Recent advances in screening of enzymes inhibitors based on capillary electrophoresis
Mengxia Cheng,Zilin Chen
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery,Ministry of Education,and Wuhan University,School of Pharmaceutical Sciences,Wuhan 430071,China
Keywords:Capillary electrophoresis Enzyme inhibitor screening Pre-capillary enzyme assays Electrophoretically mediated microanalysis Immobilized enzyme microreactor
ABSTRACT Capillary electrophoresis with many advantages plays an important role in pharmaceutical analysis and drug screening.This review gives an overview on the recent advances in the developments and applications of capillary electrophoresis in the field of enzyme inhibitor screening.The period covers 2013 to 2017.Both the pre-capillary enzyme assays and in-capillary enzyme assays which include electrophoretically mediated microanalysis(EMMA)and immobilized enzyme microreactor(IMER)are summarized in this article.
1.Introduction
Enzyme,a kind of biocatalyst which has high catalytic efficiency and specificity,is essential for maintaining normal life activities.Enzymes take part in the process of physiological metabolism including cells regulating homeostasis,growth and reproduction of living organisms.So some human diseases often result from abnormal regulation of enzymes,like in flammation,tumor,cardiovascular disease,central nervous system disease,and infectious disease.Therefore,we can treat these diseases with targeted therapy by intervening in enzyme pathway and pathologic expression[1,2].It has been revealed that enzymes are potential drug targets[3–9].One situation is that the enzyme expression level is higher than the expected value,resulting in disturbed body functions.One ofthe therapeutic ideas is restricting the enzyme activity by enzyme inhibitors.So the study and development of enzyme inhibitors is one way to find new drugs.Therefore,it is important to study effective methods to screen enzyme inhibitors for the treatment of human diseases.
Ultraviolet(UV)and fluorescence spectrophotometry,electrochemical method and high performance liquid chromatography(HPLC)are conventional methods,which are usually used as analytical tools to screen enzyme inhibitors.But these methodshave some disadvantages.UV and fluorescence spectrophotometry are only suitable for the situation in which substrates and products have significant difference in their spectrometric properties.The fluorescence signal could also be interfered by background absorption and auto- fluorescence in biological samples[10].Electrochemical method requires that substrates and products have electrochemical activity.HPLC can avoid these defects from the above methods.However,long elution time in HPLC limits high efficiency of inhibitor screening and the consumption of large amounts of organic solvent increases the cost.
Capillary electrophoresis(CE)has been widely applied in various fields including enzyme inhibitor screening,as it has many advantages such as high separation efficiency,quick analysis,low sample consumption,low solvent volume[11,12],automation and ability to be combined with various detection techniques[13]including UV,laser induced fluorescence(LIF)[14,15],electrochemical detectors[16,17]and mass spectroscopy(MS)[18,19].
In general,the methods for screening enzyme inhibitors by CE can be divided in two categories:(i)pre-capillary enzyme assays in which the enzymatic reaction takes place off-line and the capillary is just used as the separation channel;(ii)in-capillary enzyme assays in which enzymatic reaction takes place on-line and sampling,reaction,separation,and detection can be integrated into a single capillary.Electrophoretically mediated microanalysis(EMMA)and immobilized enzyme microreactor(IMER)are included in in-capillary enzyme assays.These methods are summarized in this review.
This review covers the literatures and gives an overview of the recent advances in the developments and applications of these methods in the field of enzyme inhibitor screening,over the period from 2013 to late 2017,which is a continuation of previous reports[20,21]and a supplement of recent reports[22,23].
2.Pre-capillary enzyme assays
In pre-capillary enzyme assays,incubation reaction and sample analysis are separate.The enzymatic reaction is initiated by mixing substrate,enzyme and cofactor,and off-line incubated for a specific time.After the incubation reaction is stopped,the reaction mixture is injected into the CE system for subsequent separation and detection.This method is easy to realize because enzymatic reaction is easy to carry out outside the CE system and there is no need to specifically modify separation capillary.And incubation reaction and sample analysis can be performed without mutual interference under respectively optimized conditions.Therefore,this method has been applied to enzyme inhibitor screening,determination of enzyme activity and kinetics,and drug metabolism studies for many years.
Iqbal et al.developed a pre-capillary enzyme assay for characterization and inhibition study of bovine carbonic anhydrase(CA)II.It was the first time to carry out CA enzyme assay by CE.The enzyme and substrate with or without inhibitors were added in a vial.After incubation at 37°C for 10 min,the reaction was terminated by freezing the reaction mixture.The reaction mixture was injected into the capillary by applying 0.5 psi for 5s,followed by the application of 15 kV voltages for separation of substrate and product.The developed method was used to determine the Michaelis-Menten kinetics of CA and inhibition constant of furosemide,a standard inhibitor of CA.The result showed it was a fast and efficient pre-capillary CA inhibitor screening method[24].Iqbal's group also applied a similar pre-capillary enzyme assay for the characterization and inhibition study ofα-glucosidase.The results obtained with the established method were in excellent agreement with data from other literatures[25].Zhang et al.combined high performance purification of HPLC with enzyme assay of CE to screen inhibitors of mammalian target of rapamycin from natural product extracts.This method facilitated in finding bioactive components among minor constituents of natural extracts[26].
