Effect of silicone oil on the microstructure,gelation and rheological properties of sorbitan monostearate–sesame oil oleogels
Mya Thet Htar Swe,Panida Asavapichayont,?
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,6 Rajamankha Nai Rd.,Amphoe Muang,Nakhon Pathom 73000,Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000,Thailand
Keywords:Oleogel Microstructure Rheology Sesame oil Silicone oil Sorbitan monostearate?Corresponding author.Department of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,6 Rajamankha Nai Rd.,Amphoe Muang,Nakhon Pathom 73000,Thailand.
A B S T R A C T Oleogelscontain oil or a non-polar liquid which is gelled with an agentcalled an organogelator.The aim of this study was to evaluate the effects of the addition of silicone oil(cyclopentasiloxane)to the gelation process and to the properties of sorbitan monostearate(SMS)–sesame oil oleogel and compared with that of SMS–sesame oil oleogel and SMS–cyclopentasiloxaneoleogel.Threedifferentoilphases;sesameoilphase,cyclopentasiloxane phase and a mixture of cyclopentasiloxane and sesame oil,were used to prepare oleogels with SMS gelator.The critical gelling concentrations(CGC)for oleogels were determined using different concentration of SMS in a range of 5%–22%(w/w).The characterization of the developedoleogelswasdoneusingFouriertransforminfraredspectroscopy(FTIR),polarized light microscope,rheometer,X-ray diffraction(XRD)and differential scanning calorimetry(DSC).The addition of cyclopentasiloxane reduced the CGC of SMS–sesame oil oleogel from 20%to 10%(w/w).In microscopic characterization,the oleogels with a mixture of oil phases showed the longer and thicker three-dimensional gel network than that of oleogels with sesame oil and cyclopentasiloxane.FTIR studies demonstrated that this network formation was mainly due to hydrogen bonding.Rheological measurements revealed that the combination of cyclopentasiloxane and sesame oil produced strong gel with higher complex modulus values and longer linear viscoelastic region than oleogels prepared with sesame oil and cyclopentasiloxane.In addition,oleogels with the combination of the two oils had higher enthalpy(ΔHm)and entropy(ΔSm)thus could increase thermodynamic stability of the oleogels.Therefore,the addition of cyclopentasiloxane can improve the physical,ther-mal properties and stability of SMS–sesame oil oleogel,provide greater sensory profile and better product aesthetics.The developed oleogel can be a novel carrier for topical drug delivery.
1. Introduction
Oleogel is a promising base for numerous applications such as pharmaceutical,food and cosmetic preparations[1].It is gaining extensive interest in pharmaceutical industry.Oleogel base is a good candidate for dermal pharmaceutical formulations due to the lipophilic property leading to enhancing the penetration of drugs,particularly lipophilic drugs,via the stratum corneum[2].Moreover,edible oleogels are used to encapsulate and deliver drugs or nutraceuticals because their lipid medium increases the bioavailability of lipid soluble drugs and gel matrix offers sustained or controlled release of the drug molecules[3].Oleogels or organogels consist of gelator and oil or organic liquid which is physically immobilized within the network of self-assembled aggregates of gelator molecules.They are formed by dissolving gelators in hot solvents forming solutions/dispersions.During the cooling stage,the af fi nity between gelators and solvents reduced which results in self-assembly of gelator molecules,leading to wellde fi ned aggregates such as tubules,rods and fibers.Entanglements and connections of aggregates attribute to the formation of three-dimensional network and gelation[4,5].The extent and properties of self-assembled aggregates are dependent on various forces such as hydrogen bonding,dipole–dipole interactions,π-stacking,electrostatic interactions and London dispersion forces[5].The solubility of the gelator depends on types of the liquid phase or solvent.The change in polarity of solvents strongly affects the critical gelling concentration(CGC)and gelation process.The liquid phase influences the microscopic and macroscopic properties of the gels.The optimum solubility/insolubility balance of the gelator is required to obtain the dissolved or dispersed gelator at high temperature and crystallized or self-assembled aggregates at cooling stage[1,4,6].Various groups of solvents for oleogel preparations are synthetic oils(mineral oil),long chain synthetic esters(isopropyl myristate,ethyl oleate),vegetable oils(sesame oil,olive oil,soybean oil)and organic solvents.The animal and vegetable oils are commonly used solvents for oleogel preparations.The used oils were fish oil[7],soy-bean oil[8],sesame oil[9,10],sun flower oil[6,11–13],olive oil[6,13,14],hazelnut oil[15],and castor oil[6,13,16].Among these natural oils,sesame oil has been extensively used in pharmaceutical and cosmetic applications as a result of antioxidant,antiseptics,anti-in fl ammatory and antibacterial properties[17,18].It contains long-chain fatty acids which contribute to the high viscosity of the oil.In addition,the major components of sesame oil(sesamin,sesamol,sesamolin and phytosterols)provide a remarkable stability of the preparations[10,18].However,oleogels have the major drawback of greasy and unpleasant feature due to the oily nature of natural oils[2].The attempt to reduce the drawback has been initiated in this study.The combination of synthetic and vegetable oil for oleogel preparations has been applied and never been explored.The synthetic oil,volatile silicone oil(cyclopentasiloxane),offers complementary bene fi ts in topical preparations[19].It is widely used for topical pharmaceutical products because it is compatible with a broad range of lipophilic products.The low surface tension properties of cyclopentasiloxane could improve the skin coverage and increase the bioavailability of active ingredients[20–23].The combination of vegetable oil and cyclopentasiloxane can provide better moisturizing and occlusive properties,improving spreadability and aesthetic properties,and reducing stickiness of the formulations[19].Girboux and Courbon reported that the addition of 10%cyclopentasiloxane in borage oil greatly reduced the surface tension and viscosity,and improved sensory attributes[24].In addition,the strong siloxane bond contributed to the high degree of thermal and oxidative stability of cyclopentasiloxane enabling to the stable formulations[25].The stability of vegetable oils can also be improved by reducing the unsaturation with less unsaturated oils.The addition of 1mg/kg of methyl silicone in sunfl ower oil showed significant effect on preventing oxidation by lowering the degree of foaming and reduction in oxidation products[26].
