Metastatic patterns and surgical methods for lymph nodes No. 5 and No. 6 in proximal gastric cancer
Jinou Wang, Pei Wu, Zhenning Wang, Kai Li, Baojun Huang, Pengliang Wang, Huimian Xu, Zhi Zhu
1Department of Pathology, Shengjing Hospital of China Medical University, Shenyang 110004, China; 2Department of Surgical Oncology, the First Hospital of China Medical University, Shenyang 110001, China
Correspondence to: Zhi Zhu, MD, PhD. Department of Surgical Oncology, the First Hospital of China Medical University, No. 155 North Nanjing Street, Shenyang 110001, China. Email: zhuzhi@yeah.net; Huimian Xu, MD, PhD. Department of Surgical Oncology, the First Hospital of China Medical University, No. 155 North Nanjing Street, Shenyang 110001, China. Email: xuhuimian@126.com.
Abstract Objective: The current surgical treatment guidelines for early proximal gastric cancer (PGC) still lack agreement. Lymphadenectomy of lymph nodes No. 5 and No. 6 is the major difference between total and proximal gastrectomy. We elucidated the appropriate surgical procedure for PGC by investigating the pathological characteristics and prognostic significance of lymph nodes No. 5 and No. 6.Methods: In total, 333 PGC patients who underwent total gastrectomy were enrolled in this study. We investigated their clinicopathological characteristics and the metastatic patterns of the lymph nodes. Patients with metastasis in lymph nodes No. 5 and No. 6 were combined into one group and we compared the difference in survival between those with and without metastasis in lymph nodes No. 5, 6 (lymph nodes No. 5 and No. 6 in any group of metastasis) for different subgroups.Results: The metastatic rates for lymph nodes No. 5 and No. 6 in PGC were 9.91% and 16.11%, respectively.The metastatic rate for both lymph nodes No. 5, 6 was 20.42%. Multivariate analysis showed that positive metastasis in lymph node No. 4, depth of invasion, and tumor size were independently correlated with the presence of metastasis in lymph nodes No. 5, 6.Conclusions: When lymph node No. 4 is positive (intraoperative pathology) or tumor size ≥5 cm or T4 stage,lymphadenectomy should be performed for lymph nodes No. 5 and No. 6, and total gastrectomy is recommended.
Keywords: Gastrectomy; lymph nodes No. 5 and No. 6; metastasis; prognosis; proximal gastric cancer
Introduction
Gastric cancer is a common digestive system malignant tumor throughout the world, and it had the fifth highest incidence and the third mortality rate after lung cancer(1,2). Proximal gastric cancer (PGC) occurs in the upper third of the stomach and it accounts for about 23% of cases of gastric cancer, which is the second highest after distal gastric cancer. PGC is characterized by a large tumor size,strong invasive ability, high incidence of lymph node metastasis, and a poor prognosis (3). The incidence of PGC has increased significantly in China in recent years (4).
Radical surgery is still the most effective cure for PGC and the Japanese Gastric Carcinoma Association (JGCA)guidelines (14th edition) suggest that patients with PGC should accept D2 lymphadenectomy radical surgery, but the lymphadenectomy ranges are not the same because the surgical approach differs, and the choice of total gastrectomy or proximal gastrectomy is still controversial(5). D2 total gastrectomy includes lymph nodes No. 1-7,8a, 9, 10, 11p and 12a, and thoracic surgeons advocate D1 +proximal gastrectomy, including lymph nodes No. 1, 2, 3a,4sa, 4sb, 7, 8a, 9 and 11p. Lymph nodes No. 5 and No. 6 are defined as the third metastatic region, which indicates a low overall survival (OS) rate and poor prognosis (6).However, few studies have focused on the clinicopathological features and metastatic patterns of lymph nodes No. 5 and No. 6. Metastasis is frequently detected in lymph nodes No. 5 and No. 6 in advanced cancer patients,and some clinicopathological factors related to metastasis in lymph nodes No. 5 and No. 6 could be used as predictive factors.