The real-time analysis of the metabolism of 6-mercaptopurine by xanthine oxidase in the absence and presence of inhibitor allopurinol was evaluated using CE by Lu et al.It is worthy for us to study metabolism of 6-mercaptopurine,which is a drug used in the treatment of acute lymphatic leukemia,Crohn's disease,and ulcerative colitis[27,28].Nehméet al.used the pre-capillary enzyme assay to evaluate tau phosphorylation by the glycogen synthase kinase 3-βfor the first time.This work showed that it is possible to follow phosphorylation of tau in vitro by CE assays in a rapid and safe manner.It also showed its potential to screen different modulators(inductors or inhibitors)of tau phosphorylation which may efficiently treat neurodegenerative diseases[29].Malina et al.carried out pre-capillary phosphofructokinase-1(PFK-1)catalytic reaction which is about the rate-limiting step of glycolytic pathway in polypropylene microcentrifuge tubes.Inhibition study of PFK-1 was performed by aurintricarboxylic acid.The separation by CE avoided the spectral interference from inhibitors[30].Nowak et al.revealed the potential of the CE-based enzyme assay in plant membrane enzyme-chlorophyllase.The reaction was conducted in a vial placed directly on the thermostated sample tray.While the incubation was happening,the separation and detection were simultaneously carried out.The product accumulation could be monitored at any time.The proposed method demonstrated a great potential of CE as a convenient analytical tool for monitoring enzyme reaction progress,determining activity of membrane enzymes and screening enzyme inhibitors[31].
Sun et al.developed a facile chiral ligand exchange capillary electrophoresis(CLE-CE)system with Zn(II)-L-alanine as the chiral selector in the presence ofβ-cyclodextrin for enantioseparation of dansyl amino acids.It was applied to study the activity of tyrosinase and determine the inhibitory effect of kojic acid on tyrosinase.The results implied that the proposed CLE-CE system was a useful tool for studying enzymatic reaction of tyrosinase,investigating the substrate specificity,and providing a new strategy for screening tyrosinase inhibitors[32].A new CLE-CE system with L-leucine as the chiral ligand,Zn(II)as the central ion andβ-cyclodextrin as the additive was developed by Su et al.The substrates of tyrosinase,D,L-tyrosine were well separated.The method has been applied in tyrosinase inhibitor screening with chalcones as the model compounds[33].Then Su et al.constructed dual chiral selectors with Mn(II)–[1-butyl-3-methylimidazolium][L-alanine]amino acid ionic liquids(AAILs)complex and β-cyclodextrin.The CLE-CE system was further developed for enantioseparation of dansyl D,L-amino acids and applied to screen tyrosinase inhibitors[34].In addition,new kinds of AAILs with pyridinium as cations and L-lysine as anion were also developed as the available chiral ligands coordinated with Zn(II)in CLE-CE by Sun et al.This system was successfully applied to study the specificity of substrates and the kinetic constants of L-amino acid oxidase[35].
Some pre-capillary enzymatic reactions were mainly carried out with free enzyme solutions.These methods were often hampered by the low operational stability and brought difficulties in recovery and reuse of enzymes.In addition,separation and detection of substrates and products sometimes can be interfered by free enzymes.Therefore,it was significant to explore a simple and efficient approach to improve the stability and reusability and reduce interference.Mu et al.provided a facile and efficient approach to fabricate the enzyme functionalized magnetic nanoparticles modified by a reactive polymer poly(glycidyl methacrylate)[36].The immobilized D-amino acid oxidase(DAAO)presented high enzyme activity,good reusability and satisfactory stability.A CLE-CE system with AAIL as the chiral ligand was applied to screen inhibitors of DAAO which is related to schizophrenia[37].Zhang et al.prepared an IMER in capillary which was just used for enzymatic reaction.Gold nanoparticles(AuNPs)were covalently attached to surface of the pores of the porous polymer capillary monolith via the formation of an Au–S bond.And αglucosidase was then immobilized onto AuNPs through the strong affinity of gold with amino groups of the enzyme.Substrate solution was injected by the syringe and the ef fluent was collected for the subsequent analysis by CE[38].
Therearesomelimitationsto thepre-capillary mode.Firstly,the enzymatic reaction which is very fast must be terminated by adding quenching reagents or changing the reaction conditions before analysis by CE system.Secondly,although only nanoliter-scale sample is consumed by CE,the precapillary enzyme assay requires a large number of reactants to initiate the reaction,which would cause the waste of reagents especially for those expensive enzymes.Thirdly,enzymatic reaction and analysis by CE are separate in the pre-capillary mode which increases complexity of operation and is also time consuming.
3.In-capillary enzyme assays
In in-capillary enzyme assays,the capillary can be used not only as a separation channel but also as a microreactor.Injection of reactants,mixture,enzyme reaction,separation and detection of analytes can be integrated into a single capillary,which improves the analysis efficiency.The in-capillary enzyme assay realizes automatic operation,short analysis time,low consumption of sample and low cost[39].It can be divided into two categories:electrophoretically mediated microanalysis(EMMA)and immobilized enzyme microreactor(IMER).
3.1.EMMA
Bao and Regnier[40] firstly reported the in-capillary enzyme assay known as electrophoretically mediated microanalysis(EMMA),in which the reactants were mixed and the enzymatic reaction was triggered by utilizing the difference of electrophoretic mobility of each reactant under electric field.EMMA was generally divided into two major modes according to the pattern of sampling and mixing:the continuous engagement EMMA(long contact mode)and transient engagement EMMA(plug-plug or short contact mode).
In the long contact mode,the capillary is initially completely filled with either substrate or enzyme and the second reactant is introduced as a plug(zonal sample introduction)[40]or in continuous flow(moving boundary sample introduction)[41].The product was continuously produced after applying the voltage which brings the electrophoretic mixing of enzyme and substrate.
In the plug-plug mode,plugs of enzyme and substrate are consecutively introduced into the capillary.The enzymatic reaction is initiated by the application of voltage,because the difference of their electrophoretic mobility under electric field brings interpenetration among zones.It has been the most commonly used EMMA mode because of less consumption of reactants compared with long contact mode.However,in classical plug–plug mode,the buffer is required to be suitable for enzymatic reaction and separation,which limits its application when the separation buffer is incompatible with the enzymatic reaction buffer.To solve this problem,additional plugs of incubation buffer were injected between reactants and running buffer.This approach is termed as“partial filling mode”,as shown in Fig.1A[42].
