Subcellular expression of maspin-from normal tissue to tumor cells
Laura Banias,Ioan Jung,Simona Gurzu
Laura Banias,loan Jung,Simona Gurzu,Department of Pathology,University of Medicine,Pharmacy,Sciences and Technology of Tirgu-Mures,Tirgu Mures 540139,Romania
Laura Banias,Simona Gurzu,Department of Pathology,Clinical County Emergency Hospital,Tirgu Mures 540139,Romania
Abstract
Key words: Maspin; SerpinB5; Prognosis; Cancer; Tumor suppressor
INTRODUCTION
Maspin,also known as SerpinB5,is a member of the serine protease inhibitor family,which was identified by Zouet al[1]in 1994[2-4].In most of the studies,it acted as a tumor suppressor through inhibitory effects on invasion,motility,and angiogenesis and through stimulation of a mitochondrial apoptosis pathway[1-4].This negative impact on tumor cells is supposed to be p53-linked[5].
Maspin can also induce epigenetic changes like cytosine methylation,deacetylation,chromatin condensation,or histone modulation[3].Recentin vitrostudies focused on maspin secretion[6,7].These studies tried to prove that maspin is a soluble free or an exosome cargo protein,which might be chemically synthesized and used as a future medical drug[6,7].In vitro,maspin influenced the peritumoral microenvironment by enhancing macrophage secretion of inflammatory cytokines[6,7].
In the human body,maspin is expressed in many tissues or organs and is down or upregulated in malignant tumors.As maspin shows different subcellular localizations(cytoplasmic and nuclear),in both normal and tumor tissues,it is difficult to appreciate its exact role in tumorigenesis,tumor invasion,or progression[8].The aim of this review was to perform a complex synthesis regarding maspin expression in different organs,from normal tissue to non-tumor disorders and malignant transformation.The organ-related subcellular expression was also emphasized.
METHODOLOGY
The present paper represents a narrative review of the literature on the serine protease inhibitor maspin,focusing mainly on its immunohistochemical (IHC)expression in different tissues and pathologic processes.
The online search consisted of browsing the PubMed/MEDLINE database using the MeSH terms and keywords “maspin” and “serpinB5” to identify articles published between 1994 and the beginning of 2019.Eligible for inclusion were only publications written in English,studies for human species,and with full-text availability (Figure 1).
Besides the detailed presentation of data,summary tables regarding maspin immunoexpression in different organs in various conditions were constructed based on the data published in the included articles (Tables 1-3).
TISSUE-AND ORGAN-RELATED MASPIN EXPRESSION,IN NORMAL AND PATHOLOGIC CONDITIONS
Placenta
Dokraset al[9]first evaluated IHC expression and mRNA levels of placenta maspin,in 2002.Placentas obtained after first and second trimester pregnancy and after caesarian deliveries at term were included in their observations.The maximum values of maspin mRNA level were detected in the third trimester of pregnancy.On the other hand,negative expression was observed in the immortalized first trimester cytotrophoblasts and choriocarcinoma cell lines with high invasive ability.Similar to the mRNA levels,IHC expression showed patchy staining of the cytotrophoblastic layer in the first trimester,uniform cyto-and syncytiotrophoblastic layers in the second trimester,and more intense expression in the third trimester[9](Table 1).
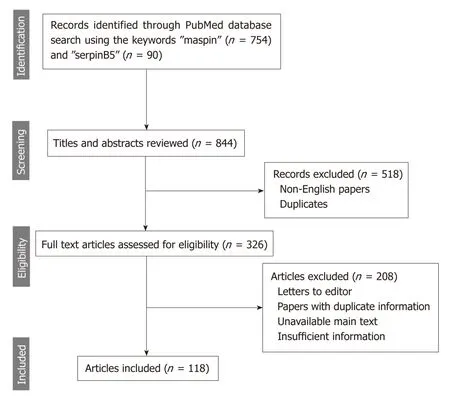
Figure 1 The methodology (PRlSMA flow diagram) used for this review.
In preeclamptic (PE) placentas,both mRNA and protein levels were upregulated(Table 1) and correlated with modifications observed with Hematoxylin and Eosin stain[10].It was intimal enlargement of the vessel wall,thickening of the syncytiotrophoblast membranes,and increased number of syncytial knots.It was concluded that hypomethylation of the maspin promoter might be the causal factor of the increased expression of maspin in PE placentas[10].