Total gastrectomy with D2 lymphadenectomy is indicated in patients with advanced PGC as the standard treatment in the JGCA guidelines (14th edition). Some reports suggest that proximal gastrectomy can be performed radically and safely for early PGC, with survival rates equivalent to those of total gastrectomy while also preserving the physiological functions of the gastric remnant (7,8). Lymphadenectomy of lymph nodes No. 5 and No. 6 plays a key role in the decision regarding surgical treatment.
According to these considerations, we analyzed the pathological characteristics and prognostic significance of metastasis in lymph nodes No. 5 and No. 6 for PGC patients.
Materials and methods
Patients
In total, we enrolled 649 patients with PGC who underwent D2 total gastrectomy between January 1980 and December 2012 (follow-up to November 2015) at the Department of Surgical Oncology, the First Affiliated Hospital of China Medical University. The enrolled patients comprised 258 males and 75 females who were aged from 26 to 83 years, with a median age of 57 years.Lymph nodes were divided into groups for pathological examination, and the site and number of metastatic lymph nodes was recorded after operation in the pathology report.All the patients underwent a standard follow-up procedure by means of outpatient clinic consultation,and/or communication with patients through telephone or letter, once every 3 months for the first 3 years and then every 6 months thereafter. Follow-up of the entire study population was conducted until death or the cut-off date(November 30, 2015). Of these, four died in the postoperative period, 25 with non-R0 resection were excluded, and none received neoadjuvant therapy. At the time of the last follow-up, six were lost. These patients were also excluded in this study.
Surgical approach
D2 total gastrectomy includes lymphadenectomy of lymph nodes No. 1-7, 8a, 9, 10, 11p, and 12a, with Roux-en-Y reconstruction according to the gastric cancer protocol revised by the JGCA in 2013 (14th edition).Clinicopathological data were classified according to the 8th edition of the American Joint Committee on Cancer TNM staging system (2015).
The study was approved by the Research Ethics Committee of China Medical University (Shenyang,China). Written informed consent was obtained from all patients.
Evaluation of clinical parameters
We analyzed the risk factors for metastasis in lymph nodes No. 5 and No. 6 in PGC using univariate and multivariate analyses. OS was compared in the patients with and without metastasis in lymph nodes No. 5 and No. 6.Tumor size and T stage subgroups were analyzed separately.
The specificity, sensitivity, accuracy, positive predictive rate and negative predictive rate for metastasis in lymph node No. 4 when predicting metastasis in lymph nodes No.5, 6 were analyzed by χ2analysis.
Statistical analysis
OS was determined by Kaplan-Meier survival analysis.Statistical differences in the survival curves were estimated using the log-rank test. Relevant factors that affected the prognosis were analyzed with the log-rank test. Two-tailed χ2-tests were used for statistical comparisons in the univariate analysis. Logistic regression analysis was performed to identify independent factors correlated with metastasis in lymph nodes No. 5 and No. 6 by multivariate analysis. P<0.05 was considered statistically significant.
All of the statistical analysis and graphical plots were performed using IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA).
Results
Patient characteristics
This study included 333 PGC patients who underwent total gastrectomy, where 33 (9.91%) patients were positive in No. 5 lymph nodes, 51 (16.11%) patients were positive in No. 6 lymph nodes, with an overlap for 16 patients who had metastasis in both lymph nodes No. 5 and No. 6, and 68 (20.42%) patients were positive in lymph nodes No. 5, 6(lymph nodes No. 5 and No. 6 in any group of metastasis).Our data showed that the clinicopathological factors comprising tumor size and depth of invasion were risk factors for metastasis in lymph nodes No. 5 and No. 6 according to univariate analysis (Table 1).
Lymph node metastatic discipline
The rates of metastasis in the lymph node groups in advanced PGC ranged from high to low in the lymph nodeNo. 3, No. 1, No. 2, No. 7, No. 4, No. 8a, No. 10, No. 11,No. 6, and No. 5 groups. Univariate analysis and further multivariate analysis showed that No. 4, No. 8a and No. 11 was an independent predict factor for No. 5, 6 lymph nodes as shown in Table 2. Logistic regression analysis showed that lymph node No. 4, tumor size and T stage were independent risk factors for metastasis in lymph nodes No.5, 6 (Table 3).