In the typical EMMA,reactants were mixed by difference of electrophoretic mobility under electric field.However,its application is limited when some enzymes are sensitive to the electric field or electrophoretic mobility of some reactants is similar.In these cases,techniques of longitudinal diffusion and transverse diffusion of laminar flow profiles(TDLFP)are applied to mix enzyme and substrate at the inlet of the capillary.The techniques have no requirements for electrophoretic mobility of reactants and reactants were mixed by simple diffusion.The schematic of the three mixing modes of reactants in the capillary is shown in Fig.2.
EMMA is a very useful tool for the study of enzyme kinetics and inhibition,and screening enzyme inhibitors.Pochet et al.determined the inhibitory potency of argatroban toward thrombin by EMMA combined with short-end injection,partial- filling mode and rapid polarity switching technology[43].The mixing method which is performed by alternative application of positive and negative potentials to move the sample plugs back and forth in the capillary is termed rapid polarity switching[44].The schematic diagramsofshort-end injection and rapid polarityswitching technology are shown in Figs.1B and C.Nehméet al.developed the in-capillary enzyme assay,which was mixed by TDLFP to screen protein kinase inhibitors.It was the first time for those four human kinases to be assessed by CE.Compared with conventional radiometric enzyme assays,this method was eco-friendly since no radioactivity was required[45].Later,Nehméet al.developed a TDLFP-based method again in order to assess tau phosphorylation and to study the effect of modulators such as glycosaminoglycans,heparin sulfate and heparin on phosphorylation[29].Wang et al.combined EMMA and LC–MS/MS for screening of protein kinase inhibitors in natural extracts.Baicalin was successfully screened as an inhibitor of protein kinase A with the method[46].
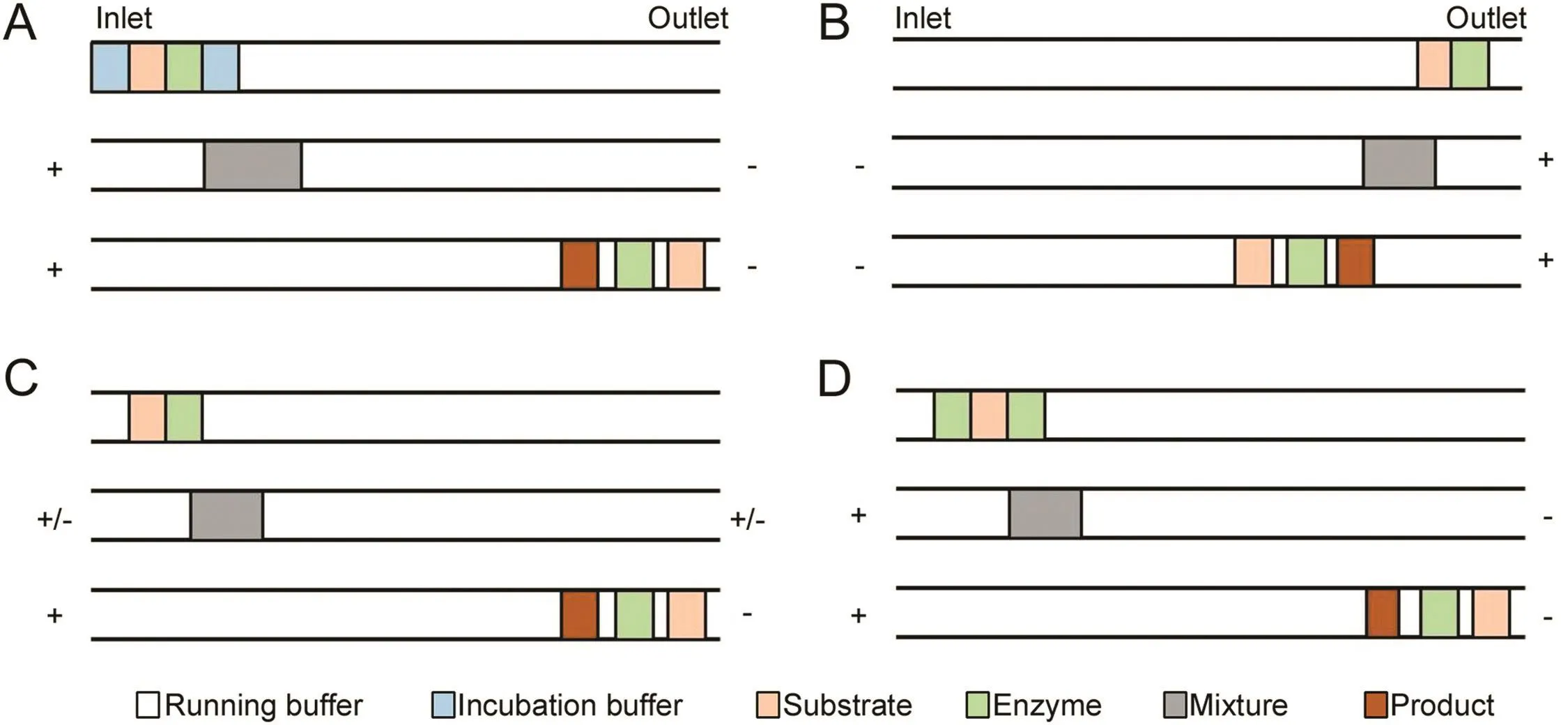
Fig.1.EMMA combined with partial filling mode(A),short-end injection(B),rapid polarity switching technology(C),and sandwich mode(D).