Qiet al[11]evaluated the plasmatic level of unmethylated maspin DNA in a population consisting of women with normal pregnancies,PE,and gestational trophoblastic disease.Unmethylated maspin DNA was not detected in healthy nonpregnant women and in those with the trophoblastic gestational disease.The level was higher in women with severe PE than in those with normal third trimester pregnancies and presented a gradual increase with the gestational age[11].
Methylated and unmethylated maspin DNA blood concentrations may be useful for identification of noninvasive fetal trisomy 18 beginning in the first trimester[12].Methylation of the maspin gene induces downregulation of maspin protein expression and subsequently inhibits migration and invasion of the first trimester extravillous trophoblast cell line through interaction with the proangiogenic factors such as mismatch repair proteins (e.g.,MMP2) or vascular endothelial growth factors(VEGF-A and VEGF-C)[13].This interaction might lead to the occurrence of PE[13].
Regarding maspin subcellular localization,nuclear expression was limited to the chorionic plate with significant downregulation in the extravillous trophoblasts[14].Cytoplasmic positivity can be seen in endothelial cells and trophoblasts[9,14](Table 1).
Mammary gland
Maspin expression was evaluated in both normal tissues,especially during pregnancy and carcinomas of the mammary gland[15-20].In late pregnancy,a peak of expression is seen during lactation and the level decreases and remains constant after the lactation period[20].Almost all cells presented cytoplasmic staining with infrequent nuclear positivity (Table 1),which can be an indicator of epithelial growth factor induced maspin phosphorylation[20].
In breast carcinomas,there are several maspin-related studies,but in most of them no data about the subcellular localization of staining were included.In these tumors,maspin IHC positivity (independently by the localization) was directly correlated with larger tumor size,younger age,high histologic grade,negative expression of estrogen receptor and/or progesterone receptor,positivity for p53,and a lymphocyterich stroma[15-18].In other studies,it was hypothesized that maspin is not involved in breast cancer histogenesis,at least in those carcinomas with extremely aggressive behavior[21].

Table 1 Maspin expression in placenta,mammary gland,and urogenital organs
Examination of the subcellular localization (Table 1) revealed that maspin cytoplasmic positivity is observed in 36% of invasive ductal carcinomas and 7% of lobular carcinomas,the latter being mostly maspin-negative[18,21].The nuclear expression is related with estrogen receptor and progesterone receptor positivity,while the cytoplasmic location is an indicator of negativity for hormone receptors,high S-phase fraction,and aneuploidy[19].Maspin cytoplasmic positivity was suggested to be a poor prognostic indicator of invasive breast cancer,independently of the histologic subtype[19].
Machowskaet al[22]presented nuclear location as a better prognostic factor in cases with invasive ductal carcinoma.Nuclear positivity was an indicator of Ki-67 negativity or low expression[22].In a study by Strienet al[23],which compares luminal subtype A and B breast cancers,it was shown that maspin expression was lost in metastases,in the majority of maspin-positive primary tumors,or presented translocation from cytoplasmic to nuclear positivity.No differences between subtype A and B were noted.
Wakaharaet al[24],examining four categories of maspin expression [cytoplasmic only,nuclear only,mixed (cytoplasm + nuclei),and negative] and their correlations with histone deacetylase 1,showed that maspin cytoplasmic only represents an independent negative prognostic factor,thus being an indicator of higher histological grade,negative progesterone receptor expression,shorter disease-free survival,and higher histone deacetylase 1 compared with the mixed expression group.They suggested that inhibition of histone deacetylase 1 could represent an inhibitory mechanism for maspin[24].
Recently,Umekitaet al[25]demonstrated that maspin mRNA expression in sentinel lymph nodes represents an independent factor of nonsentinel lymph node metastasis.Maspin was shown to act upon peritumoral stroma and to increase collagen production as a cause for doxorubicin resistance[26].
Urogenital system
Ovary:Expression of maspin was not present in the normal ovary[27-30].In ovarian carcinomas,maspin expression in over 50% of the tumor cells was associated with higher tumor grade,positive peritoneal effusion cytology,lower survival rate,and positivity for the proangiogenic factors VEGF-A,-C,and-D[27,28].Most of themalignant tumors presented with cytoplasmic only expression,but those with low malignant potential showed mixed positivity (cytoplasm and nucleus)[29].The localization of maspin expression might have therapeutic importance because the cytoplasmic positivity associates with cisplatin sensitivity[30](Table 1).