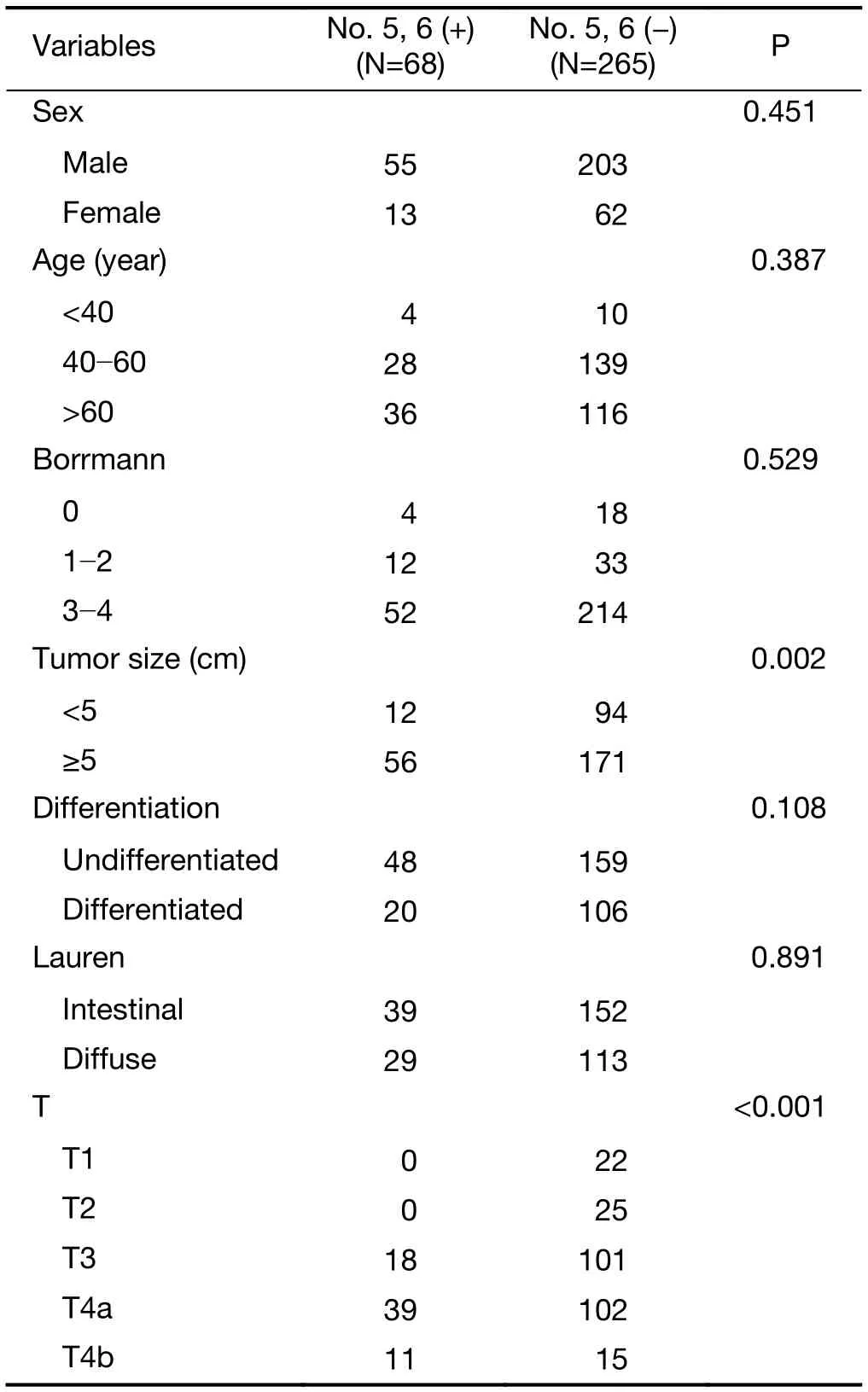
Table 1 Clinicopathological characteristics of PCG patients who underwent D2 total gastrectomy according to No. 5, 6 lymph nodes status
Survival analysis
PGC patients who underwent total gastrectomy had a long-term survival rate of 42% and a median survival time of 42 months.