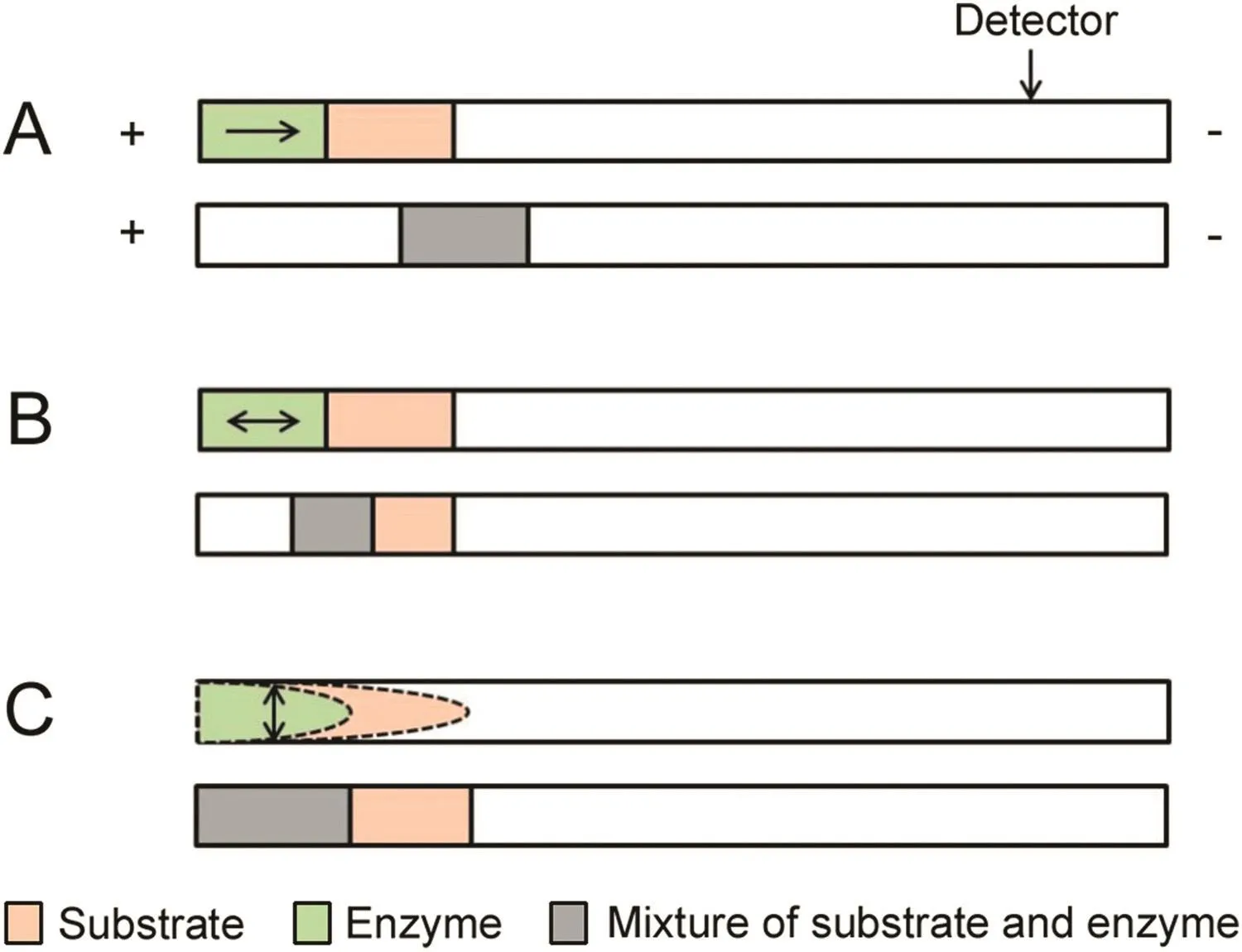
Fig.2.Mixing of reactants in the capillary:mixing by electrophoresis(A),longitudinal diffusion(B),and TDLFP(C).
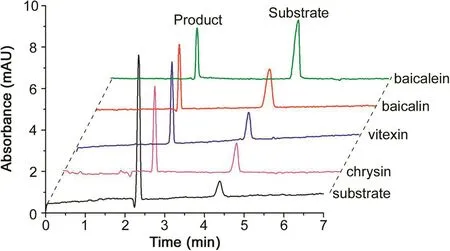
Fig.3.The overlaid electropherograms for neuraminidase inhibitor screening by online enzymatic inhibition assay.The figure was reproduced with permission from Ref.[48].