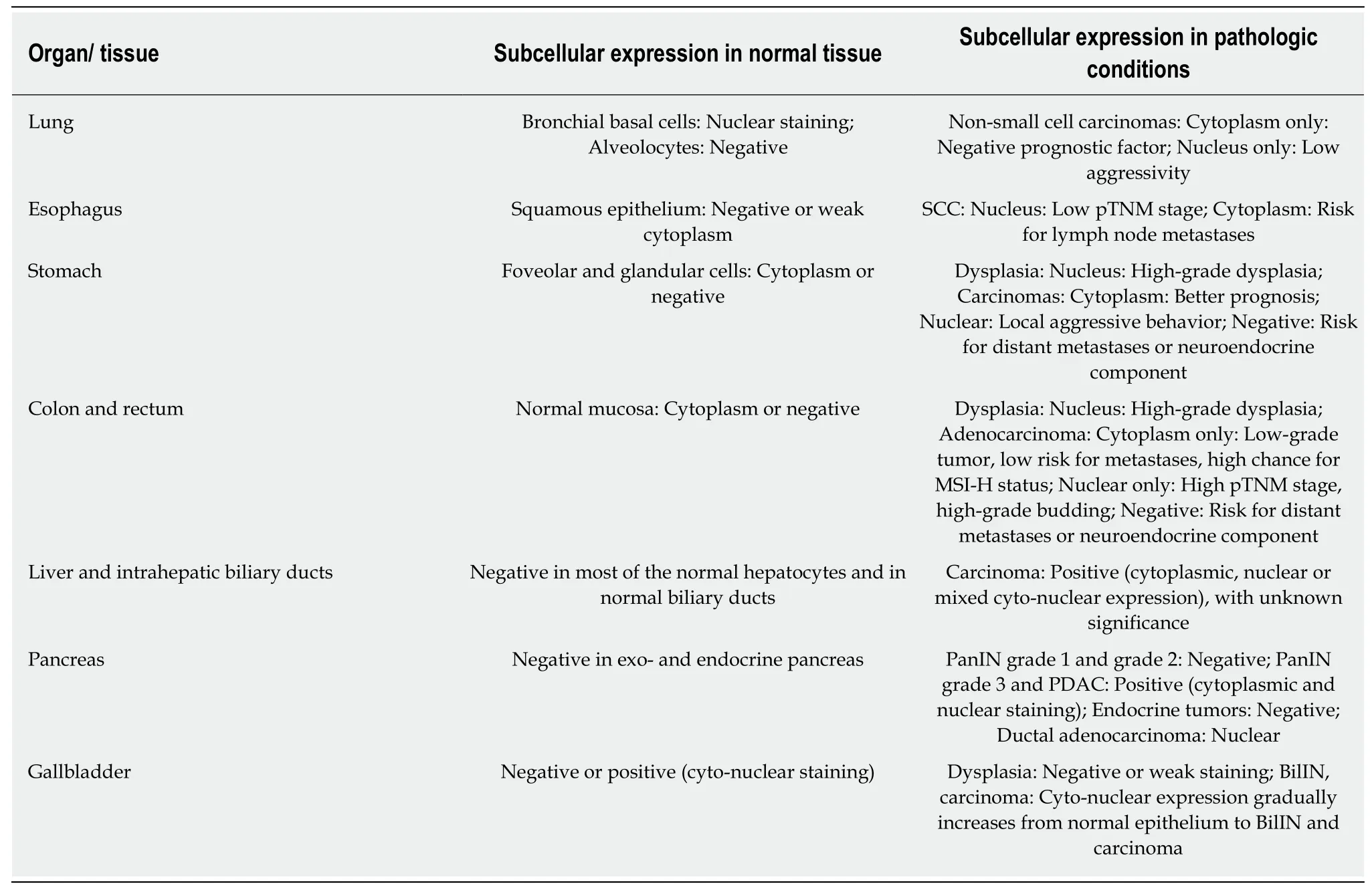
Table 2 Maspin expression in organs of the respiratory and gastroenteropancreatic system
Uterine cervix:Maspin is expressed both in the cytoplasm and nucleus of the normal squamous cervical epithelium[31,32].The cytoplasmic expression is downregulated in premalignant disorders such as cervical intraepithelial neoplasia grade 3 and even more downregulated from microinvasive to invasive squamous cell carcinoma(SCC)[31,32].
