Simultaneous determination of steroid drugs in the ointment via magnetic solid phase extraction followed by HPLC-UV
Ydollh Ymini,Meysm Sfri,Mrym Shmsyei
aDepartment of Chemistry,Tarbiat Modares University,P.O.Box 14115-175,Tehran,Iran
bDepartment of Chemical Engineering,Kermanshah University of Technology,Kermanshah,Iran
Keywords:Magnetic nanoparticles Steroid drug Ointment High performance liquid chromatography Self-assembled monolayer
ABSTRACT The copper-coated iron oxide nanoparticles with core-shell were produced by deposition of a Cu shell on Fe3O4NPs through reduction of Cu2+ions in solution using NaBH4.Subsequently,the organosulfur compound,bis-(2,4,4-trimethylpentyl)-dithiophosphinic acid(b-TMP-DTPA),was used to form self-assembled monolayer in order to modify sorbent's surface via covalent bonding between Cu and thiol(–SH)terminal groups.The prepared magnetic nanoparticles were characterized by using Fourier transform infrared(FT-IR)spectroscopy,scanning electron microscopy(SEM),transmission electron microscope(TEM),vibrating sample magnetometer(VSM)and thermo gravimetric analysis(TGA).Then,the application of this new sorbent was investigated to extract the steroid drugs in ointment samples with the aid of ultrasound.An external magnetic field was applied to collect the magnetic nanoparticles(MNPs).The extracted analytes were desorbed using acetonitrile.The obtained extraction solution was analyzed by HPLC-UV.The main affecting factors on the extraction efficiency including pH,sonication time,amount of sorbent,salt concentration,and desorption conditions were optimized in detail.Under the optimum conditions,good linearity was obtained in the range of 2.5–250.0μg/L with reasonable linearity(R2>0.99)and the limits of detection(LODs)ranged between 0.5 and 1.0μg/L(based on S/N=3).Repeatability(intra-day precision)based on five replicates and preconcentration factors were calculated to be 3.6%–4.7%and 87–116,respectively.Relative recoveries in ointment samples at two spiked levels of the target analytes were obtained in the range of 90.0%–103.2%.The results illustrated that the Fe3O4@Cu@b-TMP-DTPA NPs have the capability of extraction of steroid drugs from ointment samples.
1.Introduction
Nanocomposites with core-shell structure consisting of two or more different nanoscale materials have aroused more interests among researchers.Core-shell nanocomposites not only provide the stability for the nanocomposites in solution but also facilitate their binding with various ligands for various applications such as drug delivery,cancer hyperthermia treatments magnetic,and separation[1–5].Comparison with other nanocomposites,the surface of coreshell nanocomposites is more uniform and can be functionalized conveniently.The synthesis and applications of magnetic nanoparticles(MNPs)coated with a metallic shell[6–10]have been studied in recent years due to their usefulness and versatility in various scientific fields[11–17].Various studies are focused on the fabrication of MNPs with core-shell nanostructure,such as Fe3O4@Al2O3[18],Fe3O4@SiO2[6],Fe3O4@Ag[19],Fe3O4@Au[20],Fe2O3@Au[21]and Fe3O4@Pt[22].With metal coatings,the core-shell MNPs not only can act as a good platform to conjugate many molecules more efficiently,but also still have good magnetic properties.Au,Ag and Pt have become the most utilized coating due to their low reactivity,high chemical stability,and good affinity for binding to thiol(–SH)or amine(–NH2)terminal groups[19,23–25].However,some problems are still present in the preparation of Au,Ag and Pt core-shell MNPs,such as their high cost and complex preparation processes.
On the other hand,Cu nanoparticles(NPs)have been considered as one of the best candidates for building novel magnetic nanocomposite.Incorporation of Cu and a magnetic Fe3O4core(Fe3O4@Cu)could combine the advantages of chemical stability,inexpensive and good affinity for binding thiol(–SH)terminal groups[26].Thiols(-SH)are probably the most utilized functional groups for stabilizing and modifying Fe3O4.Thiol groups form stable bonds with copper on the surface of Fe3O4NPs.Therefore,b-TMP-DTPA can beeasilyassembledontothesurfaceof Fe3O4@Cu.Herein,they have been applied to provide a suitable coating for MNPs,used as sorbents for cleaning and separating steroid drugs from ointments samples.Therefore,MNPs were applied successfully for magnetic solid phase extraction(MSPE)(Fig.1).
