Effects of Short-term High Dose Atorvastatin on Left Ventricular Remodeling in Patients with First Time Attack of Anterior Acute Myocardial Infarction
Zhijian Liu, Gaopin Hu, Meiying Fei, Zao Yin, Quanxing Shi, Fei Sun*
1Department of Cardiology, the 210th Hospital of People’s Liberation Army,Dalian, Liaoning 116021, China
2Department of Cardiology, the 306th Hospital of People’s Liberation Army,Beijing 100101, China
Key words: atorvastatin; acute myocardial infarction; high-sensitivity C-reactive protein;Matrix metalloproteinases; left ventricular remodeling
LEFT ventricular (LV) remodeling in response to myocardial infarction (MI), defined as changes in geometry, structure and function of the heart, is associated with increased risks of heart failure and cardiovascular death.1Some studies have demonstrated that excessive inflammation2and matrix metalloproteinase (MMP) levels3were associated with adverse LV remodeling. In addition to the decrease of low-density lipoprotein-cholesterol(LDL-C), statins have been recognized to have properties of modifying inflammatory responses, endothelial function and plaque stability, and these pleiotropic lipid-lowering effects of statins may be independent.4,5Statins possess properties of anti-inflammatory6and inhibiting MMPs activity,7which is relevant to their cardioprotective effects. Previous studies have showed that normal dose statins reduce plasma levels of C-reactive protein (CRP)8and endothelin-1 (ET-1),9and appears to beneficially attenuate LV remodeling after MI.10,11As intensive statins therapy lead to a lower CRP level compared with normal dose statins,12and anterior MI patients experience more pronounced post-infarction LV remodeling and dysfunction than non-anterior MI patients,13we conducted this trial to explore whether a treatment with intensive statins could affect LV remodeling after anterior MI via its effect of anti-inflammatory and activity of inhibiting MMPs.
PATIENTS AND METHODS
Study population and protocol
This study was approved by the institutional ethic committee. Written informed consents were acquired from all patients. Inpatients with first time attack of ST-segment elevated acute anterior MI (AAMI) from March 2013 to July 2016 were candidates for the study. The inclusion criteria were: age ranged from 18 to 75 years; MI was diagnosed based on typical chest pain, cardiac enzyme elevation and persistent ST-segment elevation in 2 contiguous electrocardiographic leads; underwent successful primary percutaneous coronary intervention (PCI) within 12 h after chest pain onset. Exclusion criteria were: history of myocardial infarction; history of treatment with statins; accompanied with non-sinus rhythm; cardiogenic shock; died during hospitalization; accompanied with hepatic disease, or creatinine level >2.0 mg/dl, or clinical and/or laboratory evidence of inflammatory disease. The eligible patients were randomly assigned into two groups:the intensive treatment (IT) group, who received atorvastatin 40 mg once daily immediately after diagnosis for 1 week, followed by 20 mg once daily; the standard treatment(ST) group, who received atorvastatin 20 mg once daily after the admission.
All PCIs were performed using femoral artery approach with Iopamidol (Bracco Sine, Shanghai,China). All patients were administrated with aspirin 300 mg/day plus clopidogrel 600 mg/day before PCI,and aspirin 100 mg/day plus clopidgrel 75 mg/day after PCI. Successful PCI was identified if angiographic residual stenosis of the involved vessel was <20% by visual estimation, without any in-hospital major complication (acute myocardial infarction, repeat PCI, or death).
Blood sampling and laboratory analysis
Multiple peripheral blood samplings were performed on admission, at 1 week, 2 weeks after initial onset of AAMI, and at 6 months follow-up. Whole blood was immediately collected into tubes containing heparin sodium and ethylene diamine tetraacetate(EDTA). The heparin sodium blood samples were used to examine high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol(LDL-C), Creatine kinase-myocardial isoenzyme (CKMB) by a clinical automatic analyzer (Hitachi 7600,Tokyo, Japan). The EDTA blood was centrifuged at 1500 g for 15 min at room temperature and the plasma were stored at ?80°C until use. The plasma levels of high-sensitivity C-reactive protein (Hs-CRP)was quantified using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit (Yingchuang Biotechnology, Houzhou, China). ELISA kits were used to quantify Malonaldehyde (MDA) (Jiancheng Biotechnology, Nanjing, China), ET-1 (Bangding taike Biotechnology, Beijing), MMP-2 and MMP-9 (both Meridian Life Science, Saco USA). N-terminal probrain natriuretic peptide (NT-pro-BNP) was measured using an electro-chemiluminescence immunoassay(Roche Cobas E601, Mannhein, Germany). All the measurements followed the manufacturer’s instructions.