In SCC,cytoplasmic maspin can be colocalized with cytoplasmic testisin,a serine protease normally found in testicular germ cells,which inhibits the tumor suppressor activity of maspin[33].Maspin positivity is correlated with advanced stage,increased lymphatic microvessel density,and the presence of lymph node metastases[31,32].Nuclear expression increases in cervical intraepithelial neoplasia grade 3 but significantly decreases in SCC cells[31,32].Maspin expression is decreased or lost in intravascular emboli from SCCs[31,32].In adenocarcinomas of the uterine cervix,cytoplasmic expression of maspin was found to be an indicator of aggressive behavior[34](Table 1).
Uterine body:The normal endometrium is maspin negative or localizes to the nucleus[35-37].Maspin is positive in most of the cases diagnosed as atypical hyperplasia or endometrioid endometrial adenocarcinoma (nuclear and/or cytoplasmic staining).Maspin expression is also correlated with lymph node metastases and FIGO stage in endometrioid endometrial adenocarcinoma[35-37].Nuclear subcellular localization was correlated with squamous cell differentiation of endometrioid endometrial adenocarcinoma and with better prognosis,while concurrent cytoplasmic positivity represents an indicator of a more aggressive tumor[36,38](Table 1).
Prostate gland:In normal prostate,maspin marks basal but not secretory cells[39-43].Its expression is upregulated in high-grade prostatic intraepithelial neoplasia and downregulated during progression to invasive carcinoma[41](Table 1).In prostate carcinomas,maspin exerts a tumor suppressing role[39,40].Negative or decreased IHCexpression was correlated with p53 positivity and a higher tumor grade and stage[39,40](Table 1).
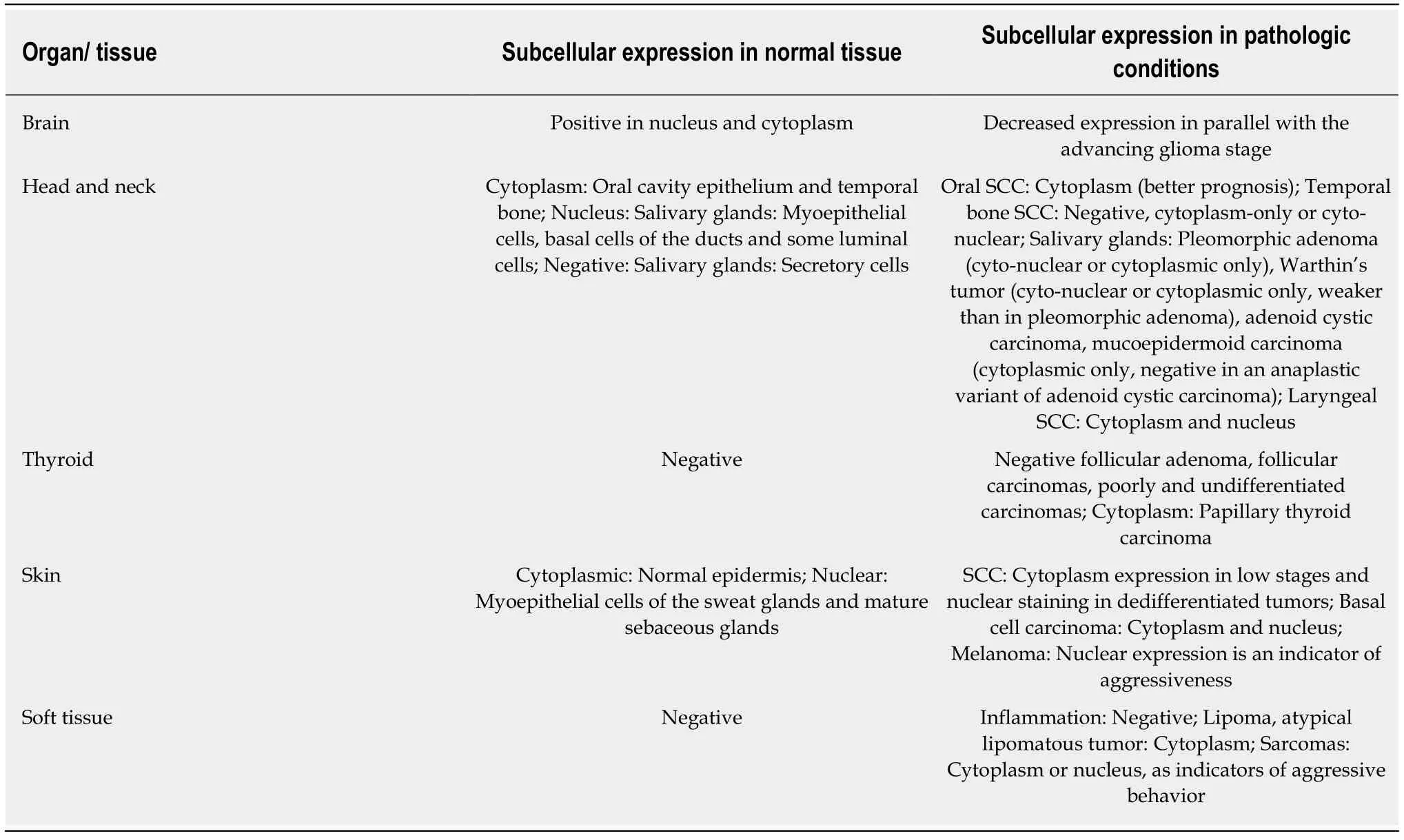
Table 3 Maspin expression in brain,organs of the head and neck area,skin and soft tissues
Positive immunostaining was noted in tumors that showed a histological response to therapy administered before prostatectomy[39,40].Maspin also proved its ability to enhance the sensitivity of hormone-resistant prostate cancer cells to curcumin treatment by modulating levels of proapoptotic proteins Bad and Bax[42].The experimental studies proved that maspin can influence prostate carcinoma host immune response through stimulation of neutrophil maturation at both the systemic and intratumoral levels along with antibody-dependent cytotoxicity and decreased lymphatic vessels formation[43].