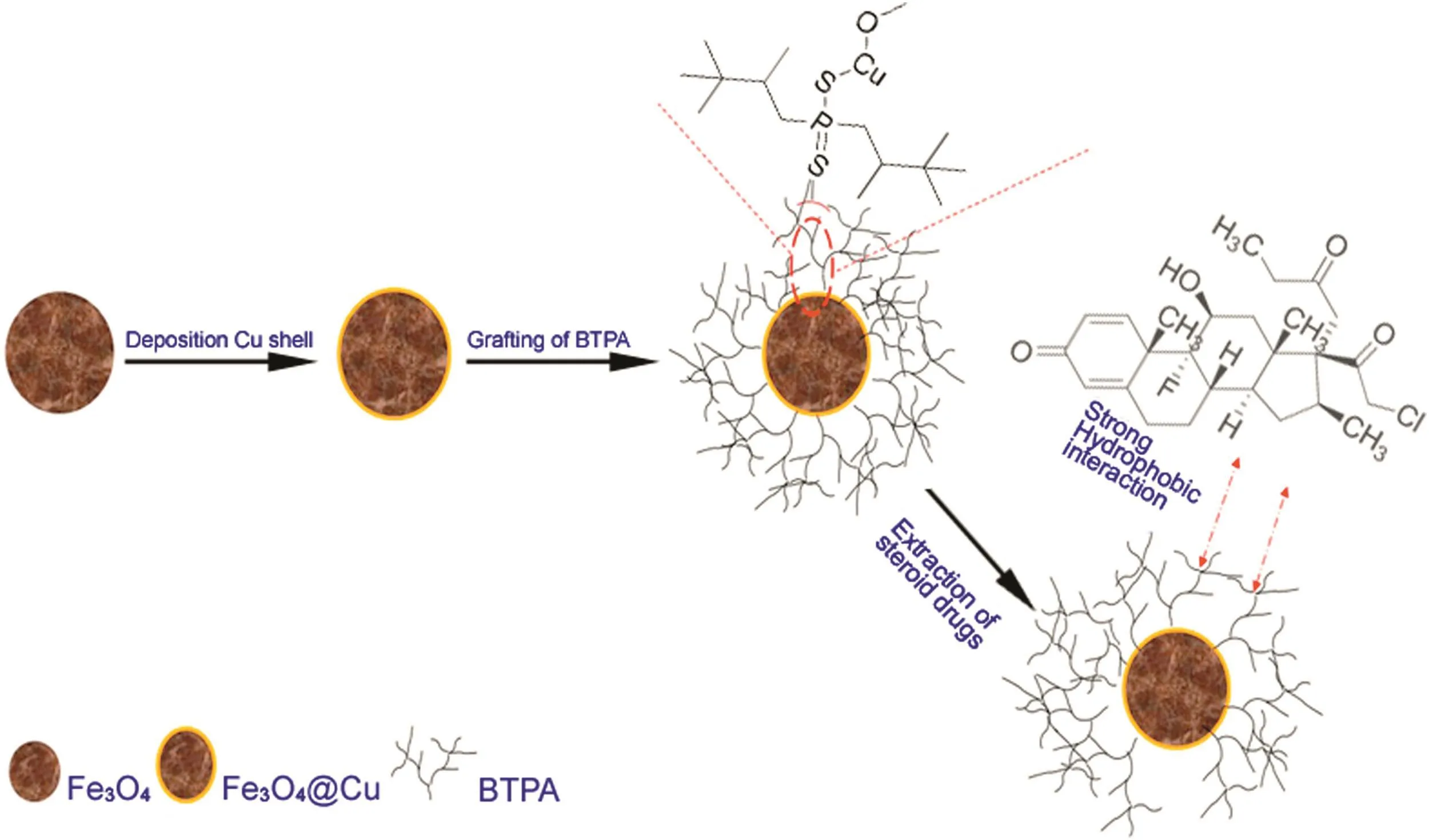
Fig.1.Schematic illustration of extraction procedure.
Corticosteroids have been garnering attention as highly effective drugs widely used in dermatology for the treatment of inflammatory diseases,in the form of creams,gel,solution and ointments.These corticosteroids have severe potential side effects such as permanent skin atopy and pustular psoriasis.Also,some systematic side effects of corticosterids are hypertension,diabetes mellitus,osteoporosis,allergic contact dermatitis,Cushing's syndrome,etc.[27,28].The risk of these side effects increases with long-term use and high dosage,especially if used without medical supervision.Therefore,this study aimed to develop and validate analytical methods allowing the separation and rapid determination of steroid drugs.Up to now,the main approaches for the detection of steroid drugs include high performance liquid chromatography(HPLC),high performance liquid chromatographymass spectrometry(HPLC-MS)and ultra high performance liquid chromatography coupled with tandem mass spectrometry(UHPLC-MS/MS)[29].In these methods,HPLC is a common and useful method for trace determination of analyzes in complex samples.Sample preparation techniques,such as liquid-liquid extraction(LLE)[30]and solid-phase extraction(SPE)[31],can enhance the sensitivity and selectivity of HPLC.
In this study,new MSPE was investigated to evaluate its performance by extraction of three steroid drugs(clobetasol propionate,desoxycorticosterone and fluorometholone)from the ointment samples by assisted ultrasound.To the best of our knowledge,this is the first report on describing the synthesis and also the application of the self-assembling technique of sulfurcontaining compound onto Fe3O4@Cu nanomaterials for extraction of steroid drugs from ointments samples.
2.Experimental
2.1.Chemicals and reagents
Copper nitrate,sodium borohydride,ferrous chloride,ferric chloride,ammonia solution(25wt%),methanol,acetonitrile,and 1-propanol were purchased from Merck(Darmstadt,Germany).b-TMP-DTPA was obtained from Sigma–Aldrich(Milwaukee,WI,USA).
A stock standard solution of 1000 mg/L of each steroid drug was prepared in HPLC grade methanol.The standard working solutions were prepared daily by appropriate dilution of the stock standard solutions with ultra-pure water to the required concentrations.The concentration of steroid drugs in the preliminary experiments was 100μg/L.Ointments were purchased from a local market(Tehran,Iran).
2.2.Apparatus
Particle size and morphology of the fabricated NPs were determined by a scanning electron microscope(SEM)model EM3200 from KYKY Zhongguancun(Beijing,China)and a transmission electron microscope(TEM),EM10C(Zeiss,Germany).Magnetic property was measured by a vibrating sample magnetometer(VSM)model AGFM/VSM 3886 from Kashan University(Kashan,Iran).The thermal degradation/stability of the Fe3O4@Cu@b-TMPDTPA was studied with a thermo-gravimetric analysis instrument,model TGA-STA 1500,from Rheometric Scientific STA(Los Angeles,CF,USA).The analysis was performed from the room temperature to 600 °C at a heating rate of 10 °C/min in an argon atmosphere.