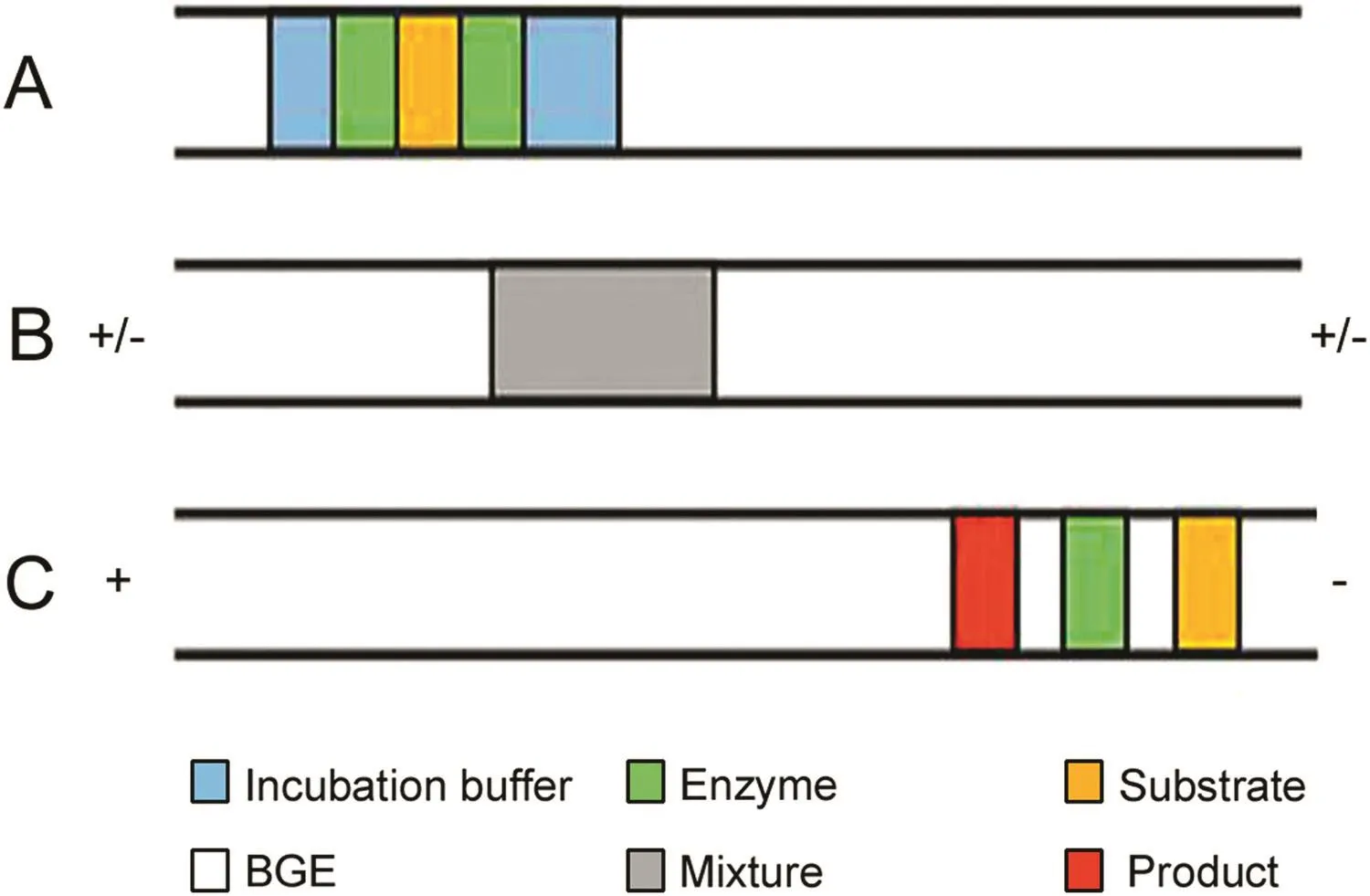
Fig.4.Schematic overview of EMMA.(A)Sequential injection of the incubation buffer,tyrosinase,L-DOPA,tyrosinase,incubation buffer,BGE.(B)Mixing by applying a voltage switch sequence.(C)Mixture separation and product detection.The figure was reproduced with permission from Ref.[49].
Our group has made a great contribution to the development of EMMA in enzyme inhibitor screening.Zhao and Chen developed an EMMA method with partial filling mode for screening aromatase inhibitors in traditional Chinese medicine(TCM).A long plug of coenzyme reducedβ-nicotinamide adenine dinucleotide 2′-phosphate hydrate was hydrodynamically injected into a fused silica capillary followed by the injection of reaction buffer,enzyme,and substrate solution.The reaction was initiated with a voltage of 5 kV for 40 s.Then the voltage was switched off for 20 min to increase the product amount and switched on again to separate all the analytes at the voltage of 20 kV.With the method,seven compounds were found to have potent inhibitory activity for aromatase[47].Then Zhao and Chen screened neuraminidase inhibitors by in-capillary enzyme assays again.The enzyme,substrate and inhibitors were sequentially injected,mixed by TDLFP,incubated and separated in the same capillary.To enhance the efficient mixture of reactants,+5 kPa and-5 kPa at the capillary inlet was alternately applied.To eliminate the interference from natural compounds,dual-wavelength detection was employed.The method has been applied to determine the kinetic constant of neuraminidase and four compounds have been screened as neuraminidase inhibitors,as shown in Fig.3[48].When it is hard to get the electrophoretic mobility of the reactants,sandwich mode injection was applied to further facilitate mixture of reactants.Its injection sequences are like substrate-enzyme-substrate or enzyme-substrate-enzyme(Fig.1D).As shown in Fig.4,Tang et al.integrated EMMA with sandwich mode,partial filling,and rapid polarity switching technique to screen tyrosinase inhibitors from TCM[49].Han and Chen developed a cathepsin B inhibitor screening method that integrated longitudinal diffusion and TDLFP to efficiently mix reactants.Twelve potential compounds were evaluated and dauricine showed inhibitory potential for cathepsin B.This work provides a new approach to screen cathepsin B inhibitors[50].
Besides enzyme inhibitor screening,EMMA can also be used for the determination of enzyme activity and stereospecificity of enzymes,the understanding of enzyme-mediated metabolic reactions and other determination which is related to enzymatic reaction.Nowak et al.applied the EMMA method to realize routine,high-throughput and cost-effective assessment of the activity of plant membrane enzyme chlorophyllase[31].Harada et al.developed an in-capillary enzyme assay for the determination of enzyme activity of lignin peroxidase using micellar electrokinetic chromatography(MEKC)for separation of reaction mixture,which combined with sandwich mode and partial filling mode for injection.This method is more sensitive than conventional spectrophotometry since the background originating from the enzyme and the culture medium can be removed via MEKC separation[51].Zhu et al.developed EMMA combined with partial- filling mode to determine the stereoselective reduction of L-methionine sulfoxide diastereomers by methionine sulfoxide reductase(Msr)and validated using fluorenylmethyloxycarbonyl(Fmoc)-L-methionine sulfoxide as substrate.The assay was applied for the analysis of stereoselective reduction of Fmoc-L-methionine-(S)-sulfoxide by human MsrA and of the Fmoc-L-methionine-(R)-sulfoxide by MsrB.It was demonstrated for the first time that Fmoc-L-methionine-(R)-sulfoxide is a substrate of MsrB[52].An enzyme mediated fluorescence-amplification method by EMMA with LIF detector was developed for glucose quantification in human serum samples by Guan and Zhou.The samples spiked with a novel fluorescent reagent named 2-[6-(4′-amino)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid(APF)and the mixed enzyme solutions of glucose oxidase and horseradish peroxidase(HRP),were individually injected into the capillary with the sandwich mode[53].Then they developed a similar method again to determine the plasma total cholesterol.In this study,the diluted samples were also spiked with APF and the mixed enzyme solutions contained cholesterol oxidase and HRP[54].All recent studies related to EMMA are summarized in Table 1.