Urinary bladder:In normal bladder,maspin expression can be seen in epithelial cells[44-47].Maspin downregulation in bladder carcinoma cells has been shown to be significantly associated with a lower progression-free survival rate[44-46](Table 1).Elevated levels inhibited proangiogenic factors such as insulin-like growth factor binding protein-2 or VEGF-C and upregulated the apoptosis rate of cancer cells[44-46].Maspin increased the sensitivity of bladder cancer cells to cisplatin therapy by enhancing its inhibitory effect on tumor cell proliferation[44-46].
Induction of maspin was suggested to be the mechanism through which Prostatederived E-twenty six factor (decreased in tumor cells compared with the normal bladder) inhibit tumor development and invasion along with repressing epithelialmesenchymal transition by upregulating E-cadherin expression and downregulating vimentin,SNAIL,SLUG,and N-cadherin[47].
Studies of IHC expression observed contradictory results.In some studies,maspin was mostly positive in low-grade tumors and associated with better survival.Others showed an important increase in maspin expression in high-grade bladder tumors[8].
Lung
In normal bronchial cells,maspin expression can be seen in the nuclei of basal cells[48-52].In non-small cell carcinomas,both SCC and adenocarcinomas,subcellular localization of maspin proved to be correlated with some clinicopathological parameters (Table 2).
Cytoplasmic expression was an independent negative prognostic indicator and was correlated with the micropapillary component,higher pTNM stage,shorter diseasefree survival,and low disease-specific survival[48-51].On the other hand,nuclear only staining (without synchronous cytoplasm positivity) was correlated with earlier pathological stage,absence of aggressive invasion,and negative p53[48-51].Maspin mRNA expression appeared to be upregulated in adenocarcinoma cells compared to the adjacent normal lung with higher levels of mRNA in advanced stages[52].
Gastrointestinal tract
Esophagus:Maspin can show infrequent cytoplasmic positivity in squamous cell epithelium[53-55](Table 2).In SCC cells,downregulation of maspin was noted compared with the adjacent normal epithelium.Strong nuclear staining is associated with favorable prognosis,increased patient survival,and a lower pTN stage while high cytoplasmic staining correlates with the presence of lymph node metastases[53,54].Based on anin vitrostudy,which used esophageal SCC cell lines,it was hypothesized that the inhibitory effect of maspin is based on switching the metabolic phenotype to low glycolysis through disrupting the hypoxia inducible factor 1α[55].