Fouriertransform infrared (FT-IR)spectroscopy wasinvestigated by Thermo Scientific Nicolet IR100(Madison,WI,USA).The spectra were recorded in the range of 4000–400 cm-1.The samples were dried and mixed with KBr and made into pellets.Ultrasonic device(TECNO-GAZ,Parma,Italy)equipped with digital timer and temperature controller was used for the sonication.Separation and determination of the three steroid drugs were carried out on a Varian HPLC system(Walnut Creek,CA,USA)equipped with a Varian 9050 UV–Vis detector.The chromatographic system consisted of a 9012 HPLC pump(Mulgrave Victoria,Australia),and a six-port Valco HPLC valve(Houston,TX,USA)with a 20μL sample loop.Chromatographic data were recorded and processed by Chromana data analysis software(Version 3.6.4).The chromatographic separation of the analytes was performed using an ODS column(250 cm × 4.6 mm,with particle size of 5 μm)from Teknochroma(Barcelona,Spain)at 25°C.The detection wavelength was 240nm and the mobile phase was a mixture of water and acetonitrile(25:75,v/v),and the flow rate was 1mL/min.
2.3.Preparation of Fe3O4@Cu core-shell magnetic nanoparticles
The process of preparation of Fe3O4@Cu@b-TMP-DTPA NPs is illustrated in Fig.1.First,the Fe3O4nanoparticles were fabricated by chemical coprecipitation method according to a previously reported method with some modifications[25].Brie fly,in a threeneck round bottom flask,8.3 g of FeCl3·6H2O and 2.1 g of FeCl2·4H2O were dissolved in 400 mL deionized water under N2atmosphere.Then,the solution was heated to 85°C in a water bath.20 mL of ammonia solution(25 wt%)was added quickly with vigorous stirring under N2atmosphere protection.A black precipitate formed instantly and the reaction was allowed to proceed for another 5min.The obtained MNPs were rinsed several times with deionized water until a neutral pH was reached and then resuspended in 400 mL deionized water.
Fe3O4@Cu core-shell NPs were fabricated by reduction of Cu2+ions in the presence of bare MNPs.20 mL of 0.64 mM CuNO3was added to 70mL of the mixture containing 1 g MNPs.A solution of 0.32 mM sodium borohydride(50 mL)was added dropwise to the suspension.The mixture stirred for 30 min after the addition of NaBH4.As the MNPs were gradually coated by Cu,the color of the mixture changed from black to reddish brown.The resultant MNPs(Fe3O4@Cu)were collected by a magnet and washed with deionized water several times.Finally,the Fe3O4@Cu was suspended in ethanol for the next step.
For fabricated Fe3O4@Cu@b-TMP-DTPA,2 mL of b-TMP-DTPA was added to 100 mL suspension of Fe3O4@Cu NPs.Finally,the mixture was stirred for 32 h at room temperature.In the end,Fe3O4@Cu core-shell NPs were washed with deionized water and ethanol several times and then dried at 50°C under vacuum for 3 h.
2.4.Ultrasound assisted extraction procedure
MSPE was carried out as follows:About 40 mg of magnetic adsorbent was activated by adding 200μL of methanol into a 15 mL conical tube by fierce vortex.Then,they were transferred into to 30 mL of aqueous solution containing steroid drugs.Then,the mixture was exposed to ultrasound at the frequency of 40 kHz(15 min),which led to the best dispersion of adsorbent into the solution,and the raising temperature simultaneously led to enhancement coefficient and progress in the mass transfer.Thereafter,the MNPs were collected from the resulting solution with an external magnet(10 cm×5 cm×4 cm with 1.4 T magnetic fields).The supernatant was decanted and the residue solution and magnetic adsorbent were transferred to a 15 mL conical tube.The magnetic adsorbent was again aggregated by a magnet to remove the residue solution completely by a syringe.The analytes were eluted from the magnetic adsorbent with 100μL of acetonitrile by vortexing for 1 min.Finally,20μL of the eluate was drawn out by a Hamilton syringe and directly injected into the HPLC-UV for analysis.
3.Results and discussion
3.1.Characterization of Fe3O4@Cu@b-TMP-DTPA NPs
The FT-IR spectra of Fe3O4@Cu@b-TMP-DTPA and Fe3O4are shown in Fig.2A.It can be seen from Fig.2A that the observed representative peaks of b-TMP-DTPA,such as the adsorption of Fe–O–Fe vibrations(580 and 400 cm-1),the C-H bending vibration of CH3(2916 cm-1),the stretching of O–H bond(3428 cm-1),stretching vibrations of C-C(1373cm-1)and the C-P bonds(800 cm-1),confirm the b-TMP-DTPA encapsulates Fe3O4NPs as the shell on the surface.
