Treatment of undifferentiated thyroid carcinoma with darafini and trametinib: a case report and literature review
Nan Nan , Tao Chi, Xiao-Juan Yan,*
Treatment of undifferentiated thyroid carcinoma with darafini and trametinib: a case report and literature review
Nan Nan1, Tao Chi1, Xiao-Juan Yan1,*
1Department of Oncology, Taiyuan Traditional Chinese Medicine Hospital, Shanxi 030009, China.
Undifferentiated thyroid carcinoma progresses rapidly and has a poor prognosis. The median progression-free survival is only about half a year, and the effect of conventional radiotherapy and chemotherapy is poor. Some patients may be associated with BRAF V600E mutation. Dabrafenib and trametinib were approved by the FDA for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations. The combination of the two may make patients receive a better benefit. A phase III clinical trial showed that in patients with advanced malignant melanoma with positive BRAF-V600E mutations, the combination of dabrafenib and trametinib can effectively improve progression-free survival and overall survival in patients. This article describes a case describing a patient with BRAF V600E-mutated thyroid undifferentiated carcinoma that was treated with darafini and trimetinib, and the relevant literature on the combination of the two drugs was analyzed.
Undifferentiated thyroid carcinoma, Darafini, Trimetinib, BRAF mutation
The treatment of undifferentiated thyroid cancer is still a problem to be explored.
Combination therapy with drugs may be a new strategy for the treatment of undifferentiated thyroid cancer.
Introduction
Anaplastic Thyroid Cancer (ATC) is a type of malignant tumor with rapid development, aggressiveness, a high degree of malignancy, and poor prognosis in thyroid tumors [1]. Most patients are diagnosed at stage IV [2]. It has been demonstrated that surgery, radiotherapy, chemotherapy, I131, and other treatments usually cannot control disease progression, so the research centers continue to explore effective treatments [3]. Especially targeted therapy has become a hot research topic. On May 4, 2018, the USA FDA approved the combination of dabrafenib (Tafinlar) and trametinib (trakininib, Mekinist) for the treatment of v-Raf murine sarcoma viral oncogene homolog B1(BRAF) V600E mutation in patients received excision or patients with metastatic ATC. This is based on phase II clinical trial (NCT02034110) of rare cancer with BRAF V600E mutation currently under study. The results of the study showed that 16 previously treated undifferentiated thyroid carcinomas with BRAF V600E mutations were treated. Among the patients, the total response rate was 69%, 1 case of completely relieve, 10 cases of partially relieve. However, the median progression-free survival and overall survival were not obtained. The most common adverse reactions during the study were fatigue, fever, and nausea [4]. This article reports a case of darafini combined with trimetinib in the treatment of thyroid undifferentiated carcinoma and combined with this case to analyze the research status of the two-drug combination therapy.
Medical record information
The patient, female, 60 years old, was admitted to the hospital because of "pre-neck mass with hoarseness in February, aggravated for 1 week". At the end of July 2018, the patient had no obvious cause of pre-neck lumps with hoarseness. The patient was treated at the Cancer Hospital of the Chinese Academy of Medical Sciences, and the relevant imaging examinations were improved. The thyroid color ultrasound showed multiple nodules, considering malignancy. However, lymphoma and undifferentiated carcinoma are still uncertain, and cervical lymphadenopathy is considered for metastasis. CT showed that the size of the irregular mass in the right lobe of the thyroid was about 3.6 × 3.1 cm, breaking through the capsule, multiple metastases in both lungs, and the large metastatic lesion was 1.2 × 1.0 cm (Fig. 1a, 1b). Goiter thyroid biopsy combined with immunohistochemistry results: AE1/AE3 (2 +), CK18 (2 +), TTF-1 (-), PAX8 (2 +), TG (-), CT (-), GATA3 (individual cells +), P53 (-), Ki-67 (50%), consider undifferentiated thyroid carcinoma. On September 12, the peripheral blood gene test revealed that the BRAF15 exon p.V600E missense mutation and the TP53 exon 4 frameshift mutation. On September 27, oral dalafini 150mg 2 times/day, trametinib 2mg 1 time/day treatment started. During taking the drug, high fever occurred frequently. No routine abnormalities such as blood routine examination and procalcitonin were observed. Considering the side effects of the drug, the physical cooling was improved, and no other adverse reactions occurred. After 1 month of treatment, the patient's neck mass was significantly reduced, and the hoarseness improved. On October 31, the CT scan showed that the size of the right thyroid tumor was reduced to 2.2 × 1.9 cm, and the multiple lung metastases were significantly reduced (Fig. 1c, 1d). During treatment, the patient developed a cough and a large amount of white sputum. According to the sputum culture and drug susceptibility results, levofloxacin anti-infective treatment was combined, and the symptoms improved after treatment with bromhexine and atomized sputum-containing antibacterial solution.Patient female, 60 years old, CT findings before and after targeted therapy. A, CT suggests that the right lobes of the thyroid are enlarged, and irregularly enlarged tumors are visible, and the trachea is displaced by pressure (yellow arrow). B, Nodules in the lungs (red arrow). C, After 1 month of oral targeted drug treatment, the thyroid mass was significantly reduced, and the trachea was centered (yellow arrow). D, After 1 month of oral targeted drug therapy, multiple lung metastases were reduced (red arrow).