Stomach:Maspin expression can be absent or in the cytoplasm of normal epithelium and increases in gastric epithelial cells with intestinal metaplasia likely as a result of demethylation of the maspin gene promoter[56-62].Nuclear maspin marks cells with high-grade intraepithelial neoplasia and is one of the factors that plays a role in the progression of intramucosal clusters of signet ring cells to signet ring cell carcinoma,especially multifocal carcinomas[57,58](Table 2).
In gastric adenocarcinoma cells,maspin expression can be lost,which is an indication of a high risk for distant metastases[21,59].Complete loss of maspin was also observed more frequently in elderly patients,poorly cohesive carcinomas,and poorly differentiated adenocarcinomas located in the distal part of the stomach[58-61].Maspin is negative in neuroendocrine components of adenocarcinomas and is not involved in tumorigenesis of gastric neuroendocrine tumors[62].
Cytoplasm only staining is rarely seen in clinical practice in poorly cohesive carcinomas[58].In adenocarcinomas,cytoplasm expression is as an indicator of lower pTNM stage and high angiogenic phenotype and correlates with positivity for p53,Bax,Ki-67 and E-cadherin[58].
Nuclear positivity (with or without associated cytoplasm expression) is predominant in undifferentiated intestinal type carcinoma and poorly cohesive carcinoma[58].Nuclear positivity is associated with locally aggressive behavior and high risk for lymph node metastases and is more frequent in young patients[21,58,59,62].It is associated with Bax,p53,and Ki-67 negativity and lower angiogenesis[58].In daily practice,we use nuclear maspin for a better approach of the depth of invasion (pT stage) of poorly cohesive carcinomas (personal unpublished observations).
Colon and rectum:Similar to the gastric epithelium,in colorectal segments maspin expression can be absent or present in the cytoplasm of normal epithelium and increases in epithelial cells with high-grade dysplasia[62-73].Maspin serum levels are increased in patients with high-grade dysplasia and carcinomas and might be used as an indicator for colonoscopy[69,70].
In colorectal segments,maspin does not mark neuroendocrine tumors[62],but its subcellular expression has a great value in the assessment of adenocarcinomas[72,73].Although there are studies that proved that maspin expression is correlated with carcinoembryonic antigen serum levels,infiltrative borders,and high histological grade and stage in colorectal adenocarcinomas[65],few articles regard maspin subcellular expression.We use this marker for daily diagnosis and data showed in this review are based on personal observations (over 200 cases were revised) and literature data (Table 2).
Maspin cytoplasmic only positivity is correlated with the absence or a low number of lymph node metastases,low-grade buddings,and absence of p53 positivity[72,73].It is important to consider a case with cytoplasmic only staining is necessary to have no nuclear positivity (in both tumor center and invasion front).
Maspin nuclear staining is an indicator of aggressive tumor behavior,high tumor grade,high budding grade,high pTNM stage,high risk of local recurrence,or lymph node metastases and absence of peritumoral lymphoid reaction and p53 and VEGF-A positivity[63,64,72,73].As the maspin nuclear expression is characteristic for tumor buds[73],we use this marker to diagnose patients because maspin is more efficient than cytokeratins (personal observations).For stage II and III colon cancer patients,nuclear maspin staining is an independent predictor of sensitivity for adjuvant chemotherapy with 5-fluorouracil and levamisole[67,68,74].
Although it was postulated that elevated nuclear maspin is associated with microsatellite instability[63],we observed that it is associated with low microsatellite instability.The high microsatellite instability (MSI-H) cases usually show cytoplasmic or mixed (cyto-nuclear) maspin positivity[72].In a few cases,nuclear predominance can be seen in MSI-H cases,but this pattern is observed in p53 negative carcinomas only[74].It was even suggested that MSI-H carcinomas with nuclear maspin might respond to 5-fluorouracil-based therapy[74].For patients who received preoperative neoadjuvant chemoradiotherapy,maspin should be downregulated as a result of chemotherapeutic influence[66].