3.2.IMER
IMERs mean that the enzyme is immobilized in the first part of the capillary for enzymatic reaction,while the remaining part of the capillary is used for separation of analytes.Compared with the enzymatic reaction in solution,the immobilized enzyme has someadvantages.First,the immobilized enzyme is reusable which avoids the waste of enzyme and reduces the experimental cost.Second,the stability of the immobilized enzyme may be improved.Third,the efficiency of enzymatic reaction can be increased.Fourth,because the enzyme is immobilized,adsorption of the enzyme on the inner wall of capillary can be avoided,which is helpful to increase reproducibility of separation.But the IMERs are not suitable for enzymatic reaction system in which the incubation buffer and separation buffer are different.In addition,preparation of IMERs is complicated and different immobilization methods have their own defects.Generally,there are three methods for enzyme immobilization:physical adsorption,covalent attachment and encapsulation[55].Physical adsorption has advantages of low cost,easy operation,mild immobilization conditions and moderate harm for the enzyme.And the capillary can be reused by rinsing with NaCl,HCl and NaOH.Enzymes immobilized by covalent attachment have good stability,but the activity of enzymes may decrease because of chemical bonding.Encapsulation brings large enzyme adsorption and keeps high enzyme activity[56,57],but the operation is complicated and hard.
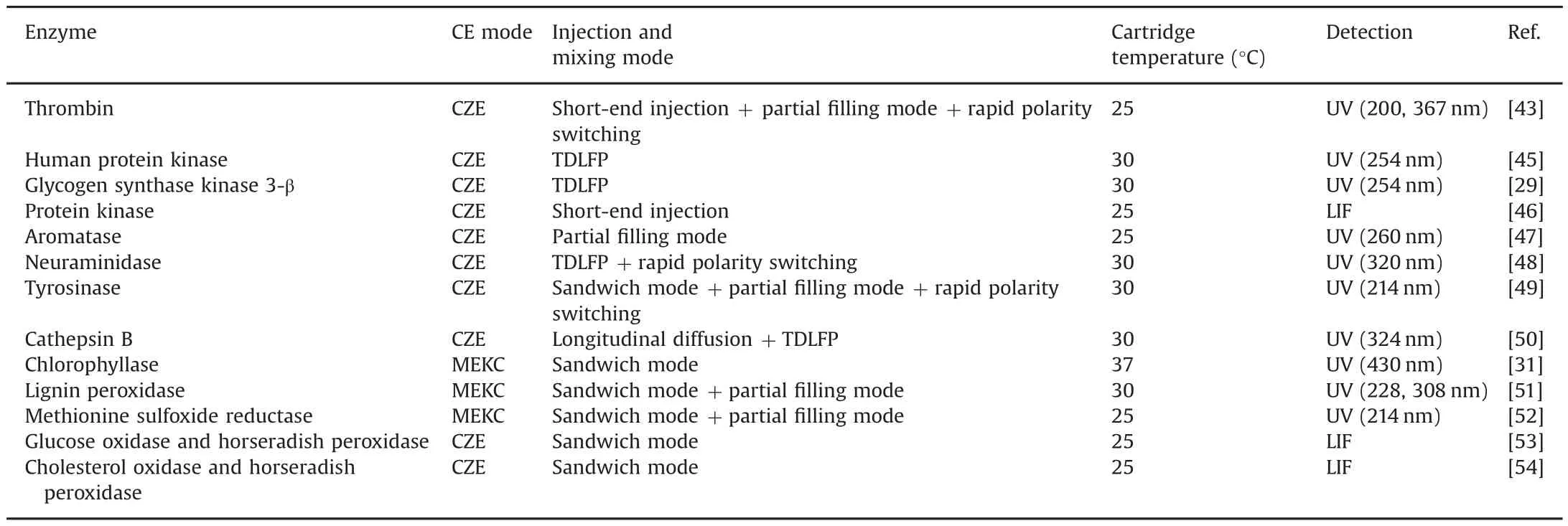
Table 1 Summary of electrophoretically mediated microanalysis.