The gelators or organogelators used were monoacylglycerols,diacylglycerols,triacylglycerols[4],fatty acids,fatty alcohols[27],waxes,wax esters[15,28]and sorbitan monostearate[4,10,14,29].Sorbitan monostearate(SMS),FDA approved non-ionic surfactant,is a commonly used gelator for oleogel preparations.It is biocompatible,non-irritant and has the ability to act as a structuring agent for developing formulations of cosmetics,pharmaceuticals and food applications.SMS can form gel with a variety of solvents such as cyclohexane,octane,isopropyl myristate,ethyl oleate,sesame oil and olive oil.The gel structures of SMS based formulations are formed by solid fiber mechanisms and they are thermodynamically stable for a long time[4,10,29].Therefore,the objective of this study was to develop the topical SMS–sesame oil oleogel formulation with improved aesthetic and sensory profiles by the addition of cyclopentasiloxane.The physicochemical properties such as microstructural characteristics,crystalline behaviors,thermal and rheological properties of the developed oleogel were evaluated.The comparison was made between the preparations with and without cyclopentasiloxane.
2. Materials and methods
2.1. Materials
Cyclopentasiloxane(TSF 405?)was obtained from Momentive Performance Materials(Thailand).Sorbitan monostearate(span 60,Nonion SPA 60?)was from NOF Corporation,Japan.Sesame oil,refined was obtained from Henry Lamotte Oils,GMBH.Other vegetable oils(sweet almond oil,castor oil,saffl ower oil,olive oil and sun flower oil)were purchased from P.C.Drug Center Co.,Ltd.,Thailand.
2.2. Preliminary study to choose the best miscible oil pair
Castor oil,olive oil,saf flower oil,sesame oil,sweet almond oil and sun flower oil were studied for their miscibility with cyclopentasiloxane.Anequalvolume(2.5ml)ofcyclopentasiloxane and each vegetable oil were mixed in a screw-cap glass vial.The mixture was stirred vigorously for 5 minutes using a magnetic stirrer(Schott,CAT)at 1500rpm at 25°C.Phase separation of the oil pairs was observed visually after 24h.The pair of sesame oil and cyclopentasiloxane was chosen for further determination of miscibility and CGC using 5 different ratios of sesame oil and cyclopentasiloxane;1:4,2:3,1:1,3:2,and 4:1.The CCG is the minimum gelator concentration required to form gel and started failing to flow when the tubes were inverted after keeping at 25°C for 24h.In CGC testing,the range ofconcentrationofSMS(5%–22%,w/w)wasusedtoinvestigate with five different ratios of sesame oil and cyclopentasiloxane.The microscopical characteristics of oleogels formed by CGC of SMS with five different ratios of sesame oil and cyclopentasiloxane were examined.The best ratio of sesame oil and cyclopentasiloxane which produce oleogels with stronger network formation was selected for preparation of oleogel.The physicochemical properties of the developed oleogel were comparedwiththatofsesameoiloleogelandcyclopentasiloxane oleogel.
2.3. Viscosity of the oil components
Viscosity of sesame oil,cyclopentasiloxane and their combination were measured using a rheometer(Kinexus Pro,Malvern Instruments,UK).Cup and bob(C25SW 1130 ss)geometry was selected and 25ml of samples were sheared at the shear rate range from 0.1 to 100s-1at temperature of 25±0.1°C;controlled by a Peltier cylinder cartridge.All measurements were done in triplicate.Mean and standard deviation(SD)were used to analyze the data.
2.4. Determination of CGC for oleogels
CGC of SMS was determined by heating the mixture of oil phase(sesame oil,cyclopentasiloxane or a mixture of cyclopentasiloxane and sesame oil)and SMS gelator(5%–22%,w/w)at 55°C in glass tubes with screw caps.The formulations were considered as oleogels,if they failed to flow when the tubes were inverted.The CGC of SMS that induces gel formation were used for preparation of oleogels.
2.5. Preparation of oleogels
Table 1 shows the composition of oleogels.The formulations consisted of SMS,sesame oil and cyclopentasiloxane.Oleogels containing sesame oil was designated as OG1,cyclopentasiloxane was coded as OG2 and sesame oil and cyclopentasiloxane combination was designated as OG3.The concentrations of SMS gelator for OG1,OG2 and OG3 were selected based on the CGC results.Oleogels were prepared by dissolving CGC of SMS in three different oil phases(sesame oil,cyclopentasiloxane or a mixture of cyclopentasiloxane and sesame oil).The mixture of gelator and oil was heated in a temperature controlled water bath at 55°C and stirred at 300rpm until a clear homogenous solution was obtained.Afterwards,the solution was allowed to cool to room temperature to induce gel formation.The samples were stored at 25±1°C for further characterization.The physical appearances of the obtaining oleogels were observed visually and their physicochemical properties(FTIR,microstructure,rheology,X-ray diffraction and DSC)were investigated.
2.6. FTIR analysis
FTIR spectra of the ingredients and oleogels were analysed using a Fourier transform infrared spectrometer(Nicolet 4700,Thermo Electron Corporation,USA).Solid samples were recorded as an intimate mixture with powdered KBr.Oil and gel samples were operated in attenuated total reflectance(ATR)mode.The scanning range was 4000–600cm-1and spectral manipulations of the samples were performed using Omnic software(version 8,Thermo Electron Corporation,USA).
2.7. Microstructure studies
The microstructure of the oleogels was observed under polarized light using a CX-31 optical microscope(Olympus Optical Co.,Ltd.,Japan)equipped with a Canon EOS(Rebel T5i)700D camera.Thin smears of oleogels were prepared on glass slides and visualized under 40×magnification.
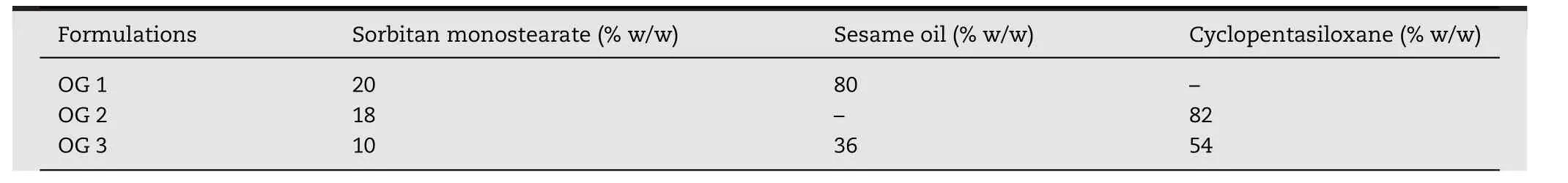
Table 1–Composition of oleogels.
