Effect of Red Ginseng Extract on the Pharmacokinetics of Aspirin Metabolite in Sprague Dawley Rats
Yutao Xue, Ning Tan, Gangyan Yu, Li Tan, Yang Lu
Pharmaceutical Department, School of Chinese Materia Medica,Beijing University of Chinese Medicine, Beijing 100029, China
Key words: red ginseng extract; aspirin; salicylic acid; pharmacokinetics
CARDIOVASCULAR and cerebrovascular diseases (CCVD) are the leading cause of mortality for middle-aged and elderly people.1The best way to reduce the incidence
of CCVD is to give appropriate medicines. It has been proved that aspirin is effective for primary and secondary prevention of CCVD. Long-term use of low dose aspirin (75-150 mg) can distinctly reduce the risk of many vascular diseases.2,3It has been suggested that people aged 40 to 69 should use aspirin chronically for primary CCVD prevention.4,6Oral administration of aspirin is rapidly converted into active metabolite salicylic acid after absorption, so it is difficult to detect in-vivo aspirin rapidly and accurately. Therefore, pharmacokinetics of aspirin is often studied by measuring the concentration of salicylic acid in blood.7,8
Red ginseng is produced by steaming and drying from ginseng (the root of Panaxginseng C.A. Mey.). It has been generally used in the older people for health care as a tonic medicine and nutraceutical food in Asian countries, such as China, Korea and Japan. It has been estimated that about 10% people take red ginseng everyday and more than 50% people eat red ginseng every month in Korea.9As a nutraceutical food,red ginseng has many beneficial properties, including anti-inflammation, anti-hyperlipidemic, anti-thrombotic,anti-platelet, anti-aging and anti-oxidative.10-14In practice, many middle-aged and elderly people in Asia often use aspirin and red ginseng together. However, there is no related report on the effects of drug combination. In this study, pharmacokinetics of salicylic acid after co-administering aspirin with red ginseng extract to Sprague Dawley (SD) rats was investigated.
MATERIALS AND METHODS
Drugs and reagents
Standard salicylic acid (Lot No. 100106-201104) was provided by the National Institute for Food and Drug Control, China. Aspirin powder was purchased from Xi’an Yue Lai Medical Technology Co., Ltd (China). Other reagents including methanol and acetonitrile were of HPLC grade.
Red ginseng slices (Baishan, Jilin Province, China)were bought from Tongrentang Pharmaceutical Factory(China). Red ginseng extract was prepared as follows:red ginseng powder (4.8 g) diluted in 48 ml water was extracted by using refluxing extraction method for 30 minutes for twice. The decoction was concentrated up to 32 ml (equivalent to the crude drug of 0.15 g·ml-1).
Instrumentation and chromatographic conditions
Chromatography was carried out on a Shimadzu HPLC LC-20A system (Japan) equipped with a LC-20AT pump, a DGU-20A5online degasser, a SPD-20A ultraviolet-visible (UV) dual-wavelength detector, a SIL-20A auto-sampler, and a CTO-10AS VP column oven(at 30°C). Samples were separated on a Welchrom C18column (4.6 mm×150 mm, 5 μm particle size) with mobile phase consisting acetonitrile, acetic acid, and water (25:2:73, v/v/v) at a flow rate of 1.0 ml·min-1at room temperature (25°C±2°C). UV detection was performed at a wavelength of 303 nm. HPLC data were analyzed by LC solution software.
Validation of the HPLC-UV method
We investigated the parameters including specificity,linearity, accuracy, precision, extraction recovery and matrix effect, and stability to validate the reliability of the method.
Preparation of standard solution
Standard stock solution of salicylic acid was prepared by dissolving 10.03 mg of salicylic acid in 10 ml acetonitrile. The working standard solutions of salicylic acid was prepared at the concentrations of 2.508, 5.015, 10.03,20.06, 30.09, 50.15, 100.30, 200.60, and 300.90 μg·ml-1respectively by diluting stock solution in acetonitrile.
Specificity
Specificity of the proposed method was verified through chromatographic runs of blank plasma solution, salicylic acid standard solution and plasma sample solution to check the interference from the components of plasma solution and salicylic acid solution.