Patients with metastasis in lymph node No. 4 as well as metastasis in lymph nodes No. 5, 6 had an the 5-year OS of 31.7%, which was lower than that in the patients without metastasis in lymph nodes No. 5, 6 (36.7%). Though the difference between the two groups was not statistically significant (P=0.192). When lymph node No. 4 was negative, there was no significant difference between the patients with and without metastasis in lymph nodes No. 5,6. The survival curves are shown in Figure 1.
Subgroup analysis of tumor size identified a significant difference in survival between patients with and without metastasis in lymph nodes No. 5, 6 when the tumor size was ≥5 cm (43.7% vs. 57.6%, P=0.041), but not in those with a tumor size <5 cm (58.3% vs. 59.8%, P=0.867)(Figure 2).
In the T1-T3 stage, there was no significant difference between the 5-year survival rate of patients with and without metastasis in lymph nodes No. 5, 6 (48.7% vs.49.3%, P=0.755). In the T4 stage, the OS of patients without positive results for lymph nodes No. 5,6 was 35.7%, which was significantly higher than that for those with negative results for lymph nodes No. 5,6 (20.4%,P=0.036) (Figure 3).
Metastasis in lymph node No. 4 was a better significant predictor based on intraoperative pathological examination,with a sensitivity of 81.25% and specificity of 85.44%,compared with that of tumor size and T stage (Table 4).
Discussion
PGC is characterized by a large tumor size, deep invasion,high malignancy, and low differentiation. The prognosis is worse than that for other forms of gastric cancer. The current guidelines for PGC comprise lymphadenectomy of the gastric cancer located in the upper third of the stomach,but the recommendations for the distal second station lymph nodes No. 5 and No. 6 are not clearly stated. Thus,there is some controversy regarding the surgical procedures for proximal gastrectomy or total gastrectomy for PGC (9-11). We combined metastasis in lymph nodes No. 5 and No. 6 in the analysis performed in this study.

Table 2 Univariate and multivariate analyses of independent factors correlated with positive No. 5, 6 lymph node metastasis
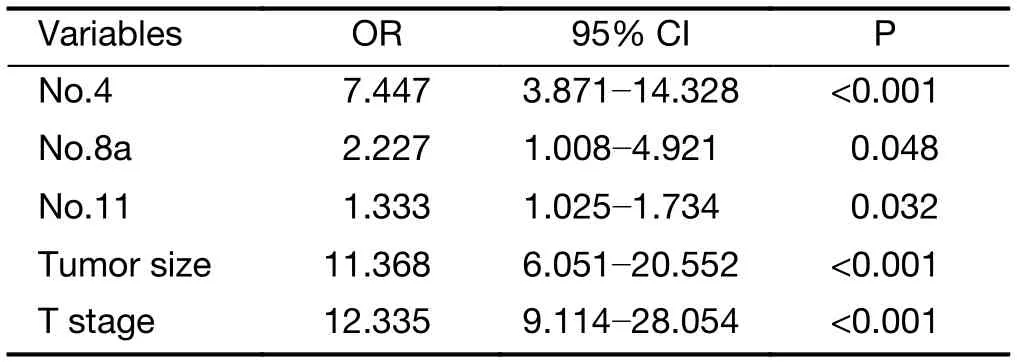
Table 3 Multivariate analysis of factors predicting No. 5, 6 lymph node metastasis
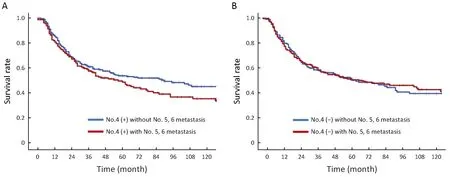
Figure 1 Comparison of survival curves between gastric cancer patients who underwent total gastrectomy with and without No. 5, 6 lymph node metastasis when No. 4 was positive (P=0.192) (A) and negative (P=0.881) (B).