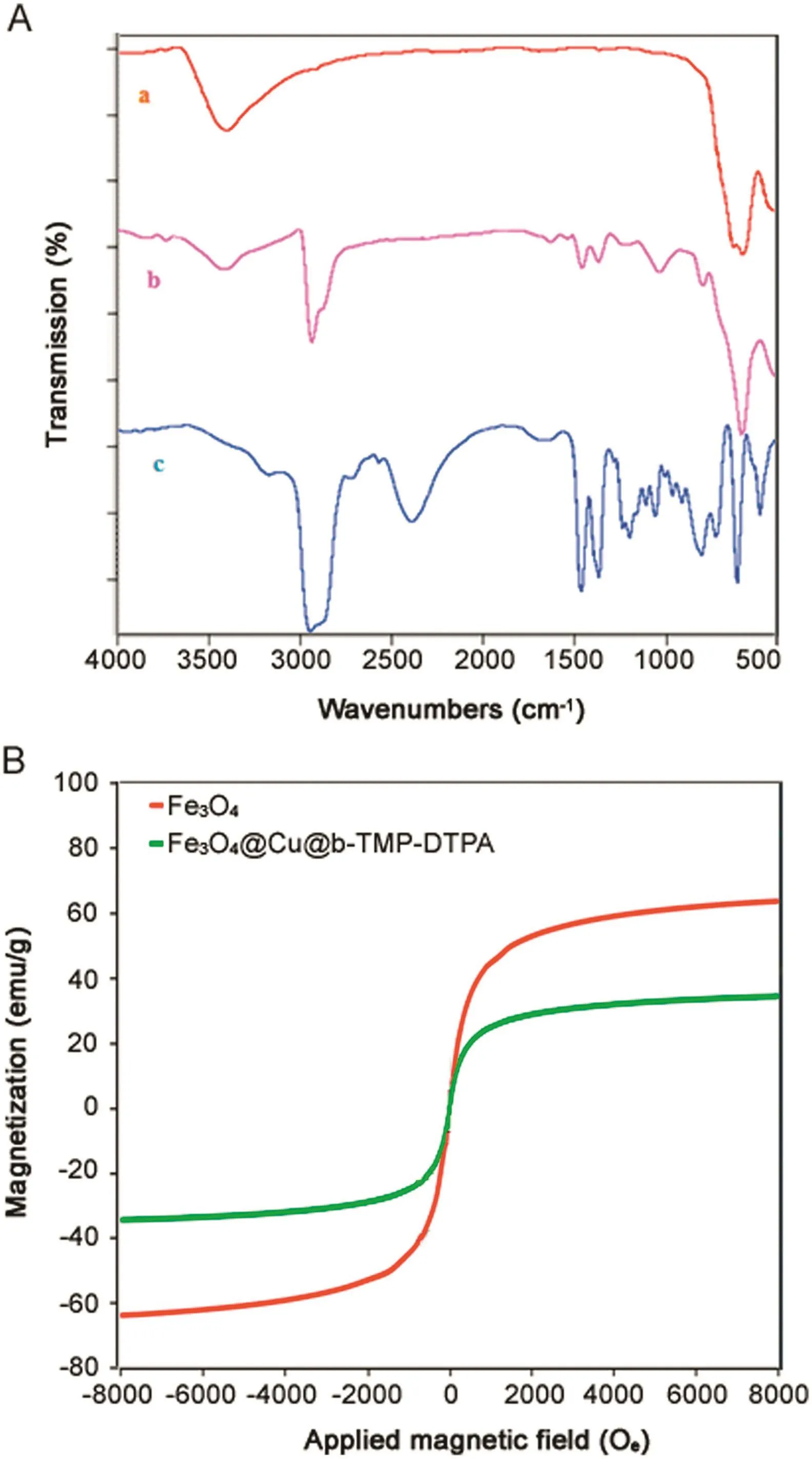
Fig.2.(A)FT-IR spectra of(a)Fe3O4,(b)Fe3O4@Cu@b-TMP-DTPA,and(c)b-TMPDTPA.(B)VSM curves of Fe3O4and Fe3O4@Cu@b-TMP-DTPA.
ThemagnetizationcurveofFe3O4@Cu@b-TMP-DTPAwas measured using a supper conducting quantum interference device by cycling the field between–8 and 8 kOe.The magnetic hysteresis loop was S-like curves.Fig.2B shows VSM magnetization curves of bare Fe3O4NPs and Fe3O4@Cu@b-TMP-DTPA NPs at room temperature.The b-TMP-DTPA shell resulted in decreased range and magnetic strength of the NPs due to the weight contribution from the nonmagnetic b-TMP-DTPA.The large saturation magnetization of Fe3O4NPs and Fe3O4@Cu@b-TMP-DTPA NPs was 63 and 28 emu/g,which is sufficient for magnetic separation with a conventional magnet.
The shape and size of the synthesized particles were observed by TEM(Fig.3A).The TEM images of Fe3O4@Cu@b-TMP-DTPA particles show that an obvious b-TMP-DTPA coating is immobilized on the surface of Fe3O4NPs.The coated b-TMP-DTPA layer is clearly seen due to the different electron densities of magnetic nanoparticles core(with dark color)and b-TMP-DTPA coating(with light color)in TEM micrograph.As can be seen,the synthesized Fe3O4@Cu@b-TMP-DTPA NPs were nearly spherical in shape with an average diameter of about 5–10 nm,and they tended to aggregate to larger particles.The morphology of the Fe3O4@Cu@b-TMP-DTPA NPs was also determined by SEM.As shown in Fig.3B,the Fe3O4@Cu@b-TMP-DTPA NPs have a nearly spherical shape with a smooth and uniform surface morphology.To confirm the presence of b-TMP-DTPA on Fe3O4@Cu NPs,a thermogravimetric analysis(TGA)of Fe3O4@Cu@b-TMP-DTPA NPs was conducted.In the TGA curve of Fe3O4@Cu@b-TMP-DTPA,a weight loss of 27%was observed,of which 2%was related to the expulsion of water molecule.The remaining 25%weight loss represented destruction of organic moiety that was grafted on the MNPs(Fig.3C).
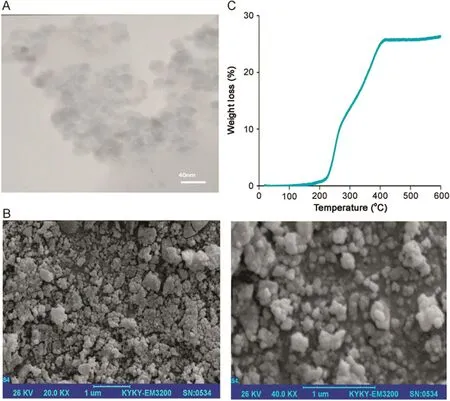
Fig.3.(A)TEM images of Fe3O4@Cu@b-TMP-DTPA NPs.(B)SEM images of Fe3O4@Cu@b-TMP-DTP NPs at 20,000× and 40,000× magnifications.(C)TGA thermograms of Fe3O4@Cu@b-TMP-DTPA.