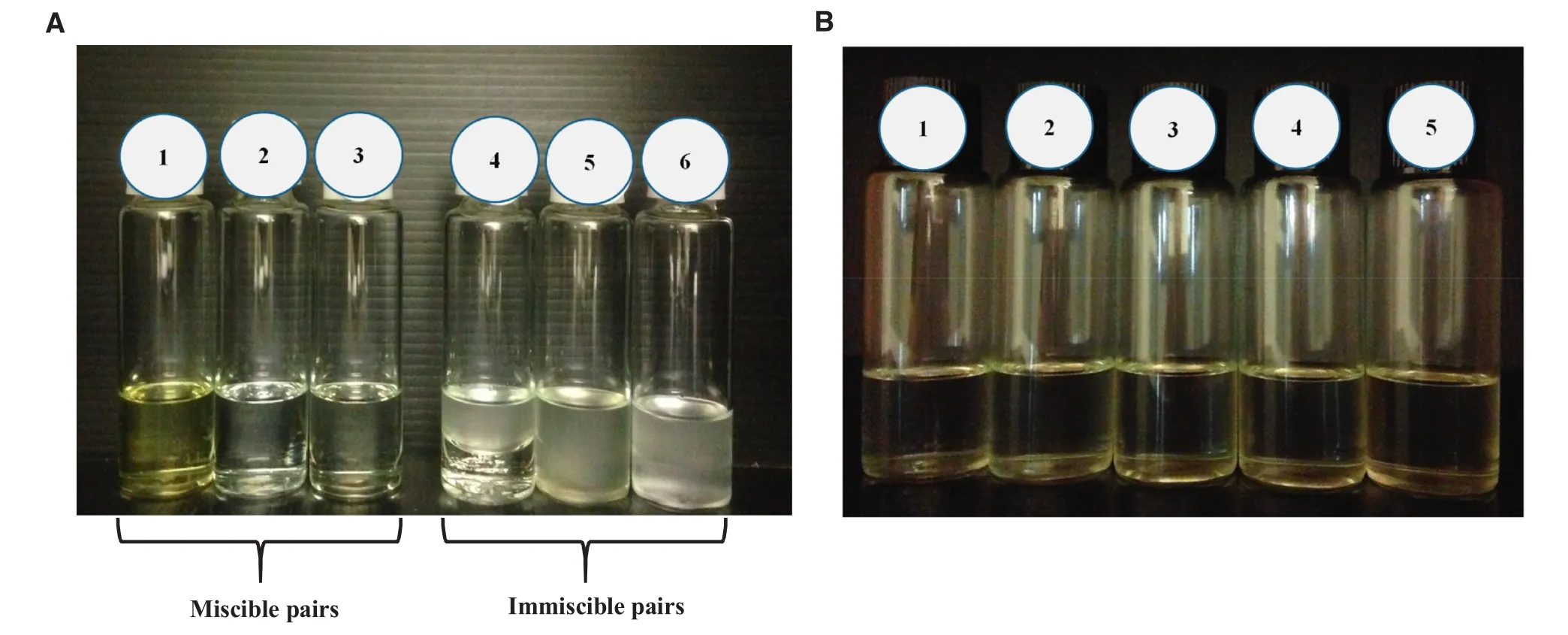
Fig.1–(A)Miscible and immiscible oil pairs of cyclopentasiloxane and(1)olive oil,(2)saf flower oil,(3)sesame oil,(4)castor oil,(5)sweet almond oil,and(6)sun flower oil and(B)miscibility study of sesame oil with cyclopentasiloxane at various ratios(1)1:4,(2)2:3,(3)1:1,(4)3:2,and(5)4:1 after keeping at 25°C for 24h.
2.8. Rheological characterization
Rheological properties of oleogels were characterized using a rheometer(Kinexus Pro,Malvern Instruments,UK)with Peltier plate and active hood cartridges to control temperature.All rheological measurements were performed at 25°C using a 40mm diameter parallel plate geometry with gap value of 1mm.The viscoelastic properties of the oleogel samples were investigated with oscillation tests.First,the amplitude sweep was run by increasing stress from 0.001 to 10Pa at a fixed frequency of 1Hz to determine the linear viscoelastic region(LVER)of the samples.Then a strain of 0.05%(within the LVER)was selected to run a frequency sweep from 0.1 to 10Hz.The flow properties were characterized using the shear rate range of 0.1–100s-1.Three step shear rate sweep(up-down-up)was used to evaluate the thixotropic behaviors of the samples during application on the skin.All measurements were performed in duplicate.
2.9. X-ray diffraction studies
The crystalline behaviors of oleogels and their ingredients were evaluated using an X-ray diffractometer(MiniFlex II;Rigaku,Tokyo,Japan)at room temperature using monochromatic CuKαradiation at 15mA and 30kV over the range of 2θ angles from 5°to 40°with the scanning speed of 2°/min.Data analysis were carried out using free trial version of Origin Pro 8 software.
2.10. Differential scanning calorimetry(DSC)
Thermal properties of the ingredients and oleogels were examined using a DSC(Pyris Sapphire DSC,Perkin-Elmer,USA).About 15mg of samples were weighed and hermetically sealed in aluminum pans.The melting and crystallization events of samples were recorded in the temperature range of 25–80 °C with a thermal scanning rate of 5°C/min.Thermal characteristics such as onset and peak maximum temperature,heat of melting(ΔHm)and heat of crystallization(ΔHc)were determined using Pyris manager software.The measurements were performed in triplicate and mean and standard deviation(SD)were used to analyze the data.
2.11. Statistical analysis
The resulting data from viscosity of oils and thermal analysis of oleogels were compared by one-way ANOVA,Tukey’s test using SPSS software version 16.0.The level of significance was considered asP<0.05.
3. Results and discussion
3.1. Preliminary study to choose the best miscible oil pair
The result of miscible and immiscible oil pairs was shown in Fig.1A.Among the 6 types of oils;castor,sun flower,and sweet almond oils were completely immiscible with cyclopentasiloxane and showed phase separation.However,olive,saf flower,and sesame oils were fully miscible with cyclopentasiloxane.From these 3 miscible pairs,sesame oil was selected to combine with cyclopentasiloxane in oleogel preparations because it has the highest antioxidant content among the 3 vegetable oils.This leads to better stability of the final oleogels.In addition,sesame oil has a wide range of applications in pharmaceutical and cosmetic fields which are the intended applications[10,18].Afterwards,the miscibility of sesame oil and cyclopentasiloxane at different ratios was investigated.The miscibility of the two oils was observed in all 5 ratios and the result was shown in Fig.1B.Fig.2 displayed the CGC determination results of these 5 ratios.The CGC values were 10,10,14,16 and 20%(w/w)at the ratios of 1:4,2:3,1:1,3:2 and 4:1 respectively.The lower CGC value of 10%(w/w)for SMS was obtained at the lower ratios of sesame oil to cyclopentasiloxane(1:4 and 2:3).This might be due to the lower solubility of SMS at the lower amount of sesame oil.The ratio of 2:3 was chosen for the preparation of oleogels because the microstructure shown in Fig.3 revealed the formation of stronger gel network at this ratio.