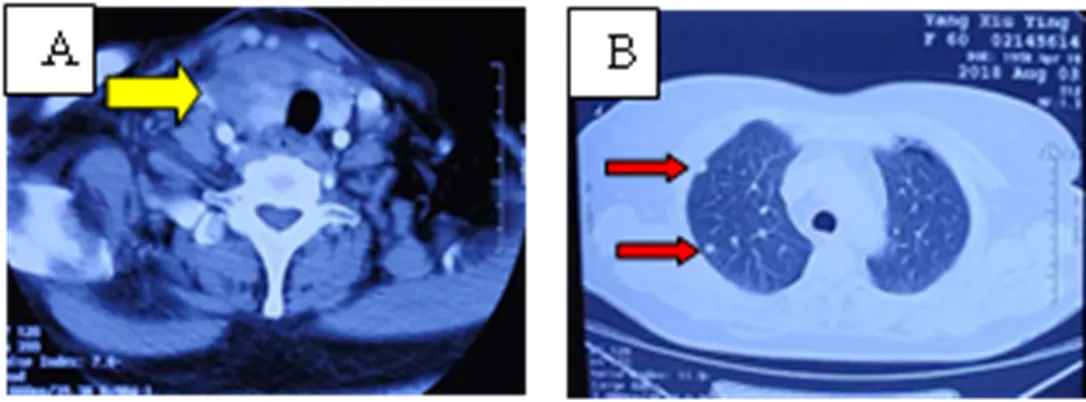
Figure 1 Patient's imaging image
Discussion
In this case, the patient was found to be undifferentiated thyroid cancer with multiple lung metastases. The stage was IVc. The current radiotherapy and chemotherapy methods are not effective, and the response rate is not obvious. Because thyroid cancer is often accompanied by BRAF V600E mutation, the patient's plasma genetic test found that the above mutations exist. Patients were treated with oral dalafinib and trametinib for targeted therapy. After one month of treatment, symptoms, signs and imaging examinations showed improvement. It can be determined that the treatment regimen has a definite effect on undifferentiated thyroid carcinoma with BRAF V600E. The side effects during the treatment of the patient were only fever and chills, which improved after symptomatic treatment. However, because of the emergence of drug resistance after targeted therapy, previous studies have demonstrated the median progression-free survival of the two combined use is 9.3-11.4 months [13, 21-24]. It may be related to genetic mutations leading to reactivation of the MAPK pathway or the role of the PI3K-Akt pathway [25]. Previous studies also found that trametinib can increase the expression of SNAI1 mRNA in cells, which may be related to drug resistance mechanisms [26]. The current treatment time of this patient is limited, and the specific time of drug resistance is not clear. If drug resistance occurs, how should the patient's next treatment plan be developed, whether it can be considered in combination with other target inhibitors [27] or HSP90 inhibitors [28], or in combination with pembrolizumab as a neoadjuvant therapy [29, 30] are currently urgent problems to be solved.