3.2.Optimization of extraction conditions
As is well known,MSPE efficiency can be affected by several working parameters including sample solution pH,sonication time,amount of NPs sorbent,salt concentration as well as desorption conditions.The effects of the above mentioned experimental parameters were investigated by one variable at a time method.All the experiments were performed in triplicates,and the mean of chromatographic peak areas was used to evaluate the in fluence of those variables on the extraction efficiency of MSPE technique.The self-assembly between the thiol and Fe3O4@Cu was based on thiol chemistry.Thiols(-SH)are probably the most utilized functional groups for stabilizing and modifying Fe3O4@Cu.Thiol groups form stable bonds with metals on the surface of Cu.Thiol chemistry was used to bind the b-TMP-DTPA to the surface of the Fe3O4@Cu.The synthesized MNPs were used as sorbents to clean and separatesteroid drugsfrom ointmentssamples.The sorbent was used for MSPE of steroid drugs mainly based on hydrophobic interaction between the sorbent and steroid drugs.
3.2.1.Effect of sample pH
Since the steroid drugs are not ionizable compounds,varying sample pH(2-12)could not affect the extraction efficiency of the analytes and then this parameter was not optimized.But effect of sample pH on stability of the sorbent was investigated and the obtained results in the wide range of pH showed that the sorbentwasstable even in very strong acidicand basic conditions,indicating very strong bonding of b-TMP-DTPA onto the Fe3O4@Cu NPs.
3.2.2.Selection of eluting solvent
The eluting solvent is an important factor that affects the MSPE procedure.A suitable solvent can effectively elute the adsorbed analytes with the minimum volume and less interfering impurities co-eluted.Also,it should not damage the nature of the adsorbent surface.In this study,some conventional solvents including methanol,1-propanol,2-propanol acetonitrile,and ethyl estate were investigated to select the optimum desorption solvent.The results shown in Fig.4A indicate that acetonitrile was the best desorption solvent.The reason for this may be that acetonitrile has a more similar polarity to the analytes and therefore has a relatively high dissolution potential for the three analytes.Therefore,acetonitrile was used as the desorption solvent for further studies.
3.2.3.Effect of adsorbent amount
Compared to conventional sorbents(micro-size particles),nanoparticles have a higher surface area.Therefore,satisfactory results can be achieved with fewer amounts of nanoparticle adsorbent.The amount of magnetic adsorbent is known to be related to the amount of analytes adsorbed in MSPE.Thus,the effect of the amount of the sorbent was examined.Fig.4B shows the changes of the extraction recoveries using NPs in the range of 10–60 mg.The extraction recoveries increase significantly when the amount of magnetic adsorbent increases from 10 to 40 mg.No significant increase in recoveries is observed when the amount of magnetic adsorbent increased from 40 to 60 mg.Thus,40 mg is adopted as the amount of magnetic adsorbent in the following studies.
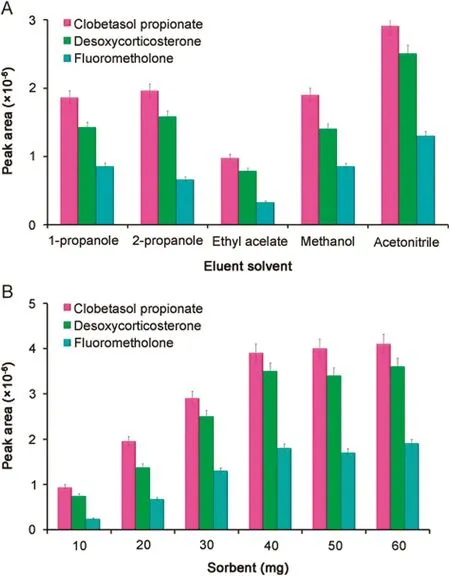
Fig.4.(A)Effect of eluent type on extraction efficiency.Extraction conditions:30 mg of the adsorbent;30 mL of sample solution containing 100 μg/L of steroid drugs at pH=7;extraction time,6 min;100 μL of eluent;and desorption time,1 min.(B)Effect of sorbent on extraction efficiency.Extraction conditions:extraction time,6 min;30 mL of sample solution containing 100 μg/L of steroid drugs at pH=7;100 μL of acetonitrile as eluent;and desorption time,1 min.
3.2.4.Effect of sonication time on the adsorption efficiency
To achieve better extraction efficiency with shorter analysis time,it is necessary to select an adsorption time that provide the equilibrium between sample solution and adsorbent.The effect of adsorption time was investigated in the range of 3–15 min.The highest recoveries of steroid drugs were obtained when the adsorption timewasselected to be 6min.No significant increase in recoveries was observed when the time increased from 6 to 15min.Finally,to be 6 was chosen as the adsorption time.
3.2.5.Effect of desorption sonication time
The effect of desorption time was studied in the range of 30–150 s.It was revealed that after 60 s,no significant change occurred in the extraction efficiency.Therefore,60 s was selected for the analyte desorption.3.2.6.Effect of eluting solvent volume
To achieve the highest enrichment and better recovery of the adsorbed analytes,the effect of the eluent volumes in the range of 100–400μL was also tested.The experimental results indicated that 100μL of acetonitrile was enough to obtain satisfactory recoveries and enrichment of steroid drugs.Therefore,100μL of acetonitrile was selected to ensure complete elution of analytes for further experiments.