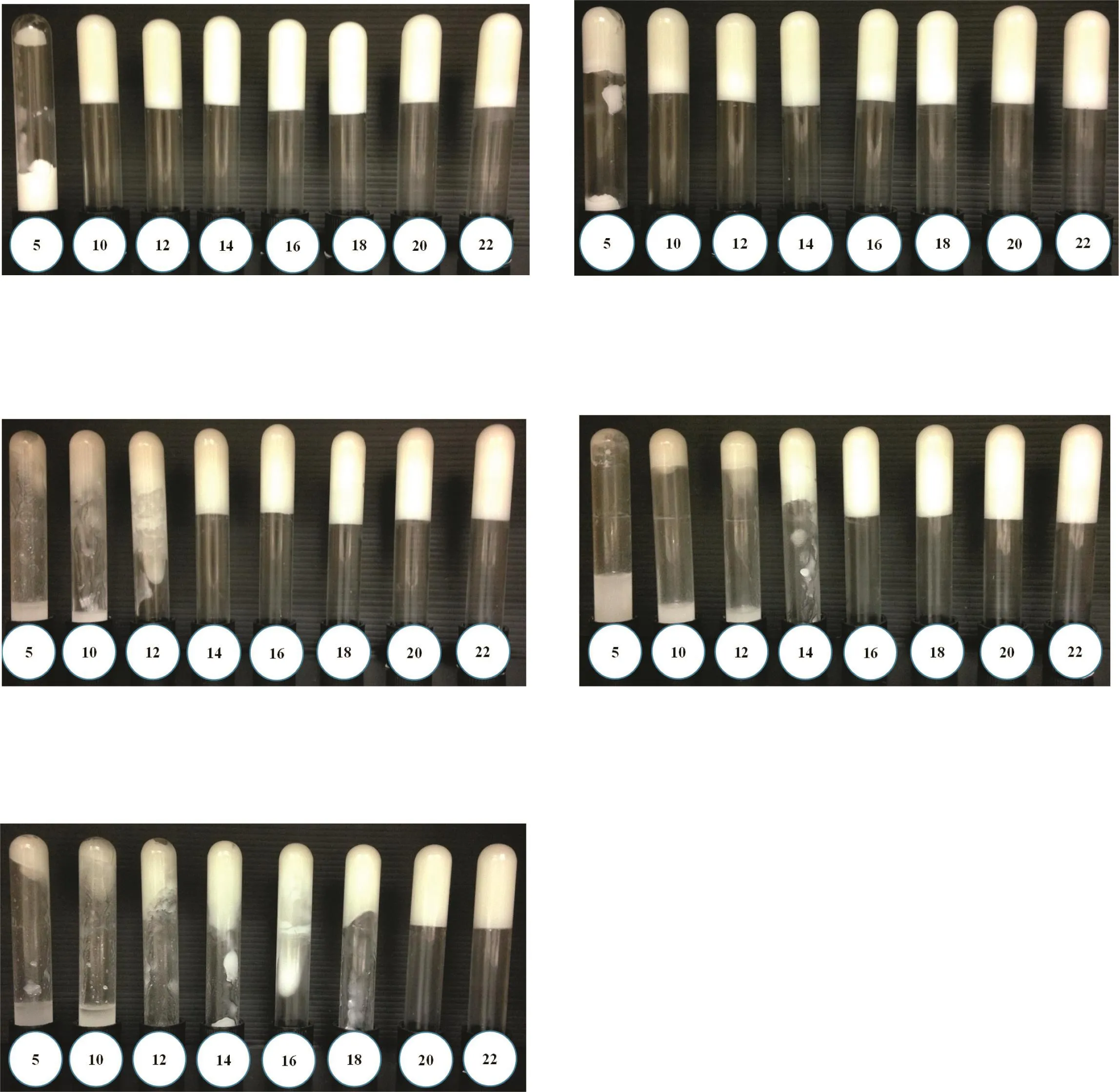
Fig.2–Determination of CGC of SMS for five different ratios of sesame oil and cyclopentasiloxane oleogels(A)1:4,(B)2:3,(C)1:1,(D)3:2,and(E)4:1.The concentration of SMS for each ratio was varied from 5%to 22%(w/w).The CGC is the minimum gelator concentration which failed to flow when the tubes were inverted after keeping at 25°C for 24h.The CGCs of A to E were found to be 10,10,14,16 and 20%w/w,respectively.
3.2. Viscosity of the oil components
Fig.3–Polarized light micrographs of five different ratios of sesame oil and cyclopentasiloxane oleogels at CGC of SMS.(A)1:4,(B)2:3,(C)1:1,(D)3:2,and(E)4:1.The gel network formed by association of tubules at contact points and appeared at all ratios as demonstrated by arrows.When the ratio of the polar sesame oil was low(1:4)or high(4:1),there was no strong connection between the tubules.The ratio 2:3 showed the strongest gel networking with association of thicker and longer tubules as compared to the ratios 1:1 and 3:2.
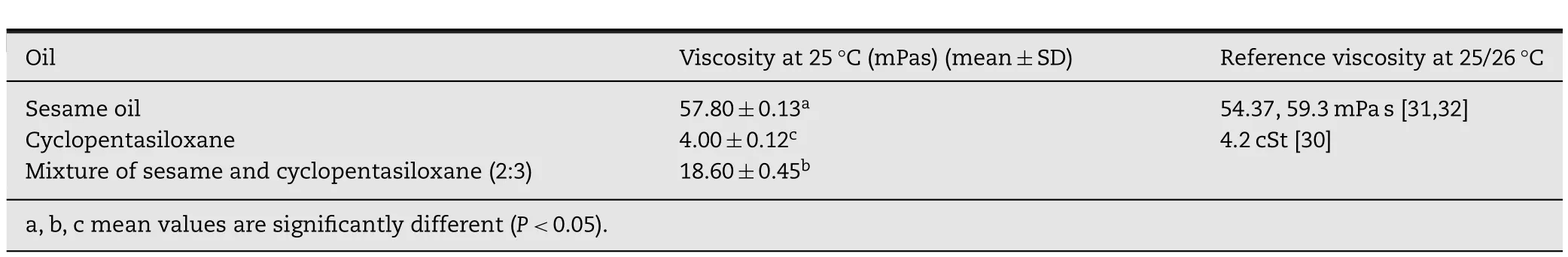
Table 2–Viscosity of oils(n=3).