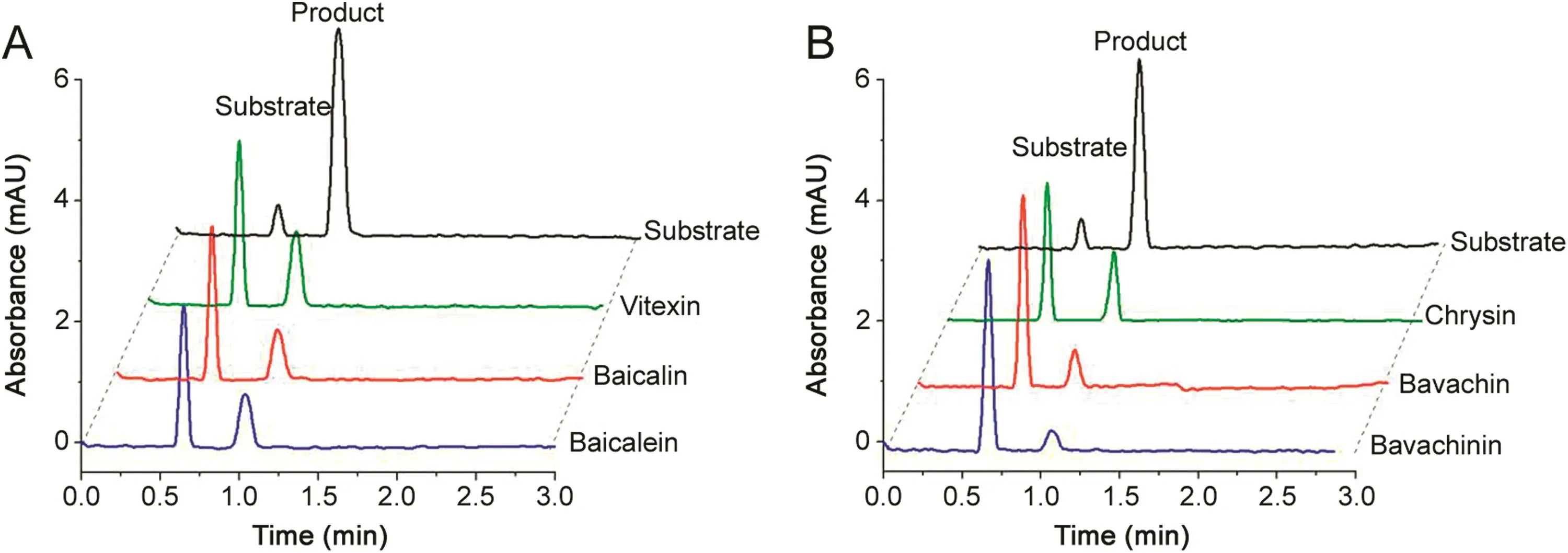
Fig.5.Overlaid electropherograms for neuraminidase inhibitor screening by on-line immobilized enzyme assay detected at 320 nm with reference wavelength of 260 nm(A)and at 320 nm without reference wavelength(B).The figure was reproduced with permission from Ref.[63].
IMERs are a good tool to study enzyme assays.Min et al.established an efficient trypsin IMER at the inlet of the capillary by glutaraldehyde cross-linking technology.The method was successfully used to study the enzyme kinetics of trypsin and on-line screen trypsin inhibitors from 19 kinds of natural extracts[58].Jiang et al.created a tyrosinase IMER based on layer-by-layer assembly for inhibitor screening.Tyrosinase was immobilized on the surface of fused silica capillary via ionic binding technique with cationic polyelectrolyte hexadimethrine bromide(HDB).Then,HDB solution with the same plug length as the tyrosinase was introduced again into the capillary to cover the immobilized tyrosinase by forming HDB-Tyrosinase-HDB sandwich-like structure.The inhibition kinetics of the immobilized tyrosinase was studied by the method[59].Camara et al.developed a multilayer IMER by layer-by-layer electrostatic assembly which can easily enhance theglucose 6-phosphate dehydrogenase(G6PDH)loading capacity.After poly(diallyldimethylammonium chloride)(PDDA)was modified on the inner surface of the capillary to create a positively charged coating,G6PDH enzyme solution was injected into the capillary to make negatively charged G6PDH electrostatically absorb on the PDDA layer.A multilayer IMER was produced by repeating the above procedures to coat PDDA and G6PDH.With the method,on-line inhibition of several green tea catechins on the G6PDH enzyme was investigated[60].Schejbal et al.developed a novel IMER based on magnetic microparticles for study of pharmacologically important cytochrome P450 2C9(CYP2C9).The CYP2C9 was attached to magnetic SiMAG-carboxyl microparticles using the carbodiimide method.The formation of an IMER at the inlet end of the capillary was ensured by two permanent magnets fixed in a cassette from the CE apparatus in the repulsive arrangement.The IMER was applied for on-line kinetics and inhibition study of CYP2C9 with diclofenac as a model substrate and sulfaphenazole as a model inhibitor[61].
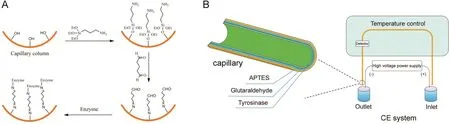
Fig.6.Procedure for immobilizing tyrosinase on capillary inner wall(A)and schematic of tyrosinase IMER assay(B).The figure was reproduced with permission from Ref.[64].
A dual-enzyme co-IMER was constructed by Lin et al.The adenosine deaminase and xanthine oxidase were firstly immobilized on AuNPs,and the functionalized AuNPs were then assembled on the inner wall of the inlet end which was treated with polyethyleneimine.The developed method could be used for on-line simultaneous screening of multiple enzyme inhibitors.This co-immobilized enzyme technology contributes to highthroughput screening of enzyme inhibitors and provides a new strategy for discovering multitargeted enzyme inhibitors as drug lead compounds[62].
Our group has been studying the CE-based IMERs for many years and has made some great achievements.Zhao and Chen fabricated a simple and effective neuraminidase IMER by glutaraldehyde cross-linking technology for screening neuraminidase inhibitors which are effective for in fluenza type A and B viruses in humans,avians and animals.With short-end injection mode,the substrate and product were separated by CE within 2 min.As shown in Fig.5,six compounds were found as potent inhibitors[63].Cheng and Chen prepared a tyrosinase IMER with the same method for screening tyrosinase inhibitors,as shown in Fig.6.Four compounds including quercetin,kaempferol,bavachinin,and bakuchiol from 15 compounds of TCM were found to have inhibitory potentials.Meanwhile,molecular docking study was carried out to further demonstrate their inhibitory potential on the basis of molecular interaction between the enzyme and inhibitors.As shown in Fig.7,it was obvious that bakuchiol fitted well into the binding pocket and several key interactions were observed[64].Cheng and Chen developed a trypsin IMER at the inlet of capillary treated with polydopamine(PDA).Based on strong noncovalent adsorption of PDA and skillful use of PDA for many years in our group,the trypsin IMER was successfully established for the first time and had good stability.Baicalin was screened as a trypsin inhibitor and molecular docking study well supported the experimental result[65].