Combining the microsatellite status with BRAF mutation and IHC expression of p53 and maspin,a classification of colorectal cancer was proposed with the best prognosis attributed to MSI-H/BRAF mutated/p53 negative cases with a high number of tumor infiltrating lymphocytes and cytoplasmic maspin expression[71].Cases with the worst prognosis were described as those being MSS/BRAF mutated/p53 positive with low tumor infiltrating lymphocytes and nuclear maspin staining predominance[71].Similar to gastric carcinomas,loss of maspin expression is an indicator of a neuroendocrine component or high risk for distant metastases[72].
Hepatic and pancreatic system
Liver and intrahepatic bile ducts:Maspin infrequently marks hepatocytes and biliary epithelium[75-78].It is downregulated in hepatocellular carcinoma cells (Table 2),but the exact mechanism is still unknown.Maspin downregulation can be the result of activation of inhibitor κB kinase α by HBx protein and can induce chemoresistance[75].Decreased maspin expression along with increased VEGF-A expression may be induced by overexpression of chloride intracellular channel 1[76].Yanget al[77]identified a correlation between patients with a C allele polymorphism of maspin rs2289520 and high Child-Pugh grade (B/C)[77].
In intrahepatic cholangiocarcinomas,a delayed progression was shown to be the benefit of maspin and Bax coexpression[78].
Pancreas:Normal pancreatic ducts,Langerhans islets,endocrine tumors,and lowgrade lesions of the pancreas do not express maspin[79-82].Maspin is localized in the cytoplasm and nucleus of the premalignant lesions such as pancreatic intraepithelial neoplasia (PanIN) grade 3 and also in pancreatic ductal adenocarcinoma(with/without cystic changes; with/without mucinous component) (Table 2) along with strong expression of carcinoembryonic antigen and p53[79-82].
In chronic pancreatitis,which causes diagnostic problems,the presence of an unmethylated maspin promoter can be used to differentiate this lesion from pancreatic ductal adenocarcinoma (PDAC)[83].In a recent meta-analysis,maspin and trefoil factor 1 were found to display significantly higher blood plasma levels in PDAC compared to normal tissue[84].Overexpression of maspin was confirmed by RTPCR in PDAC and normal adjacent pancreatic tissue[85].
Gallbladder:Although maspin is mostly negative in normal epithelium,it might be helpful for differentiating a malignant tumor from atypical reactive changes of the bile ducts (Table 2) in combination with p53[86-91].Maspin shows gradually increasing cyto-nuclear expression from regenerative atypia to biliary intraepithelial neoplasia with significant upregulation in carcinomas[86].The stepwise rise in maspin level from normal epithelium to gallbladder carcinoma is also reflected in its mRNA level[87].
For bile duct biopsy specimens,the use of an immunomarkers complex was also proposed consisting of maspin,insulin-like growth factor-II mRNA binding protein-3,S100P,and von Hippel-Lindau gene product.Positive reactions for maspin,S100P,insulin-like growth factor-II mRNA binding protein-3 along with negativity for von Hippel-Lindau gene product was suggested as a specific staining pattern for bile duct adenocarcinoma[88,89].Double IHC expressions for maspin (nuclear and cytoplasmic)and claudin-18 (membrane) may improve the diagnostic sensitivity to differentiate a bile duct carcinoma from a ductal adenocarcinoma[90].This combination was also proposed for distinguishing biliary intraepithelial neoplasia from non-neoplastic changes[91].
Brain
Normal brain tissue strongly expresses maspin in the cytoplasm and nucleus and is downregulated in parallel with increasing glioma grade (Table 3) possibly by maspin promoter methylation[92,93].
Head and neck
Maspin expression is observed in the cytoplasm or nuclei of salivary glands (myoepithelial cells) and also in oral cavity epithelium[94-105](Table 3).Maspin mRNA was identified in the corneal layers and stroma where it may exert adhesion regulatory functions between the cells and matrix molecules and where it may play a role in wound healing through regulation of the activated fibroblasts migration[105].In inflammation,maspin was hypothesized to be an indicator of invasive fungal rhinosinusitis.It was downregulated in comparison with the noninvasive type and with chronic rhinosinusitis for both cyto-plasm and nucleus[104].Nuclear reaction and a higher intensity of maspin staining were associated with benign lesions of the salivary glands[102,103].