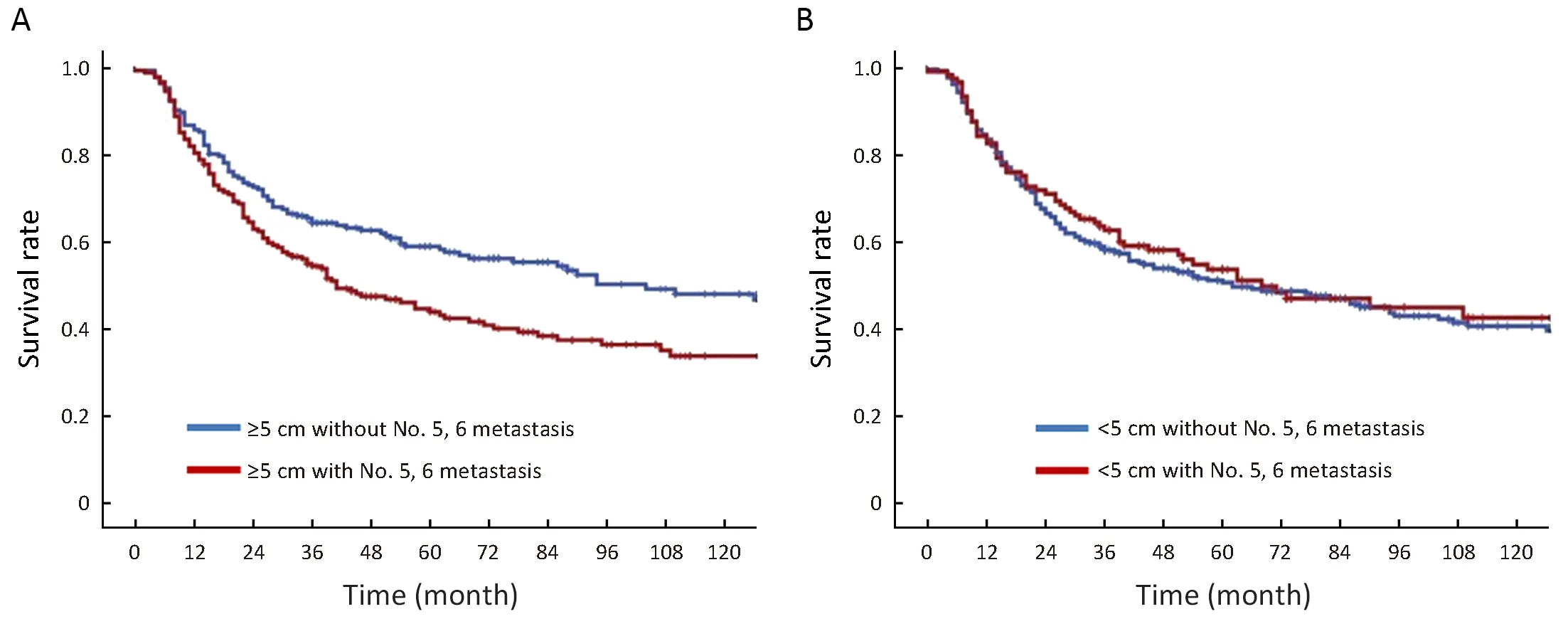
Figure 2 Survival analysis of tumor size identified a significant difference in survival between patients with and without metastasis in lymph nodes No. 5, 6 when tumor size was ≥5 cm (P=0.041) (A) and tumor size <5 cm (P=0.867) (B).