The BRAF gene is also named the V-Raf murine sarcoma viral oncogene homolog B1, is an important member of three subtypes of the RAF family (ARAF, BRAF, CRAF), which is a mitogen-activated protein kinase (A key driver of tumorigenesis in the MAPK pathway [5]. BRAF activates the RAF-MEK-ERK signaling pathway by phosphorylating the downstream MEK protein, playing a vital role in promoting cell cycle regulation, and plays a role in cell growth, proliferation, and differentiation. According to the nucleotide sequence comparison in the National Center for Biotechnology Information Gene Pool, the mechanism of BRAF V600E mutation is T to A conversion (T1799A) at position 1799 of exon 15 of BR3 kinase region of BRAF gene, which results in the substitution of the 600th proline (V) in its protein product by glutamate (E), interrupting the association of the BRAF gene-encoding product with the P-ring that maintains its inactive state [6]. BRAF is continuously activated, acting on downstream genes, causing cascade activation reactions, abnormal proliferation and differentiation of cells, ultimately leading to tumor formation [7].
Dabrafenib and trametinib were approved by the FDA on May 29, 2013, for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations. Dabrafenib is a class I BRAF inhibitor that inhibits cell viability by down-regulating MEK/ERK phosphorylation, arresting the OCUT-4 cell cycle in G0/G1 phase [8]. Trametinib, as the first mitogen-activated extracellular signal-regulated kinase (MEK1/2) reversible inhibitor, inhibits tumor cell proliferation by down-regulating the phosphorylation of ERK and blocking the MAPK signaling pathway. However, experimental studies have revealed that trametinib has a weaker inhibitory effect on cell lines with BRAF mutations [8], but it can enhance the anti-tumor effect of BRAF inhibitors [9]. It has also been observed in clinical trials that the treatment effect of trametinib on glioma [10], malignant melanoma [11] is not well, but the combination of the two may make patients receive a better benefit. A phase III clinical trial showed that in patients with advanced malignant melanoma with positive BRAF-V600E mutations, the combination of BRAF inhibitors and MEK inhibitors can effectively improve progression-free survival and overall survival in patients [12- 14], and can improve brain metastases in patients [12]. The mechanism of action is that MEK inhibitors re-suppressed MAPK signaling pathway and reduced PD-L1 production [15].
Moreover, the application of this treatment plan is not limited to malignant melanoma, and significant effects have also been obtained in the treatment of malignant tumors such as intrahepatic cholangiocarcinoma with BRAF-V600E mutation [16] and non-small cell lung cancer [17,18]. In the clinical study of the combination therapy of the two drugs, the adverse reactions of the patients mainly include general symptoms (fever, chills, fatigue, and peripheral edema), skin and subcutaneous tissue diseases, gastrointestinal disorders, nervous system diseases, respiratory diseases, musculoskeletal diseases, and tamponade [19]. Many patients have to reduce or discontinue medication due to fever, chills, nausea, rash, and decreased left ventricular ejection fraction. Studies also found that the adverse effects of the combination of the two drugs were not significantly increased compared with the single drug alone [13], but helped to reduce the incidence of squamous cell carcinoma of the skin [20].
Conclusion
Under the existing research, we still need to constantly explore new and more effective treatment methods, especially the combination of immunotherapy, to improve the efficiency and survival of cancer patients.
1. Zhang ZM, Xu ZG, Tang PZ,. Re-recognizing undifferentiated thyroid cancer. J Chin Academy Med Sci 2006, 28: 323-324.
2. Amin MB, Edge SB, Greene FL,. AJCC Cancer Staging Manual. 8th edit. New York: Springer 2017: 878-883.
3. Liu Y, Zhang SL. Advances in targeted drugs for thyroid undifferentiated carcinoma. J Surg Theory Prac 2018, 23: 169-172.
4. Vivek S, Robert JK, Zev AW,. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 2018, 36: 7-13.
5. Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res 2010, 16: 3329-3334.
6. Wan PT, Gamer MJ, Rose SM,. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutation of B-RAF. Cell 2004, 116: 855-867.
7. He YZ, Jiang Y, Yu JC,. Research progress on the relationship between BRAF gene and thyroid cancer. Chin J Surgery 2016, 54: 237-240.
8. Kurata K, Onoda N, Noda S,. Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells. Int J Oncol 2016, 49: 2303-2308.