No significant differences in maspin expression were discovered between recurrent and nonrecurrent ameloblastoma and/or ameloblastic carcinoma[94].After studying the maspin gene in a large number of participants,it was found that heterozygous TC of rs17071138 polymorphism and G-G homozygotes or heterozygotes of rs2289520 increase the susceptibility to oral cancer development[95,96].IHC-based studies on SCC of the oral cavity and tongue emphasized an association between high maspin expression and better overall survival,while the absence of maspin was correlated with high pT stage and presence of lymph node metastases[97-99].
In the temporal bone SCC cases,cytoplasmic subcellular localization of maspin expression was significantly higher in the recurrence-free group,thus representing a potential prognostic marker[100].For laryngeal SCC,a separate evaluation of cytoplasmic and nuclear immunostaining has led to an association of the nuclear positivity with a longer disease-free interval after surgery[101].
Thyroid:Maspin is one of the six gene panel proposed for distinguishing normal thyroid from papillary thyroid carcinoma,along with TIMP3,RARB2,RASSF1,TPO,and TSHR[106].In a study by Boltzeet al[107],positive maspin immunoreaction(cytoplasm and nucleus) was observed in papillary thyroid carcinomas,while the normal thyroid tissue,follicular adenomas,follicular carcinomas,and poorly and undifferentiated carcinomas were negative (Table 3).The study also presented maspin promoter methylation as a factor of the silencing mechanism of the dedifferentiation degree[107].
Skin and soft tissues
Skin:Normal epidermis and sweat or sebaceous glands are maspin positive[108-111].In SCCs,translocation of maspin immunoexpression from the cytoplasm to the nucleus in the front of invasion was seen as an indicator of tumor dedifferentiation[108,111](Table 3).All well-differentiated tumors and all cases diagnosed in pT1 stage presented cytoplasmic maspin expression only[108].
The PCR-related studies showed maspin downregulation in tumor tissues compared with the normal adjacent cutis showing the potential role of maspin in tumor development inhibition[109].Basal cell carcinoma cells variably express maspin at the cytoplasm and nucleus in the center of the nodules especially in nodular basal cell carcinoma[110].Although infrequently observed,nuclear maspin can be seen in Merkel carcinoma cells,especially in sun-exposed areas[111].A sun-activated maspininduced DNA damage was hypothesized[111].
In melanoma cases,a significant association of nuclear maspin staining with aggressive tumor behavior and shorter disease-free survival was shown,while cytoplasmic predominance was present in superficial spreading melanoma[111,112].High maspin intensity in the invasive margins of primary melanomas was correlated with an unfavorable prognosis[113].
Soft tissues and joints:Although it can act as a proangiogenic marker,maspin expression is negative in soft tissue structures and does not mediate osteoarthritis[114].For malignant soft tissue tumors,cytoplasmic expression of maspin was correlated with higher histological grade and risk for distant metastasis[115].In liposarcomas,maspin and VEGF-A seem to be angiogenic promoters[116].Negative staining was observed for most soft tissue tumors such as granular cell (Abrikossoff) tumor[117],but also for other mesenchymal tumors such as gastrointestinal stromal tumors[118].
CONCLUSION
Although several studies tried to elucidate parts of the molecular journey in which maspin influences the transformation of epithelial cells and tumor behavior,the maspin-related processes are yet to be elucidated.Experimental studies are needed before chemical synthesis of a maspin-based agent can begin.Despite several unknown areas of the effects of maspin,several aspects have been confirmed by us and others.These aspects include:maspin is a good marker of budding quantification in colorectal carcinomas; it can be used for identification of intragastric mucosa signet ring cells (in biopsic specimens) or a proper evaluation of poorly cohesive gastric carcinoma invasion; and it is a useful marker for differential diagnosis of PanIN from a ductal adenocarcinoma of pancreas.The other aspects should be elucidated by further studies.
 World Journal of Meta-Analysis2019年4期
World Journal of Meta-Analysis2019年4期
- World Journal of Meta-Analysis的其它文章
- Drug interactions of dipeptidyl peptidase 4 inhibitors involving CYP enzymes and P-gp efflux pump
- Anti-inflammatory properties of antidiabetic agents
- Effectiveness of taxanes over anthracyclines in neoadjuvant setting:A systematic-review and meta-analysis
- Safety and efficacy of percutaneous transhepatic balloon dilation in removing common bile duct stones:A systematic review
- Endoscopic management of biliary strictures post-liver transplantation