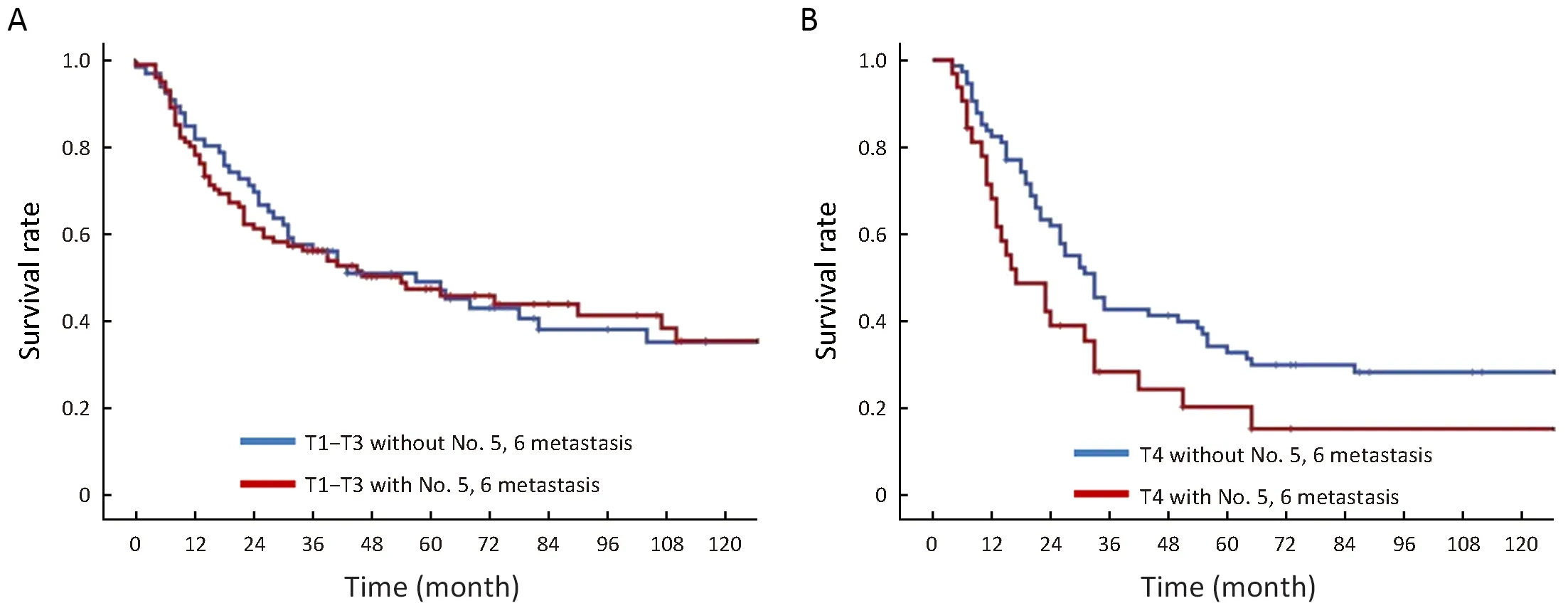
Figure 3 Comparison of survival curves between gastric cancer patients in T1-T3 stage (A) and T4 stage (B) with and without No. 5, 6 lymph node metastasis.
Previous studies determined the metastatic rate for distal lymph nodes in advanced PGC as 18.7% (12). In the present study, we found that the metastatic rates in lymph nodes No. 5 and No. 6 for PGC patients who underwent total gastrectomy were 9.91% and 16.11%, respectively.The number of patients who were positive for lymph nodes No. 5 and No. 6 was 68, with a metastasis rate of 20.42%.This result is similar to those reported in previous studies,where the metastasis rate in lymph node No. 5 was 9.14%and that in lymph node No. 6 was 10.06% (13).