3.2.7.Effect of salt addition
The effect of ionic strength on the extraction efficiency was evaluated by increasing NaCl concentration,in the sample solution,from 0%to 10%(w/v).Regarding the obtained data,salt addition has a negative effect on the extraction efficiency.It is due to the fact that the aqueous solution viscosity would increase with the addition of salt,which resulted in difficult mass transfer and low extraction efficiency.The addition of salt would also reduce the interaction of analytes with sorbent surface,thus reducing the extraction efficiency.Consequently,salt was not added into the solution in the following experiments.
3.2.8.Reusability of the Fe3O4@Cu@b-TMP-DTPA NPs
To investigate the capacity of reusability of the adsorbent,for the next extraction,the used Fe3O4@Cu@b-TMP-DTPA was soaked two times with 1mL of acetonitrile.MNPs could be used at least 5 times without obvious loss of the extraction efficiency and significant loss of extraction efficiency was observed after 5 times cycles.Thus,the Fe3O4@Cu@b-TMP-DTPA NPs could be used repeatedly 5 times(Fig.5).
3.3.Method Validation
3.3.1.Analytical performance
To evaluate practical applicability of the proposed MSPE technique,preconcentration factors(PFs)linearity(LDR),limits of detection(LOD)and quantification(LOQ),and precision were investigated by extraction of the steroid drugs under the optimal conditions.The results are summarized in Table 1.
Preconcentration factor de fined as the ratio of the slopes of calibration curve after and before extraction was attained in the range of 87–116 under the optimized conditions.Good linearity of response was observed in the range of 2.5–250.0μg/L with a determination of coefficients(R2)ranging from 0.9952 to 0.9978.The LOD(S/N=3)and LOQ(S/N=10)were found to be 0.5–1.0 and 2.5μg/L,respectively.Repeatability(intra-day precision)of the MSPE-HPLC measurements reported as RSD values(n=5)in peak areas was found to be less than 4.7%based on five-replicate analysis during 1 day at a concentration of 100μg/L.Reproducibility(inter-day precision)of the proposed method reported as RSD values(n=5 day)in peak areas was found to be less than 5.1%.
The results of a comparison made between the figures of merit of the proposed method and some of the published methods[30–33]for extraction of steroid drugs are provided in Table 2.The analytical characteristics of the developed method are compared with the other reported methods as summarized in Table 2.The linear range,repeatability and recovery among these methods are similar.However,the proposed method used markedly less organic solvents,especially when compared to the LLE.
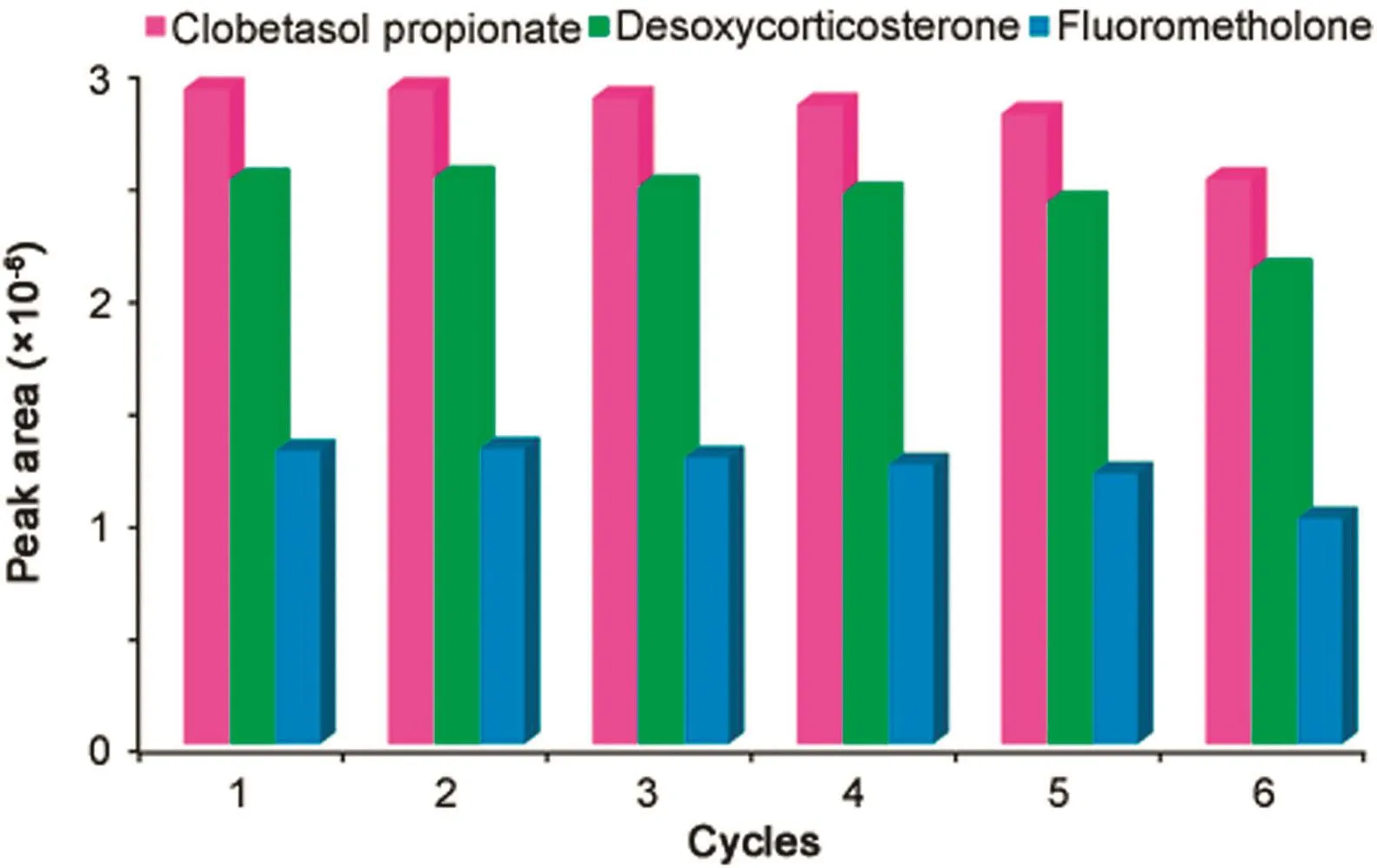
Fig.5.Reusability of the Fe3O4@Cu@b-TMP-DTPA NPs.