The viscosity of the oil components was determined using a rheometer with viscosity-shear rate ramp.All the oil samples in this study showed Newtonian flow characteristics as the viscosity was constant,independent of shear rate.The absolute viscosity of the samples was obtained from either the linear regression of the slope from shear stress(Y-axis)against shear rate(X-axis)plot or from the average readings of viscosity at different shear rates.The results were shown in Table 2.The absolute viscosity of both sesame oil and cyclopentasiloxane were comparable to those published values[30–32].Sesame oil had the significant highest viscosity value(P<0.05)as it consisted mainly of long-chain fatty acids(linolenic acid 46.9%,oleic acid 37.4%,and palmitic acid 9.1%)[10,18].Cyclopentasiloxane,a short-chain cyclic polydimethylsiloxane,has low heat of vaporization of 31cal/g and low viscosity of 4.2cSt[30].Due to very light and excellent spreading properties,cyclopentasiloxane was used to blend with high viscosity silicone oils to improve spreadability[19].In this study,cyclopentasiloxane showed low viscosity value of 4.0mPas which is equal to 4.2cSt.Addition of cyclopentasiloxane significantly reduced the viscosity of sesame oil which could improve the spreading property of the oleogel preparations.This is in accordance with the findings from previous study[24].
3.3. Determination of CGC for oleogels

Fig.4–Determination of CGC of SMS for three oleogels(A)OG1,(B)OG2 and(C)OG3.The concentration of SMS for each oleogel was varied from 5%to 22%(w/w).The CGC is the minimum gelator concentration which failed to flow when the tubes were inverted after keeping at 25°C for 24h.The CGCs of OG1,OG2 and OG3 were found to be 20%,18%and 10%(w/w),respectively.
Oleogels with 5%–22%(w/w)of SMS were prepared and the CGC of each oleogel preparation was determined.The results were shown in Fig.4.The CGCs of OG1 and OG2 were 20%and 18%(w/w)respectively which were not very different.However,the CGC of OG3 which contained combination of sesame and cyclopentasiloxane decreased by half to only 10%(w/w)of SMS.Gel formation and CGC values of SMS oleogel were mainly influenced by the nature of solvent.According to previous studies,CGC of SMS was 1%(w/v)using hexadecane as a solvent;however,higher CGC values were found in vegetable oils;15%(w/w)for sesame oil,20%(w/w)for olive oil,and 18%(w/w)for sun flower oil oleogels[4,10,14,33].Different type of vegetable oils had different composition of fatty acids which could influence the solubility of the gelator molecules[34].In this study,the CGC value of SMS in sesame oil oleogel was found to be different from previous studies.It might be due to the different sources of the oil and SMS.Since SMS is a non-ionic surfactant which comprised both polar and nonpolar segments,the higher CGC of SMS for sesame oil and cyclopentasiloxane might be due to the higher solubility of SMS in both polar sesame oil and non-polar cyclopentasiloxane.Interestingly,the combination of cyclopentasiloxane and sesame oil reduced the CGC of SMS because the combination of polar and non-polar solvent could provide an optimum solubility and insolubility balance of the solvent.Solvent modification could affect the CGC of oleogels due to the influence of solvent on the gelator solubility;hence altering the crystallization behavior and microstructure of the gel network[35].
3.4. Preparation of oleogels
Oleogelswereformedwhenthehotmixtureofgelatorandsolvent was cooled down to room temperature.The solution of gelator and solvent was transparent when hot,which slowly turned turbid and finally converted into opaque oleogels.This might be accounted to the change in the solubility of the gelator molecules.The color of the oleogels in this study was significantly different due to the different nature of solvents.OG1 was yellowish in color and very oily,which is the major drawback of vegetable oil oleogels.OG2 was white in color but did not spread evenly when applied on the skin.However,OG3 waswhiteincolor,lessoilywhenappliedandbetterinspreadability compared to OG1.This might be due to the lower surface tension,and the light and dry feel property of cyclopentasiloxane added to sesame oil.
3.5. FTIR analysis
The FTIR spectra of SMS gelator,oils(sesame oil and cyclopentasiloxane)and oleogels are shown in Fig.5.FTIR spectra indicated the presence of molecular interactions amongst the components presented in the oleogels.A shallow broad peak at 3700–3100cm-1in OG1 and OG3 revealed the presence of stretched hydrogen-bonded O–H groups.This indicated the presence of intermolecular hydrogen bonding between the hydroxyl groups present in SMS and sesame oil,resulting in the formation of the gel network[29].As this bond was absent in OG2,it might also explain the different gel network formation of OG2.The spectra of OG3 and OG2 showed additional peaks at about 800cm-1,1200cm-1,and a wide band around 1270–850cm-1.This indicated the presence of Si–C stretching and CH3deformation in Si–CH3and Si–O–Si stretching[36].The C–H asymmetric and symmetric stretching vibrations of SMS,sesame oil and cyclopentasiloxane produced absorption bands at about 2920 and 2850cm-1.The absorption band at around 1750cm-1was attributed to C=O stretching vibration due to the ester group present in SMS[10,29].

Fig.5–FTIR spectra of oleogels and their ingredients.
3.6. Microstructure studies
The microstructure of the oleogels was observed under polarized light microscope and the results were depicted in Fig.6.The oleogels were prepared by melting/dissolving the gelator in the solvent and allowing the system to cool down gradually.Upon cooling down,the solubility of the gelator in the solvent reduced according to the decreasing af fi nity between the gelator and the solvent.Consequently,gelator molecules self-assembled and toroidal vesicular tubules were formed,followed by the association of the tubules at contact points and the 3-dimensional network was developed[4].From Fig.6A,only small and thin 3-dimensional network with distinct contact points was seen in OG1 despite its high gelator concentration of 20%(w/w).Furthermore,with 18%(w/w)SMS concentration,OG2 showed only toroidal vesicular tubules aggregation of the gelator molecules without establishing contact points,as shown in Fig.6B.In contrast,the long and thick 3-dimensional network with distinct contact points was observed in OG3 with only 10%(w/w)of the gelator concentration(Fig.6C).The nature and functional groups of the solvent significantly affect the microstructure of the gelator molecules aggregation[4,37].In this study,the network formation of the oleogels was mainly driven by hydrogen bonding between the hydroxyl groups present in SMS and sesame oil.Cyclopentasiloxane was non-polar and contained no functional groups to form hydrogen bond with SMS,confirmed by the absence of hydrogen bonding in the FTIR analysis of OG2.Although there might be hydrophobic interactions between cyclopentasiloxane and the hydrophobic part of SMS(which is a surfactant),this interaction is weak and at CGC level,it might not be sufficient to produce a stable gel,leading to syneresis in the future[14].The high concentration of SMS in OG2 could only increase the viscosity of the bulk sample in the liquid phase,thus favored stronger gelator aggregate–aggregate interaction rather than solvent–gelator interaction.Consequently,only solvent entrapment occurred during the cooling stage,and gave rise to a gel with weaker strength.From the oil viscosity results,the addition of cyclopentasiloxane reduced the viscosity and also decreased the polarity of the sesame oil.The viscosity reduction as well as the polarity alteration of the solvent could influence the self-assembly of SMS and favored the formation of the long and thick 3-dimensionalnetworkwithdistinctcontactpoints,asobserved in OG3.The influence of the polarity and viscosity of the oil component on the self-assembly of the gelator molecules affected the crystallization,gelation behavior,and gel strength[4–6].Therefore,itcouldbeconcludedthatthehydrogenbonding(between the gelator–gelator and the gelator–solvent),the solvent polarity and the viscosity of the oil phase were the major factors influencing the formation of the gel network.In addition,combination of the 2 oils at a suitable ratio in this study also improved the solvent entrapment property of the gelator hence reduced the possibility of syneresis.