Linearity
The standard salicylic acid working solution of different concentrations (10 μl) was diluted with blank plasma(100 μl) and swirled for 30 seconds to reach the final concentrations of 250.75, 501.5, 1003, 2006, 3009,5015, 10 030, 20 060, and 30 090 ng·ml-1of salicylic acid respectively. The mixture solution was added to acetonitrile (190 μl), then swirled for 30 seconds and centrifugated at 2331×g for 10 minutes. Finally, 10 μl supernatant was used to quantify salicylic acid concentration by using HPLC. The limit of quantification (LOQ)was defined as the minimum level that the salicylic acid can be quantified. The limit of detection (LOD)was defined as the minimum level that the salicylic acid can be detected.
Accuracy
Accuracy of the proposed method was determined by adding known amounts of salicylic acid standard solution to plasma sample solution. The measured value of this solution was compared with the known value.Three concentration levels of salicylic acid in plasma(501.5, 3009, and 20 060 ng·ml-1respectively) were assessed, and each was analyzed in quintuplicate. Accuracy was reported as relative error (RE).
Precision
Precision of the proposed method was studied by determining intra-day and inter-day repeatability. Intra-day precision was determined by carrying out five independent assays of the test sample in a day and calculating relative standard deviation (RSD) of five standard replicates.
Inter-day precision was determined by evaluating three different concentrations of salicylic acid (501.5,3009, and 20 060 ng·ml-1, respectively) for three days and calculating RSD between three replicates for each concentration.
Extraction recovery and matrix effect
Extraction recovery and matrix effect of the proposed method was measured as follows. Firstly, three diffferent salicylic acid blank sample solutions (501.5, 3009,20 060 ng·ml-1respectively) were prepared by diluting salicylic acid standard stock solution in acetonitrile;secondly, three different salicylic acid plasma sample solutions (501.5, 3009, 20 060 ng·ml-1respectively)were obtained by adding salicylic acid standard stock solution to the blank plasma; thirdly, three different salicylic acid precipitated plasma sample solutions(501.5, 3009, 20 060 ng·ml-1respectively) were prepared by adding salicylic acid standard stock solution to the blank plasma precipitated by acetonitrile.
Extraction recovery was measured by comparing the responses of salicylic acid plasma sample solution and precipitated plasma sample solution. The matrix effect was calculated by comparing the responses of salicylic acid blank sample solution and plasma sample solution. Each level was made in quintuplicate.
Stability
Stability of the plasma sample solution was determined by comparing the responses of three different concentrations (501.5, 3009, 20 060 ng·mL-1, respectively) of salicylic acid plasma sample solution placed at different temperatures (4°C, 25°C) for 12 hours with sample that is not placed. Each level was made in quintuplicate, and RE and RSD were calculated to evaluate stability.
Grouping and drug adminstration
SD male rats (n=12) weighing 250±10 g were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. [animal license No. SCXX (JING) 2012-0001].Animals were housed in the Beijing University of Chinese Medicine Laboratory. Rats were acclimatized for 7 days before the experiments. All rats were fasted for 12 hours before experiment. All the animal studies were performed under the Guidelines for the Care and Use of Laboratory Animals and the Experimental Protocols.
They were randomly and uniformly divided into the aspirin group (n=6) and combined group (n=6).Rat in the aspirin group was intragastrically administrated 10.42 mg·kg-1aspirin (equivalent to 100 mg per 60 kg body weight, conversion factor 6.25). Rat in the combined group was given 0.5 mg·g-1red ginseng extract (equivalent to 4.8 g per 60 kg body weight,conversion factor 6.25) and 10.42 mg·kg-1aspirin by gavage.
Collection of blood samples
Blood samples (0.5 ml once) were drawn from orbit at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 10, and 12 hours after drug administration and anticoagulated with heparin.All blood samples were centrifugated at 2331×g for 10 minutes. Transfer supernatant plasma 100 μl in 0.5 ml centrifuge tube, and add acetonitrile 200 μl. The mixture was swirled for 30 seconds and centrifugated at 2331×g for 10 minutes. An aliquot of 10 μl supernatant was analyzed by using HPLC-UV.
Data analysis
Plasma concentration—time curve of salicylic acid of the aspirin group and combined group was constructed. The pharmacokinetic parameters of salicylic acid including AUC(0-t), Cmax, MRT(0-t), Tmax, t1/2z, Vz/F, and CLz/F were determined according to non-compartmental model. AUC(0-t)reflects the relative amount of the drug entering the systemic circulation; Cmaxindicates the highest concentration of the drug in the blood; Tmax(the peak time) reflects the speed at which the drug enters the body; MRT(0-t)(mean residence time) indicates the average retention time of the drug in vivo; t1/2z(half-life) refers to the time reducing the concentration of plasma drugs in half; CLz/F reflects the clear rate of the drug from the body.