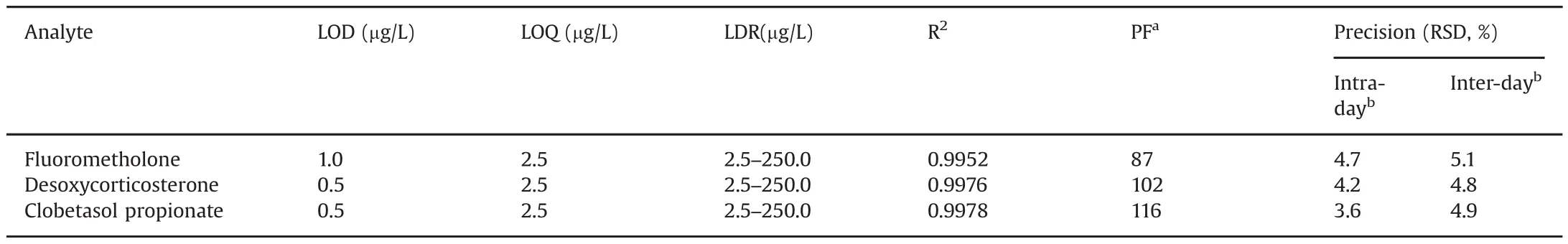
Table 1 MSPE performance and validation data.

Table 2 Comparison of developed method with the previously reported methods for the determination of steroid compounds.
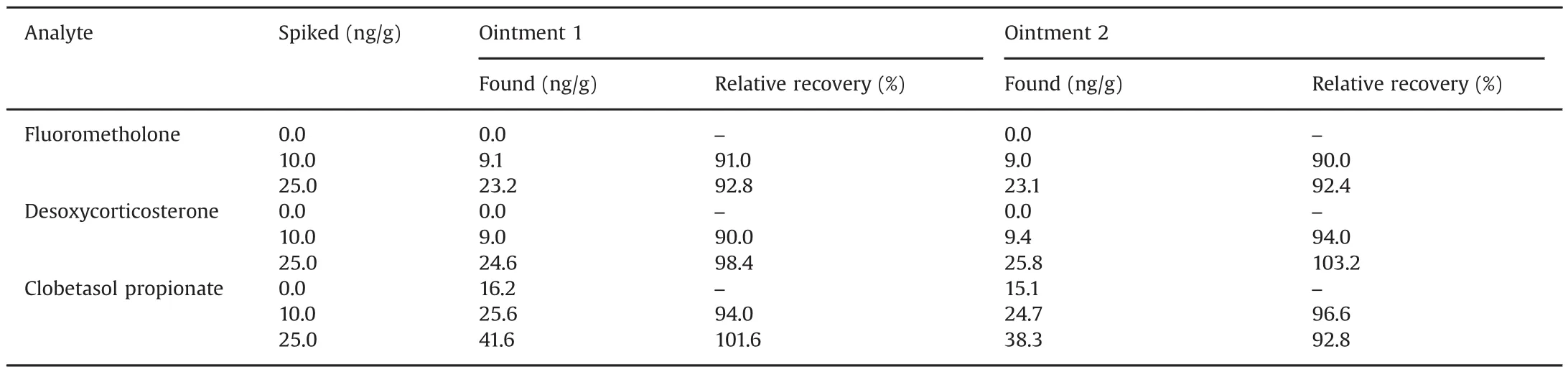
Table 3 Determination of target analytes in ointments samples by the proposed method.
3.3.2.Analysis of real samples by ultrasound assisted
To demonstrate the applicability of the MSPE adsorbent,the proposed procedure has been applied to determine three steroid drugs in ointment samples.For extraction of steroid drugs from ointments samples,20 mg of each sample was accurately weighed and separately mixed with 2.5 mL of 2-propanol and was subsequently exposed to ultrasound for 5 min.The solution was filtered through the 0.45 m cellulose acetate filter and diluted to 500 mL with deionized water in a 1000mL volumetric flask.Finally,relative recoveries of each steroid drug in ointment samples at two spiked levels(10,25 ng/g)of the target analytes were determined.According to the obtained results in Table 3,clobetasol propionate was detected in ointments samples.High relative recoveries from 90.0%to 103.2%were significantly achieved.These results demonstrated the applicability of the proposed procedure for steroid drugs determination in ointment samples.Fig.6 shows the typicalchromatogramsofthe extractedsteroid drugsfrom ointment sample before and after spiking with 10 and 25 ng/g of steroid drugs.