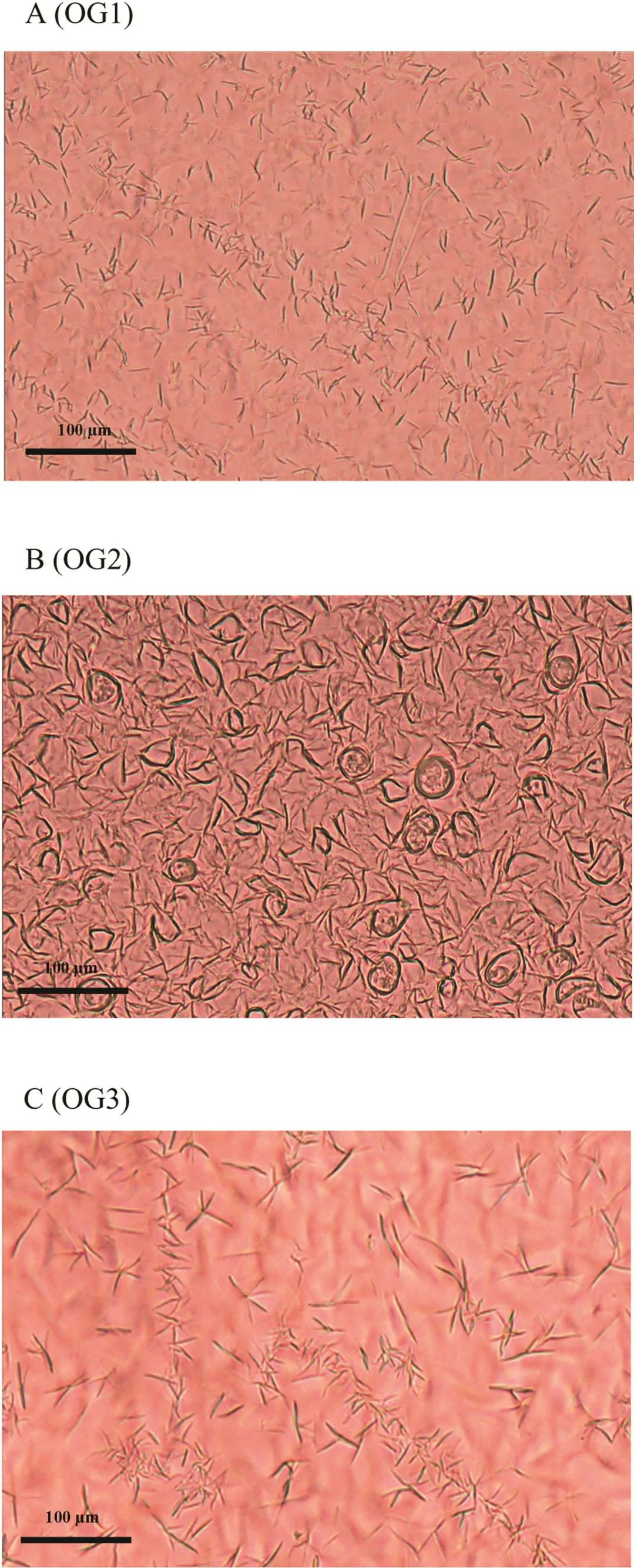
Fig.6–Polarized light micrographs of three oleogels at CGC of SMS(A)OG1,(B)OG2 and(C)OG3.OG2 showed the toroidal characteristic with less networking while the obvious network formed in OG1 and OG3.OG3 had stronger gel network with thicker and longer tubules than OG1.
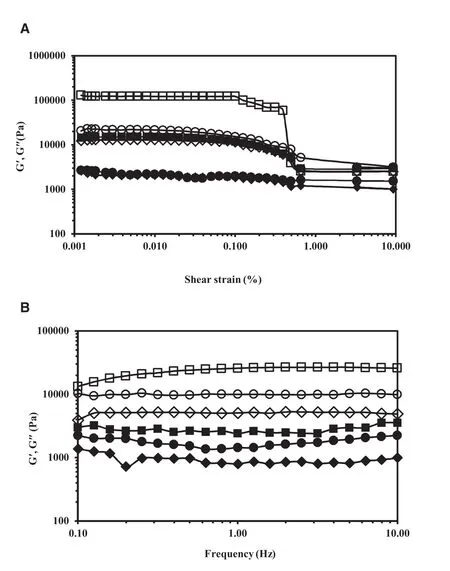
Fig.7–(A)Amplitude sweep result of oleogels:storage modulus(G′,open symbols)and loss modulus(G′′, fi lled symbols)as function of shear strain for OG1(°/●),OG2(◇/◆)and OG3(□/■).The strain range was 0.001–10Pa at a fixed frequency of 1Hz.(B)Frequency sweep result of oleogels:Storage modulus(G′,open symbols)and loss modulus(G′′,fi lled symbols)as function of frequency for OG1(°/●),OG2(◇/◆)and OG3(□/■).The frequency range was 0.1–10Hz at the strain value of 0.05Pa.
3.7. Rheological characterization
Non-destructive oscillation tests provide the rheological parameters such as the storage or elastic modulus(G′),the loss or viscous modulus(G′′),and the LVER.The viscoelastic behavior involves the structural changes within the gel formulation.It combines 2 components;G′andG′′which refer to solid-like and liquid-like properties,respectively.During an amplitude sweep,the amplitude of the shear stress is varied while the frequency is kept constant.TheG′andG′′are plotted against the strain.At low strain,G′andG′′are constant,meaning that the sample structure is undisturbed.This region is called LVER.Once the moduli start to decrease,the structure is disturbed,and the end of the LVER is reached.The plateau valueofG′intheLVER-regiondescribestherigidityofthesample at rest while the plateauG′′value implies the viscosity of the unsheared sample.Therefore,LVER provides the information involving resistance of the material to deformation[38].The long LVER presented in a product have higher stability as comparedtoproductswithaweakandsensitivestructure[39].From the amplitude sweep result in Fig.7A,all oleogels had higherG′thanG′′,about 1 decade difference,indicating that the system was more elastic than viscous at the studied frequency range,hence they had strong gel property.The plateauG′value of OG3 was the highest among all preparations,also about 1 decade higher than that of the other two,implying the highest gel strength.OG3 also possessed the longest LVER suggesting the highest stability of the gel formulation and resistance to syneresis.As mentioned in the microstructure studies,thegelformationofOG2was mainlyfromself-aggregation of the high gelator concentration and also no contact points were established.Therefore,the gel strength of OG2 was the lowest as seen from the plateauG′values in Fig.7A and a shorter LVER than that of OG3.The plateauG′andG′′values of OG1 were in between those of OG3 and OG2,meaning weaker gelstrengththanOG3butbetterthanOG2.Thiswassupported by the polarized light micrographs in Fig.6 as small and thin fi bers gel network was observed for OG1 while the long and thick 3-dimensional network with distinct contact points was obvious in OG3.