Statistical analysis
All data was analyzed by using DAS 3.0 software.Pharmacokinetic parameters data were shown as the mean ± SD. The differences between groups were determined by two independent samples t-test. Linear regressive analysis was carried out to analyze the correlation between peak area and concentration of salicylic acid. P<0.05 was considered as statistically significant.
RESULTS
Parameters of methodology
In this study, we developed a simple, sensitive and ac-curate HPLC method for determination of salicylic acid in plasm. In order to observe symmetrical peaks with better mobile phase, we optimized the chromatographic conditions, such as the ratio of acetonitrile, acetic acid and water. Fig. 1 showed no interference from the components of plasma solution and salicylic acid solution was observed and the peaks of salicylic acid were obtained at retention time of 10.00 minutes.
Linear regressive analysis revealed a linear correlation between peak area and concentration in the range of 250.75-30 090 ng·ml-1for salicylic acid. The linear regression equation was Y=6.6766 X+ 899.58 with correlation coefficient equal to 0.9998, where X is the concentration and Y is the absolute peak area. The LOQ and LOD were respectively 250.8 ng·ml-1and 75.2 ng·ml-1.
The results for the accuracy and precision investigations are shown in Table 1. Values of RE and RSD were well within the acceptable range of ±15%. The results indicated the analytical methods had a good accuracy and precision.
The results (≥70%) shown in Table 2 indicated that the pretreatment of sample had a good extraction recovery and acceptable matrix effect.
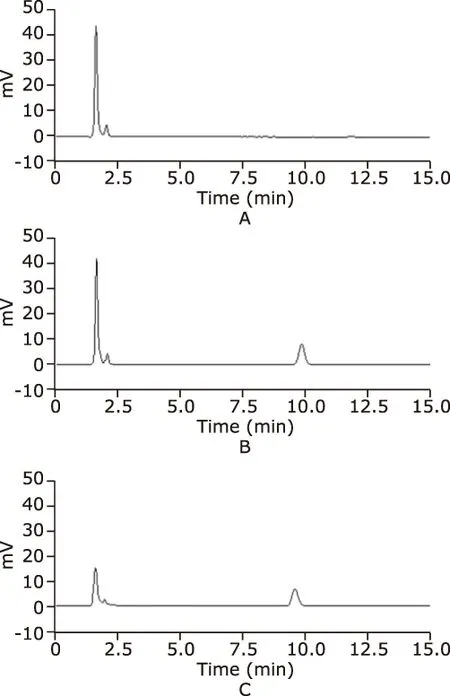
Figure 1. The representative chromatogram of blank plasma and salicylic acid solutions. A. blank plasma; B. salicylic acid standard solution; C. salicylic acid plasma solution.
Table 3 illustrated that values of RE and RSD were well within the acceptable range of ±15%, and the concentrations of salicylic acid in plasma under different conditions had a good stability.
Effects of red ginseng extract on pharmacokinetics of salicylic acid
Plasma concentration—time curves of the aspirin group and combined group are shown in Fig. 2. As demonstrated in Table 4, compared with the aspirin group,AUC(0-t)and Cmaxof the combined group increased ob-viously (all P<0.01), while CLz/F was decreased clearly(P<0.05).
