 2014, 16: 257-265.
21. Giatromanolaki A, Koukourakis MI, Kakolyris S,Focal expression of thymidine phosphorylase associates with CD31 positive lymphocytic aggregation and local neo-angiogenesis in non-small cell lung cancer. Anticancer Res 1998, 18: 71-76.
22. Ju S, Qiu H, Zhou X,CD13+CD4+CD25hi regulatory T cells exhibit higher suppressive function and increase with tumor stage in non-small cell lung cancer patients. Cell Cycle 2009, 8: 2578-2585.
Xiaoyan decoction enhances the T cell immunity in lung cancer microenvironment, and thus inhibiting lung cancer in mice.
Clinical experience has demonstrated that traditional Chinese medicine may have anti-tumor function. However, the underline mechanisms remain largely unknown. This study revealed Chinese medicine Xiaoyan decoction may inhibit lung cancer by enhance T cell immunity.
Submitted: 13 June 2019,
Online: 16 September 2019.
14 August 2019,
Funding: This work is supported by Tianjin Science & Technology Plan Projects (No. 17ZXMFSY00190), Tianjin Traditional Chinese Medicine Research Project, Tianjin health and family planning commission (No. 2017003) and the National Natural Science Foundation of China (No. 81403220).
Competing interests: Authors declare that they have no competing interests.
Copyright: ?2019 TMR Publishing Group Limited. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License.
#They contributed equally to this work.
*Correspondence: Ying-Jie Jia, Department of Oncology,First Teaching Hospital of Tianjin University of TraditionalChinese Medicine, Anshanxi Road, Nankai District, Tianjin,300193, China. Email: jiayingjie1616@163.com.