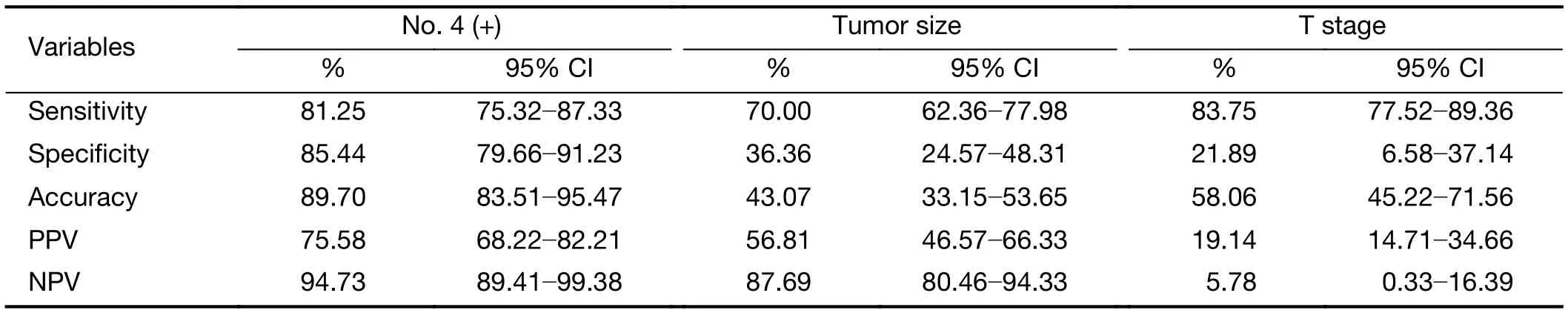
Table 4 Efficacy of No. 4 lymph node compared with tumor size and T stage in predicting No. 5, 6 lymph node metastasis
Some previous studies have suggested that total gastrectomy should be performed in advanced proximal gastric carcinoma (14). We found that positive metastasis in lymph node No. 4, a tumor size ≥5 cm, and pT4 stage were independent risk factors for metastasis in lymph nodes No.5 and No. 6. When lymph node No. 4 was positive, the positive rate was as high as 42.3% in lymph nodes No. 5 and No. 6. Therefore, if lymph node No. 4 is found to be enlarged during an operation, we suggest that intraoperative pathological examination should be confirmed and total gastrectomy is recommended with a positive result.
Many methods have been proposed for the preoperative prediction of lymph node metastases (15,16). However,clinicopathological characteristics such as the macroscopic type, size of tumor, or depth of invasion have mostly been used in these systems without considering the lymphatic flow and pattern of lymph node metastasis (17,18). No. 4 located at upstream of lymph node No. 10 and was proved to be an indicator for No. 10 (19). Similarly, we found that the metastatic status of lymph node No. 4 could be used as a predictor of metastasis in lymph nodes No. 5 and No. 6.We showed that the metastatic status of lymph node No. 4 has a significant advantage for making predictions of metastasis in lymph nodes No. 5,6 with a sensitivity of 81.25% and specificity of 85.44%.
Some studies have shown that tumors with a diameter greater than 3 cm were associated with a lymph node metastatic rate of 19.2% (13,20), thereby suggesting that lymph node metastasis is associated with tumor size. For PGC patients with a tumor size ≥5 cm, we found that the metastatic rate was as high as 24.7% in lymph nodes No. 5,6. These results suggest that a tumor diameter greater than 5 cm or deep infiltration of the PGC may indicate metastasis distal to lymph nodes No. 5, 6. When the tumor size was less than 5 cm, the positive rate was only 11.3% in lymph nodes No. 5, 6. For patients with gastric cancers at pT1-T2, no cases were observed with metastasis in lymph nodes No. 5, 6. For patients at pT1-T3, the positive rate for lymph nodes No. 5, 6 was 15.1%, and that at pT4 was 29.9%.
Conclusions
The results obtained in this study indicated that the incidence of metastasis in lymph nodes No. 5, 6 increased with metastasis in lymph node No. 4, tumor size, and pT stage, and most importantly, patients who were positive for metastasis in lymph node No. 4 had a high risk of distant lymph node metastasis. These associations with lymph node metastasis may help surgeons to determine the most appropriate surgical approach and strategy for patients with PGC in different stages.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.
2.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1-10.