Enzymatic reactions in IMERs are used not only for enzyme inhibitor screening but also for other applications.An IMER using graphene oxide as support was developed based on layer-by-layer electrostatic assembly by Yin et al.Angiotensin and bovine serum albumin(BSA)were successfully digested by the CE-based IMER[66].Liu et al.fabricated a novel CE-based IMER by particlepacking technique.The IMER was accomplished by utilizing largepore beads as the enzyme supports and perfusive silica single particles as the frits.The IMER was successfully applied for accurate analysis of trypsin inhibition and on-line digestion of standard proteins(myoglobin and BSA)[67].All recent studies about IMER mentioned above are summarized in Table 2.
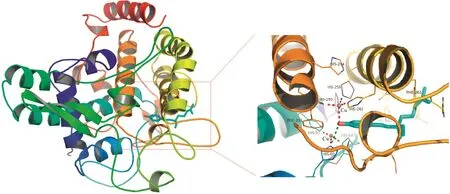
Fig.7.Binding mode of bakuchiol(in stick model)and the active site cavity of tyrosinase(PDB ID:2Y9X).The hydrogen-bonding interaction is shown in the green dashed lines.The interactions between Cu and residues are shown in red dashed lines.The figure was reproduced with permission from Ref.[64].
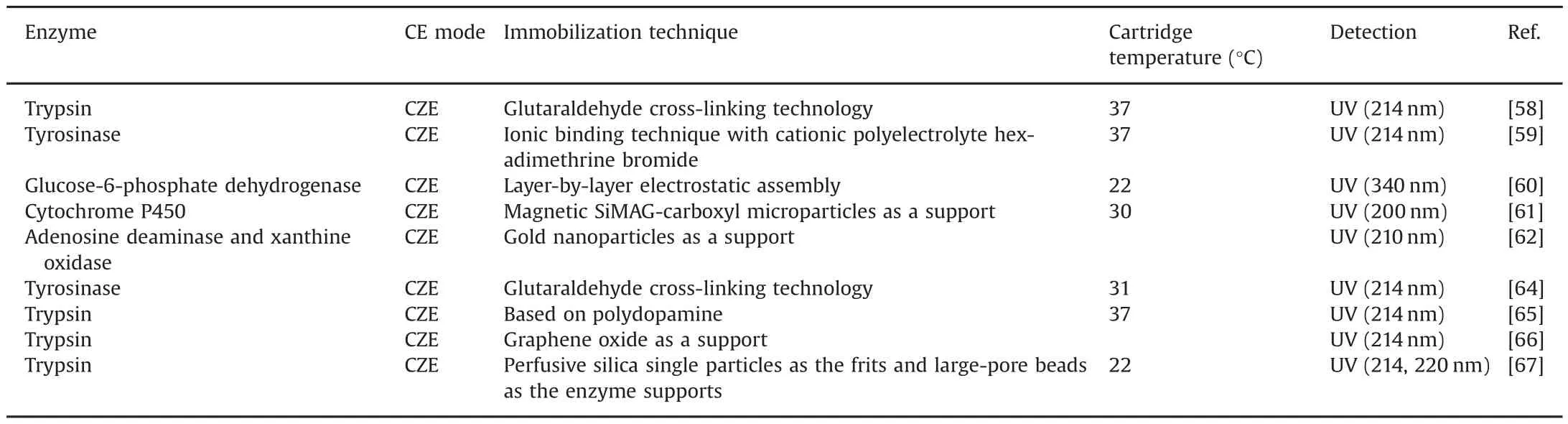
Table 2 Summary of immobilized enzyme microreactors.
4.Conclusions
As can be concluded from the literature collected in the present review article,over past years great advances have been made in the CE-based enzyme inhibitor screening.In pre-capillary assays,the enzymatic reaction conditions and separation conditions can be optimized separately,which is universal for most enzymes.In the mode of in-capillary assays,the sampling,mixture,reaction,separation and detection are integrated into one single capillary,which simplifies the operation,reduces reagent consumption,shortens analysis time,facilitates the automatization and miniaturization of enzymatic assays and realizes high-throughput enzyme inhibitor screening.With the rapid development of CE-based enzyme inhibitor screening,the CE will be more widely applied to enzyme assays for study of enzyme kinetics,understanding of enzyme-mediated metabolic reactions,study of rapid peptide mapping in proteomic and so on.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China(Grant nos.81573384 and 21375101).
 Journal of Pharmaceutical Analysis2018年4期
Journal of Pharmaceutical Analysis2018年4期
- Journal of Pharmaceutical Analysis的其它文章
- Quantitation of tadala fil in human plasma using a sensitive and rapid LC-MS/MS method for a bioequivalence study
- Duplex microRNAs assay based on target-triggered universal reporter hybridization
- Extracellular synthesis of silver nanoparticles by Pseudomonas sp.THG-LS1.4 and their antimicrobial application
- Simultaneous determination of steroid drugs in the ointment via magnetic solid phase extraction followed by HPLC-UV
- Screening potential mitochondria-targeting compounds from traditional Chinese medicines using a mitochondria-based centrifugal ultra filtration/liquid chromatography/mass spectrometry method
- Unusual retention behavior of omeprazole and its chiral impurities B and E on the amylose tris(3-chloro-5-methylphenylcarbamate)chiral stationary phase in polar organic mode