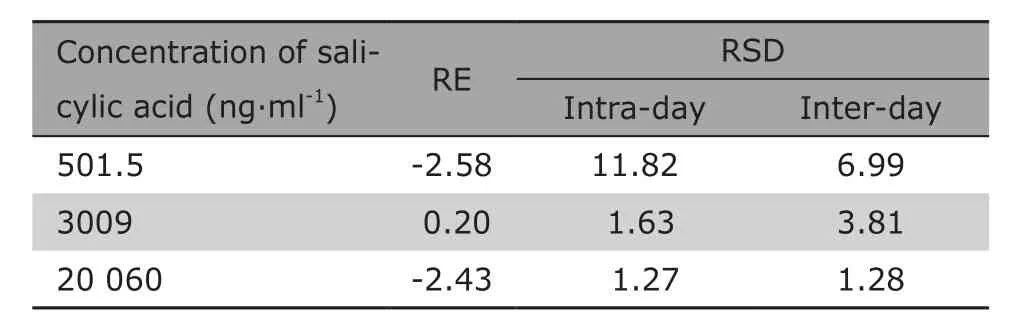
Table 1. Accuracy and precision of the method (%)
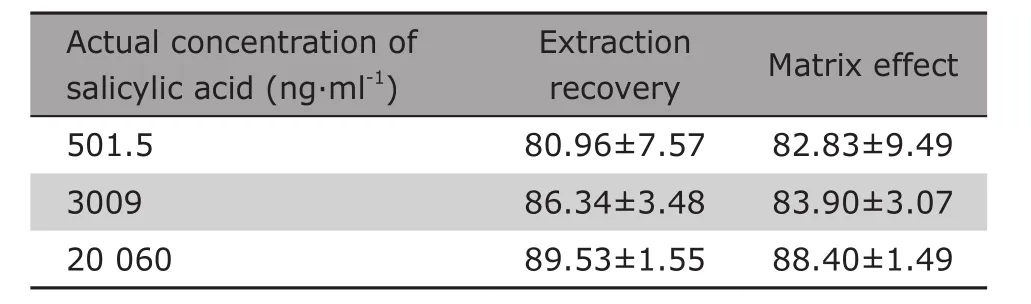
Table 2. Extraction recovery and matrix effect evaluated with salicylic acid sample solution at the three concentration levels in rat plasma§ (%)
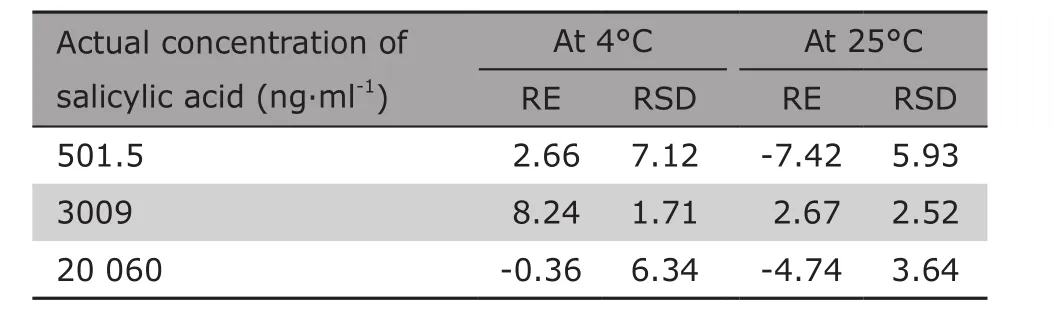
Table 3. Stability of the method determined with salicylic acid sample solution at the three concentrations in rat plasma (%)
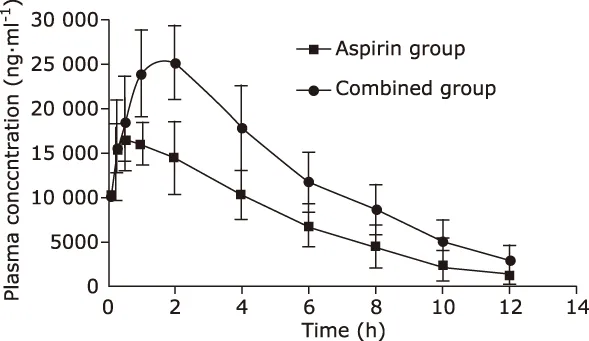
Figure 2. Plasma concentration—time curves of salicylic acid in plasma obtained from rats after intragastric administration of drugs. Data were expressed as means ± SD (n=6).

Table 4. Comparisons of pharmacokinetic parameters of salicylic acid in the plasma of the two-group rats§ (n=6)
DISCUSSION
Aspirin is widely used in the treatment of ischemic stroke, myocardial infarction, and the prevention of CCVD. During the treatment of these clinical illnesses, aspirin is often combined with other drugs.15-16In recent years, some pharmacokinetic interactions between aspirin and other drugs have been investigated.Tian et al7found that drug-drug interaction in vivo existed between aspirin and panax notoginseng saponins,the concentration of salicylic acid increased obviously when the two drugs were administered together, and the result was proved by observing the transport of aspirin and salicylic acid in MDCK-MDR1cell monolayer. In addition, Chen et al8reported that single-dose of Mailuoning injection accelerated the clearance of aspirin in rats, but this effect of multiple-dose was not distinct.
Aspirin can be easily absorbed in the gastrointestinal tract, and then quickly decomposed into salicylic acid by aspirin esterases in vivo. It has been reported that aspirin can reach the peak plasma concentration in 30 minutes after oral administration, and salicylic acid can also reach the Cmaxafter taking medicine for 1-2 hours. The elimination half-life of aspirin and salicylic acid are 20-30 minutes and 3-4 hours, respectively.17,18These data suggests that aspirin has a fast absorption and metabolism in human body.