3.Wang S, Freedman ND, Loftfield E, et al. Alcohol consumption and risk of gastric cardia adenocarcinoma and gastric non-cardia adenocarcinoma: A
References16-year prospective analysis from the NIH-AARP diet and health cohort. Int J Cancer 2018;143:2749-57.
4.Park JC, Lee YC, Kim JH, et al. Clinicopathological features and prognostic factors of proximal gastric carcinoma in a population with high Helicobacter pylori prevalence: a single-center, large-volume study in Korea. Ann Surg Oncol 2010;17:829-37.
5.Kunisaki C, Shimada H, Nomura M, et al. Surgical outcome in patients with gastric adenocarcinoma in the upper third of the stomach. Surgery 2005;137:165-71.
6.Yang K, Zhang WH, Chen XZ, et al. Comparison of modified D2 lymphadenectomy versus standard D2 lymphadenectomy in total gastrectomy for gastric cancer patients with lymph nodes involvement.Surgery 2015;158:1446-7.
7.Galizia G, Lieto E, De Vita F, et al. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery 2015;157:285-96.
8.Wang W, Zheng C, Fang C, et al. Time trends of clinicopathologic features and surgical treatment for gastric cancer: Results from 2 high-volume institutions in southern China. Surgery 2015;158:1590-7.
9.An JY, Youn HG, Choi MG, et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 2008;196:587-91.
10.Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg 2017;265:277-83.
11.Rosa F, Quero G, Fiorillo C, et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG).Gastric Cancer 2018;21:845-52.
12.Kitamura K, Nishida S, Yamamoto K, et al. Lymph node metastasis in gastric cancer in the upper third of the stomach - surgical treatment on the basis of the anatomical distribution of positive node. Hepatogastroenterology 1998;45:281-5.
13.Song W, Liu Y, Ye J, et al. Proximal gastric cancer:lymph node metastatic patterns according to different T stages dictate surgical approach. Chin Med J (Engl)2014;127:4049-54.
14.Ooki A, Yamashita K, Kikuchi S, et al. Clinical significance of total gastrectomy for proximal gastric cancer. Anticancer Res 2008;28:2875-83.
15.Choi JH, Suh YS, Park SH, et al. Risk factors of microscopic invasion in early gastric cancer. J Gastric Cancer 2017;17:331-41.
16.Pang W, Lou N, Jin C, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer.Eur J Gastroenterol Hepatol 2016;28:493-502.
17.Lee SL, Lee HH, Ku YM, et al. Usefulness of twodimensional values measured using preoperative multidetector computed tomography in predicting lymph node metastasis of gastric cancer. Ann Surg Oncol 2015;22 Suppl 3:S786-93.
18.Wu XJ, Miao RL, Li ZY, et al. Prognostic value of metastatic lymph node ratio as an additional tool to the TNM stage system in gastric cancer. Eur J Surg Oncol 2015;41:927-33.
19.Son T, Kwon IG, Lee JH, et al. Impact of splenic hilar lymph node metastasis on prognosis in patients with advanced gastric cancer. Oncotarget 2017;8:84515-28.
20.Li H, Huo ZB, Kong FT, et al. Predictive factors for lymph node metastasis and defining a subgroup treatable for laparoscopic lymph node dissection after endoscopic submucosal dissection in poorly differentiated early gastric cancer. World J Gastrointest Oncol 2018;10:360-6.
 Chinese Journal of Cancer Research2019年1期
Chinese Journal of Cancer Research2019年1期
- Chinese Journal of Cancer Research的其它文章
- STIM1 expression is associated with osteosarcoma cell survival
- Prognostic implications of epidermal growth factor receptor variant III expression and nuclear translocation in Chinese human gliomas
- Risk-stratification model to select conversion surgery for advanced gastric cancer patients
- Prognostic value of 18F-fluorodeoxyglucose positron emission tomography using Deauville criteria in diffuse large B cell lymphoma treated with autologous hematopoietic stem cell transplantation
- Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1,085 WHO classified cases in a single institution in China
- Incidence and mortality of thyroid cancer in China, 2008-2012