The frequency sweep test measures the response of a system as a function of frequency at a constant amplitude within the LVER.It provides the specific information ofG′andG′′of a viscoelastic system without disrupting its structure[40].Fig.7B displayed the frequency sweep result of the 3 oleogels.All preparations were regarded as true gels becauseG′values were higher thanG′′in all cases andG′was not dependent upon the applied frequency.
The viscosity profiles of the oleogels were demonstrated in Fig.8A.All showed pseudoplastic flow characteristic.OG3 had the highest viscosity at rest but the viscosity reduced quickly when sheared thus suggested easiest spreadability which was suited for topical applications.
Fig.9–XRD patterns of oleogels and their ingredients.
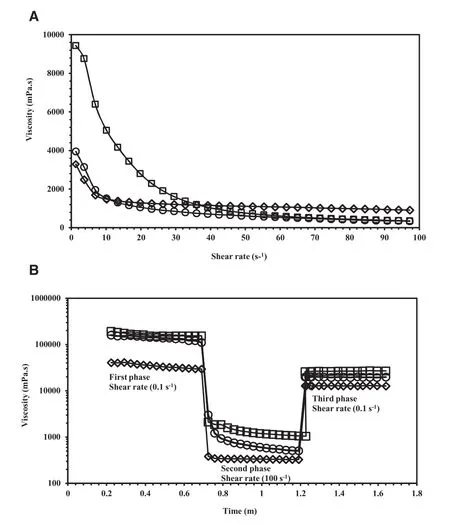
Fig.8 – (A)Viscosity profiles of OG1(ο),OG2(◇)and OG3(□)at the shear rate range of 0.1–100s-1.(B)Thixotropic behavior of OG1(ο),OG2(◇)and OG3(□)using three step shear rate sweeps.
Thixotropy refers to time-dependent decrease in viscosity at a constant shear rate with reversible structure recovery after removal of the shear stress or shear rate.The recovery can be a complete recovery or a partial recovery and can be rapid or gradual.Therefore,the thixotropic behavior is best studied by changing the shear rate stepwise and tracking the response viscosity over time[41].The thixotropic behavior of the three oleogels was measured using a specific rheology test called“three-step shear rate sweep”which consists of three consecutive phases in the control rate mode with alternate low and high shear rates.The results were presented in Fig.8B.In the first phase(at the shear rate of 0.1s-1)which represented the viscosity at rest,all three oleogels had high viscosity.In the second phase,the shear rate was rapidly shifted from 0.1s-1to 100s-1which related to the shear rate during application of semisolid product on the skin,the viscosity of all oleogels immediately dropped as the network structure was completely broken down by the high shear.In the third phase,the shear rate was quickly shifted back to 0.1s-1,representing the end of application process on the skin.The oleogel viscosity increased to different values as their structure showed some recovery depending on the gel strength.OG2 had the weakest gel strength,hence easily broke down and flowed with the lowest viscosity.Its recovery was very rapid(2s)in the third phase.This might be because no strong connection points between the tubules were established in the gel network.This result was in agreement with previous study of Doan et al,whichstatedthatweakgelsbrokeeasier, flowedatlowershear values and had higher regeneration percentage than stronger gels[42].In contrast,the gel network structures of OG1 and OG3 were stronger than that of OG2 due to the presence of hydrogen bonding,therefore the structure breakdown in the secondphasewaslessandtheviscositydecreasewasintheorder of OG3<OG1<OG2.All 3 oleogels showed very quick partial structure recovery in the third phase.The recovery time was within a few seconds.
3.8. X-ray diffraction studies
The oleogels and their components were analyzed using XRD to study the influence of solvent on crystalline behaviors of oleogels.The underlying peaks of the profiles were determined using the peak- fi tting tool of Origin Pro 8 and Gaussian model was used for peak- fi tting.The full width of the peak at half maximum(FWHM),area under the curve(AUC),and the Bragg distance(d-spacing)values were calculated using the Origin Pro 8 software and the results were tabulated in Table 3.
Fig.9 showed the presence of broad peaks,cyclopentasiloxane at 11°2θ(peak 1),and sesame oil at 19°2θ(peak 2),and single sharp peak of SMS at 21°2θ(peak 3).SMS showed a strong sharp signal with an associated Bragg distance(dspacing)of 4.11?A.When compared to the XRD patterns of the neat SMS,all three oleogels showed a dominant peak at 4.11–4.14?A which was attributed to the crystalline nature of SMS.The samed-spacing values were noted in the XRD patterns of SMS–mustard oil oleogels as reported by Sagiri and coworkers[29].Although the peak position of SMS and thed-values of all oleogels did not significantly changed,the FWHM and AUC values of oleogels had altered.
The FWHM is dependent on the crystallinity of the sample,which in turn is dependent on the interactions amongst the gelator molecules responsible for the formation of an ordered structure.For FWHM determination,the XRD profile data was smoothened by Savitzky–Golay filter using 50 points of window and polynominal order of 5.Then the width of the smoothened curve was calculated resulting the FWHM value[43].The FWHM values of the broad peaks(peaks 1 and 2)were higher than that of peak 3,indicating the comparatively amorphous nature as compared to single sharp peak of peak 3.Therefore,only peak 3(SMS)was chosen to compare the crystalline behavior of different oleogels.The FWHM values of SMS peak for OG1,OG2 and OG3 were found to be 0.56,6.67,and 3.72,respectively.The lower FWHM value relates to the higher crystallinity and vice versa[43].The low FWHM valueofOG1indicatedanincreaseinthecrystallinityfromthe high concentration of SMS(20%,w/w).In contrast,the highest FWHM value of OG2 showed the low crystalline nature although it contained the high gelator concentration of 18%w/w.This might come from decrease in the ordered structure of OG2.This finding was in agreement with the microscopic character of OG2.The AUC values of OG1,OG2,and OG 3 were 181.65,2512.72,and 349.97,respectively.The AUC results were similar to those of FWHM results.The higher AUC favored the lower crystalline nature.Therefore,it could be concluded that the combination of cyclopentasiloxane and sesame oil in OG3 exhibited crystalline behavior even with 10%(w/w)of SMS.The crystallinity of OG3 might be attributed to the presence of network structure made up of toroidal vesicular tubules formed in the solvent.