In this study, the effects of red ginseng extract on the pharmacokinetics of aspirin in rats were investigated. When comparing the results of the aspirin group,it can be found that AUC(0-t)and Cmaxof the combined group increased clearly (P<0.01), and the calculated value of relative bioavailability of the combined group was 167.58%. These results indicated that red ginseng extract can improve relative bioavailability of salicylic acid. This study also found that CLz/F of the combined group decreased obviously (P<0.05); MRT(0-t), Tmaxand t1/2zof the combined group increased, but the differences were not statistically significant (P>0.05). By analyzing the reduction of CLz/F and the enhancement of MRT(0-t)and t1/2z, it suggests that red ginseng extract can improve the residence time of salicylic acid by reducing the speed of elimination of salicylic acid.To summarize, red ginseng extract can improve the absorption of salicylic acid and slow down its metabolism in vivo.
It can be seen from Figure 2, when plasma concentration of the aspirin group began decreasing after 30 minutes, that of the combined group still increased.It means that red ginseng extract can improve the absorption of aspirin. By consulting literature materials,it shows that saponins have cell membrane permeabilising properties by solubilizing cholesterol and can influence cellular transport and permeability as absorption enhancers.16,19,20It is supposed that the ginsenosides of red ginseng extract maybe act as absorption enhancers and make it easier for aspirin to penetrate cells in the gastrointestinal tract. Besides, this study also revealed that red ginseng extract can reduce the metabolism of salicylic. Because cytochrome P450 2C9(CYP2C9) is generally thought to be related to the metabolism of aspirin,21,22and ginsenosides can inhibit the activities of CYP2C9.23It is suspected that red ginseng extract may inhibit the activities of CYP2C9 and then retard the metabolism of salicylic acid. The specific mechanism of absorption and elimination will be further studied.
In clinical practice, low dose of aspirin (75-150 mg daily) is an effective antiplatelet regimen for longterm use, however, aspirin can also injury human gastric epithelial cells (GES-1) in a concentration-dependent and time-dependent manner, and long-term use of aspirin can increase the risk of upper gastrointestinal bleeding.24-26It is indicated that red ginseng extract can apparently accelerate the absorption of aspirin and restrain the elimination of salicylic acid. The results showed red ginseng extract can help the curative effect of aspirin last longer. These findings have clinical significance in warning people to use aspirin safely and modestly when aspirin and red ginseng are administrated together. Although there is no obvious evidence suggesting reducing the dose of aspirin would reduce the incidence of gastrointestinal bleeding, the risk of gastrointestinal bleeding seems more strongly related to dose than duration of aspirin use.26According to these results, people should use aspirin cautiously and pay more attention to bleeding risk issues when combined with red ginseng.
REFERENCES
1. Collaborators M C O D. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385(9963):117-71. doi: 10.1016/S0140-6736(14)61682-2.
2. Baigent C, Kappelle LJ, Algra A, et al. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71-86. doi: 10.1136/bmj.324.7329.71.
3. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomized trials. Lancet 2009; 373(9678):1849-60. doi: 10.1016/S0140-6736(09)60503-1.
4. Baigent C. For and against: aspirin for everyone older than 50? Against. BMJ 2005; 330(7505):1440-1. doi:10.1136/bmj.330.7505.1442.
5. Bulugahapitiya U, Siyambalapitiya S, Sithole J, et al.Age threshold for vascular prophylaxis by aspirin in patients without diabetes. Heart 2008; 94(11):1429-32. doi: 10.1136/hrt.2008.150698.
6. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. preventive services task force. Ann Intern Med 2016; 164(12):777-86. doi: 10.7326/M15-2129.
7. Tian ZH, Pang HH, Du SY, et al. Effect of panax notoginseng saponins on the pharmacokinetics of aspirin in rats. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1040:136-43. doi: 10.1016/j.jchromb.2016.12.007.
8. Chen WK, Ju WZ, Tan HS. Effects of mailuoning injection in combination with aspirin on salicylic acid pharmacokinetics in rats. Pharm Clin Res 2009; 17(4):283-6. Chinese. doi: 10.13664/j.cnki.pcr.2009.04.013.
9. Cheng ZM. The study on public recognition of processed ginseng’s health-care effects [dissertation].Beijing: China Academy of Chinese Medical Sciences,2012.