4.Conclusions
The core-shell Fe3O4@Cu NPs with magnetic property were synthesized by reduction of Cu2+ions in the presence of Fe3O4nanoparticles.Then thiol chemistry was used to bind the b-TMPDTPA to the surface of the magnetic NPs.The size,morphology,composition,and properties of the prepared nanoparticles have also been characterized and determined using SEM,TEM,FT-IR,VSM and TGA to show the applicability of present core shell Fe3O4@Cu@b-TMP-DTPA.It was used in MSPE for adsorbing and separating steroid compounds in ointment samples with the aid of ultrasound.The novel magnetic core-shell has the following advantages:(i)these MNPs are environmentally friendly and reusable;(ii)the surface area is binding to amine(–NH2)or thiol(–SH)terminal groups;(iii)the process of binding is easy and safe;(iv)MNPs are inexpensive.
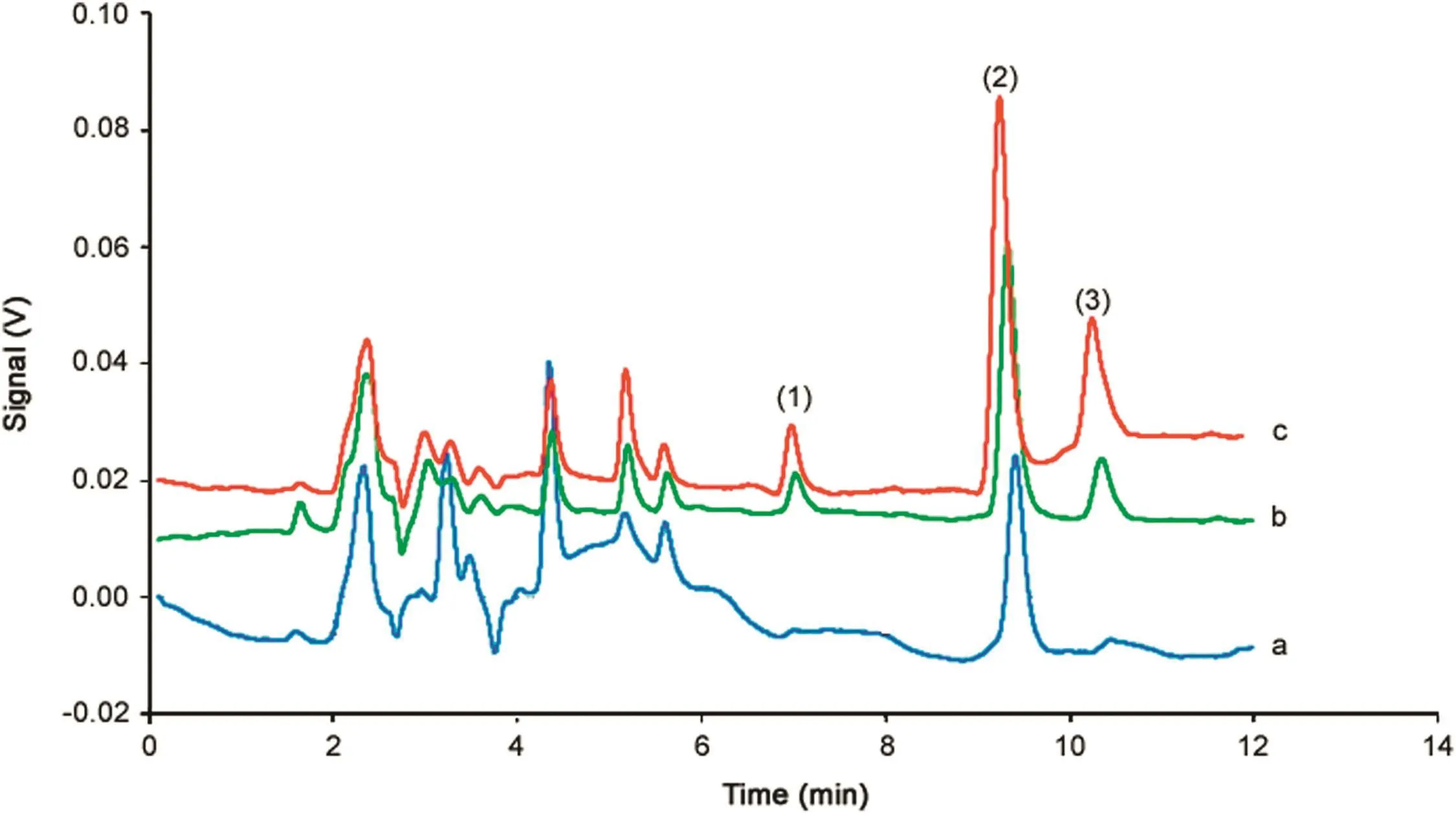
Fig.6.HPLC chromatograms obtained after extraction of ointment samples under optimal conditions:(a)nonspiked sample,(b)sample spiked with steroid drugs at concentration of 10 ng/g and(c)sample spiked with steroid drugs at concentration of 25 ng/g.Peak identification:(1)clobetasol propionate,(2)desoxycorticosterone and(3) fluorometholone.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study has been supported by grants from Tarbiat Modares University,which is hereby gratefully acknowledged.
 Journal of Pharmaceutical Analysis2018年4期
Journal of Pharmaceutical Analysis2018年4期
- Journal of Pharmaceutical Analysis的其它文章
- Quantitation of tadala fil in human plasma using a sensitive and rapid LC-MS/MS method for a bioequivalence study
- Duplex microRNAs assay based on target-triggered universal reporter hybridization
- Extracellular synthesis of silver nanoparticles by Pseudomonas sp.THG-LS1.4 and their antimicrobial application
- Screening potential mitochondria-targeting compounds from traditional Chinese medicines using a mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry method
- Unusual retention behavior of omeprazole and its chiral impurities B and E on the amylose tris(3-chloro-5-methylphenylcarbamate)chiral stationary phase in polar organic mode
- Recent advances in screening of enzymes inhibitors based on capillary electrophoresis