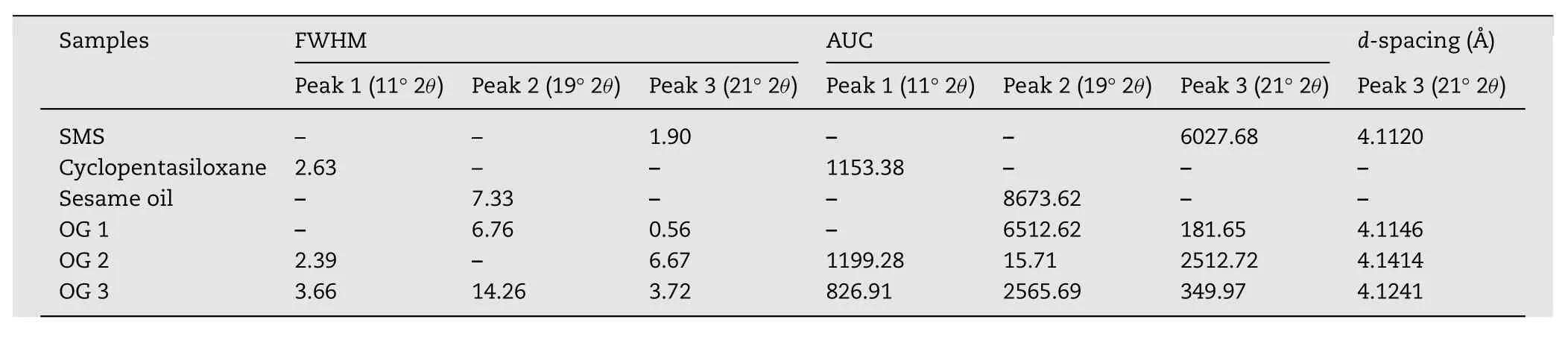
Table 3–FWHM,AUC and d-spacing values of oleogels and their components for XRD analysis.
3.9. Thermal analysis
The thermal profiles of SMS,sesame oil,cyclopentasiloxane and oleogels were characterized with DSC and the results were displayed in Fig.10.In this study,the endothermic and exothermic peaks of SMS were found to be at 52 °C and 46°C during the heating and cooling cycles,respectively.This result showedsomevariationfrompreviousstudies;51°C,endothermic[4,10]and 45°C,exothermic[10]which might be from different sources of SMS.All oleogels showed thermoreversible nature.The endothermic and exothermic peaks assigned to the melting and crystallization of SMS were found at 43–46°C and at 44–48°C,respectively in all three oleogels[10].The slight shifting of the corresponding peaks during the heating and cooling cycle might be due to the structural changes of SMS molecules.Due to thermal hysteresis property,the position of the melting and crystallization peaks might be shifted.In addition,different microstructure of oleogels affected the gel strength,which in turn affected the melting and crystallization behavior of oleogels.The melting and crystallization enthalpies were determined using the area under the respective peaks and the values were tabulated in Table 4.In general,melting enthalpy(ΔHm)and entropy(ΔSm)values increase with higher gelator concentration.This was true for SMS as confirmed by Singh et al.[10].In this study,the SMS concentration was not varied but fixed at the CGC of each oleogel formulation.The melting enthalpy(ΔHm)and entropy(ΔSm)values of OG3 were the highest among all three preparations despite the lower SMS concentration of OG3 than that of OG1 and OG2.Combination of the 2 oils in OG3 improved its thermodynamic stability even with low concentration of gelator.This might be due to the long and thick gel network formation in OG3 which lead to stronger gel strength.
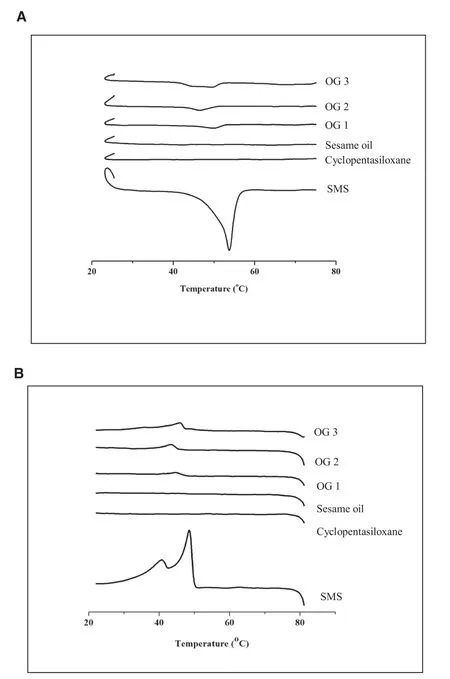
Fig.10–Thermal analysis of(A)heating cycle and(B)cooling cycle of oleogels and their ingredients.

Table 4–DSC studies of OG1,OG2 and OG3(n=3,mean±SD).
4. Conclusion
Addition of volatile silicone to sesame oil-SMS oleogel system resulted in an oleogel which was a true gel,had longer LVER and better gel strength at only 10%(w/w)gelator concentration(as compared to 20%and 18%for the single oil systems).The polarized light micrographs revealed the long and thick three-dimensional network which can enhance solvent entrapment properties of the gelator and prevent syneresis.Moreover,the obtained oleogel had thermoreversible in nature.It had pseudoplastic flow with thixotropic behavior and high viscosity at rest.It also provided better spreadability and aesthetic property,thus suitable for topical application.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgments
The authors acknowledge the financial support received from the Faculty of Pharmacy,Silpakorn University(Grant no.RGG 001/2558).
 Asian Journal of Pharmacentical Sciences2018年5期
Asian Journal of Pharmacentical Sciences2018年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Special issue:Pharmaceutical innovation
- Vesicular carriers containing phenylethyl resorcinol for topical delivery system;liposomes,transfersomes and invasomes
- Design and characterization of monolaurin loaded electrospun shellac nanofibers with antimicrobial activity?
- Formulation and evaluation of gels containing coconut kernel extract for topical application?
- The effect of surfactant on the physical properties of coconut oil nanoemulsions?
- Preparation and characterization of nanoparticles from quaternized cyclodextrin-grafted chitosan associated with hyaluronic acid for cosmetics