10. Jin L, Cho JY, Kim WK. Anti-inflammation effect of exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract 2014; 8(3):284-91. doi: 10.4162/nrp.2014.8.3.284.
11. Kwak YS, Kyung JS, Kim JS, et al. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull 2010; 33(3):468-72. doi: 10.1248/bpb.33.468.
12. Jin YR, Yu JY, Lee JJ, et al. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol 2007; 100(3):170-5. doi:10.1111/j.1742-7843.2006.00033.x.
13. Lee HS, Kim MR, Park Y, et al. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study.J Med Food 2012; 15(11):1015-23. doi: 10.1089/jmf.2012.2187.
14. Hong CE, Lyu SY. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw 2011; 11(1):42-9. doi:10.4110/in.2011.11.1.42.
15. Jinya LU, Chen J, Cai H. Clinical efficacy of shexiang baoxin pill combined with aspirin in treating elder patients with coronary heart disease. Jiangsu Med J 2015; 41(1):44-7. Chinese. doi: 10.19460/j.cnki.0253-3685.2015.01.016.
16. Zhang T. The clinical effect of xuesaitong combined with aspirin in acute cerebral infarction. J Aerospace Med 2016; 27(4):428-9. Chinese. doi: 10.3969/j.issn.2095-1434.2016.04.011.
17. Tian L. Pharmacokinetics of aspirin after single and multiple doses in healthy subjects. Chin J New Drugs 2006; 15(16):1393-6. Chinese. doi: 10.3321/j.issn:1003-3734.2006.16.022.
18. Ijaz A, Bhatti HN, Rasheed S, et al. Pharmacokinetic study of aspirin in healthy female volunteers.Pak J Bio Sci 2003; 6(16):1404-7. doi: 10.3923/pjbs.2003.1404.1407.
19. Jacob MC, Favre M, Bensa JC. Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry A 1991; 12(6):550-8.doi: 10.1002/cyto.990120612.
20. Sim JS, Zhao HL, Li DW, et al. Effects of saponins from the root bark of aralia elata on the transport of chondroitin sulfate in caco-2 cell monolayers and rats.Biol Pharm Bull 2005; 28(6):1043-8. doi: 10.1248/bpb.28.1043.
21. Wang B, Wang J, Huang SQ, et al. Genetic polymorphism of the human cytochrome p450 2c9 gene and its clinical significance. Curr Drug Metab 2009;10(7):781-834. doi: 10.2174/138920009789895480.
22. Agúndez JA, Garcíamartín E, Martínez C. Genetically based impairment in cyp2c8- and cyp2c9-dependent nsaid metabolism as a risk factor for gastrointestinal bleeding: is a combination of pharmacogenomics and metabolomics required to improve personalized medicine? Expert Opin Drug Metab Toxicol 2009; 5(6):607-20. doi: 10.1517/17425250902970998.
23. He N, Edeki T. The inhibitory effects of herbal components on cyp2c9 and cyp3a4 catalytic activities in human liver microsomes. Am J Ther 2004; 11(3):206-12. doi: 10.1097/00045391-200405000-00009.
24. Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 2000; 321(7270):1183-7. doi: 10.1136/bmj.321.7270.1183.
25. S?rensen HT, Mellemkj?r L, Blot WJ, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol 2000;95(9):2218-24. doi: 10.1016/S0002-9270(00)0104-06.
26. Huang ES, Strate LL, Ho WW, et al. Long-term use of aspirin and the risk of gastrointestinal bleeding.Am J Med 2011; 124(5):426-33. doi: 10.1016/j.amjmed.2010.12.022.
 Chinese Medical Sciences Journal2018年2期
Chinese Medical Sciences Journal2018年2期
- Chinese Medical Sciences Journal的其它文章
- Early Diagnosis of Recurrent Optic Neuritis Using Contrast-Enhanced T2 Fluid-attenuated Inversion Recovery Imaging: a Case Report
- Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study
- A Cohort Study of Incidences and Risk Factors for Thromboembolic Events in Patients with Idiopathic Membranous Nephropathy
- Effects of Short-term High Dose Atorvastatin on Left Ventricular Remodeling in Patients with First Time Attack of Anterior Acute Myocardial Infarction
- Irrationality of Allogeneic Red Blood Cell Transfusion in Intraoperative Cell Salvage Patients: a Retrospective Analysis
- Midterm Follow-up of Coronary Artery Bypass Grafting with 64-Slice Multi-detector Computed Tomography:Identification of Risk Factors Affecting Graft Patency