9. McFadden DG, Vernon A, Santiago PM,. P53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci USA 2014, 111: E1600-E1609.
10. Bavle A, Jones J, Lin FY,. Dramatic clinical and radiographic response to BRAF inhibition in a patient with progressive disseminated optic pathway glioma refractory to MEK inhibition. Pediatr Hematol Oncol 2017, 34:254-259.
11. Grimaldi AM, Simeone E, Ascierto PA. The role of MEK inhibitors in the treatment of metastatic melanoma. Curr Opin Oncol 2014, 26: 196-203.
12. Long GV, Hauschild A, Santanami M,. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-Mutated Melanoma. New Engl J Med 2017, 377: 1813-1823.
13. Long GV, Stroyakovskiy D, Gogas H,. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. New Engl J Med 2014, 371: 1877-1888.
14. Long GV, Flaherty KT, Stroyakovskiy D,. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017, 28:1631-1639.
15. Jiang X, Zhou J, Giobbie-Hurder A,. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013, 19: 598-609.
16. Lavinqia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol 2016, 7: 98-102 .
17. Rivalland G, Mitchell P. Combined BRAF and MEK inhibition in BRAF-mutant NSCLC. Lancet Oncol 2016, 17: 860-862.
18. Planchard D, Besse B, Groen HJM,. Dabrafenib plus trametinib in patients with previously treated BRAF (V600E) -mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016, 17: 984-993.
19. Sundaram VR, Abbas T. Cardiac tamponade induced by dabrafenib and trametinib combination therapy for melanoma: a case report. Med 2018, 97: e12751.
20. Dossett LA, Kudchadkar RR, Zager JS. BRAF and MEK inhibition in melanoma. Expert Opin Drug Safe 2015, 14: 559-570.
21. Long GV, Stroyakovskiy D, Gogas H,. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trials. Lancet 2015, 386: 444-451.
22. Larkin J, Ascierto PA, Creno B,. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014, 371: 1867-1876.
23. Robert C, Karaszewska B, Schachter J,. Improved overall survival in melanoma with combined dabrafenib and trametrnib. N Engl J Med 2015, 372: 30-39.
24. Curtin JA, Fridlyand J, Kageshita T,. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005, 353: 2135-2147.
25. Kakadia S, Yarlagadda N, Awad R,. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Oncol Targets Ther 2018, 11:7095-7107.
26. Kurata K, Onoda N, Noda S,. Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells. Int J Oncol 2016, 49: 2303-2308.
27. Temraz S, Mukherji D, Shamseddine A. Dual inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int J Mol Sci 2015, 16: 22976-22988.
28. Smyth T, Paraiso KHT, Hearn K,. Inhibition of HSP90 by AT13387 delays the emergence of resistance to BRAF inhibitors and overcomes resistance to dual BRAF and MEK inhibition in melanoma models. Mol Cance Ther 2014, 13: 2793-2804.
29. Cabanillas ME, Ferrarotto R, Garden AS,. Neoadjuvant BRAF- and Immune-Directed therapy for anaplastic thyroid carcinoma. Thyroid 2018, 28: 945-951.
30. Iyer PC, Dadu R, Gule-Monroe M,. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer 2018, 6: 68.
20 March 2019,
03 April 2019,
22 June 2019.
Authors declare that they have no competing interests.
?2019 TMR Publishing Group Limited. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License.
Many patients with undifferentiated thyroid cancer have no significant response to treatment, which is an important cause of tumor progression and death in patients. Genetic testing and the use of targeted drugs against mutant genes may be a viable approach to the treatment of undifferentiated thyroid cancer.
Xiao-Juan Yan, Department of Oncology,Taiyuan Traditional Chinese Medicine Hospital, No. 2,Baling South Street, Taiyuan, Shanxi 030009, China, E-mail:708860791@qq.com.
- Cancer Advances的其它文章
- The clinical efficacy analysis of radiofrequency ablation combined with chemotherapy in treating late non-small cell lung cancer
- Combination of Chinese and Western medicine in the treatment of aggressive angiomyxoma: a case report
- The differences and similarities between regional ethnic medicine and traditional Chinese medicine in the prevention and treatment of cancer pain
- Research progress in the treatment of colorectal cancer in classical prescriptions