Echocardiography and measurements
The echocardiographic studies were performed on admission, at 2-weeks follow-up and 1 year follow-up after PCI. Echocardiographic examinations were performed using a commercially available sonographer system (Vivid 7, GE, Milwaukee, WI, USA) with a 3.5-MHz phased array transducer. Performers were blind to the grouping information of each patients. Echocardiographic examinations were performed with the patient taking supine position. Two-dimensional images were recorded and measured. Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume(LVESV) were measured on the apical two-chamber view and apical four-chamber view respectively. Left ventricular ejection fraction (LVEF) was calculated using the Simpson’s method.14
Statistical analysis
Continuous variables were described as mean ± standard deviation; categorical variables were described as number and percentage. Normal distribution was tested by the Kolmogorov–Smirnov test. Differences in baseline parameters between the two groups were analyzed using independent-samples t test or the Chi-Square test where appropriate. Levels of hs-CRP,MMPs, MAD, ET-1 and the LV remodeling parameters in-group assessments were analyzed by the repeated measures test with the post hoc LSD test. Multivariate analysis of variance (MANOVA) was used in comparison of the data between the two groups with the post hoc LSD test. All probability values were 2-sided, and a P value of less than 0.05 was considered significant.All statistical analyses were performed by using SPSS 19.0.
RESULTS
Clinical information of patients
Totally 22 out of 125 illegible patients were excluded for reasons shown in the flowchart (Fig. 1), and 103 patients were finally enrolled in the study. There were no significant differences between the two groups in terms of age, gender, hypertension, diabetes Mellitus, smoking, baseline levels of HDL-C, LDL-C, peak CK-MB, time from symptom onset to PCI, single/multi-vessel disease, as well as medications after PCI(Table 1).

Figure 1. Flowchart of the study cohort.
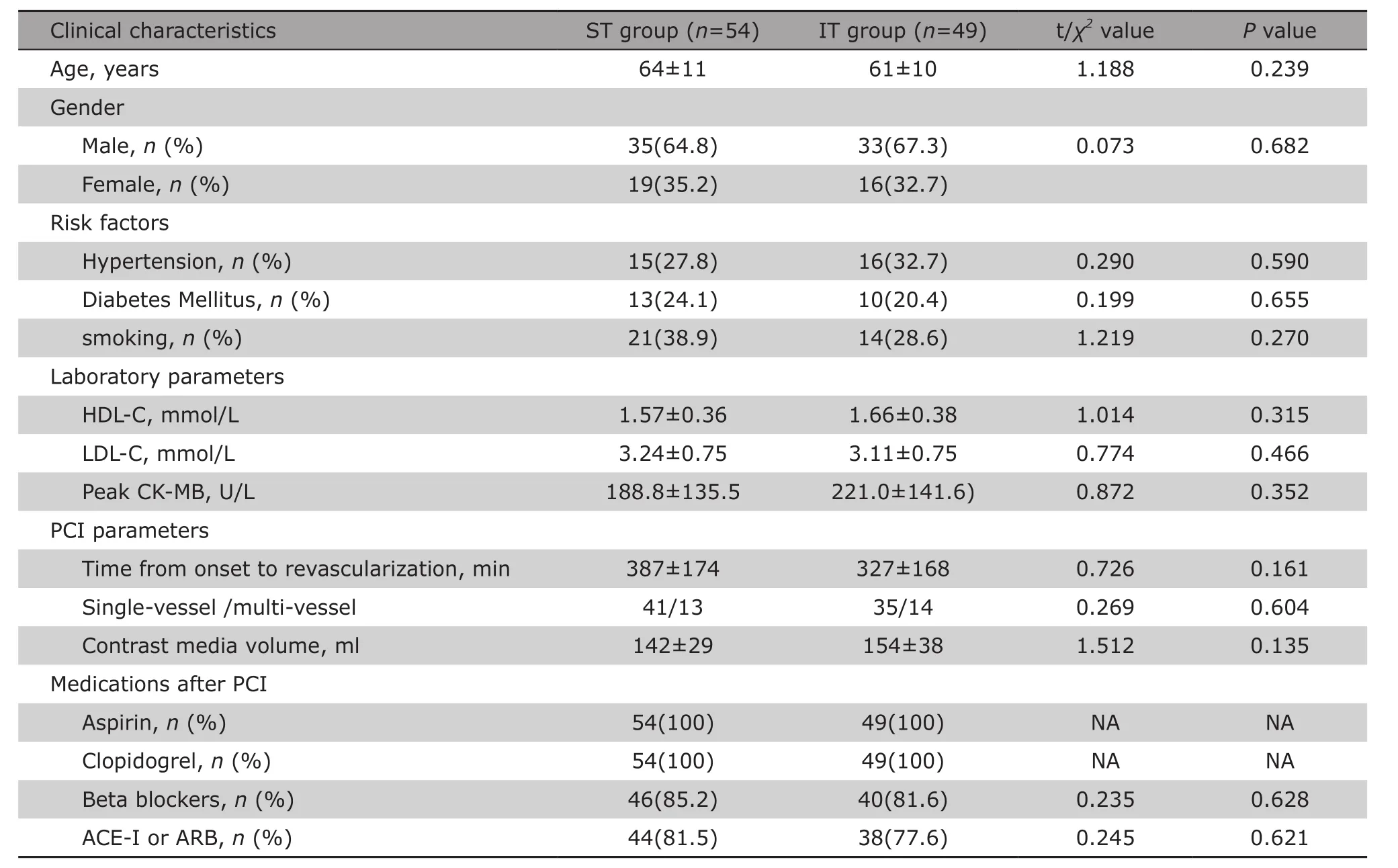
Table 1. Comparison of clinical characteristics of AAMI patient between the ST group and the IT group§
Effects of atorvastatin treatment on plasma hs-CRP, ET-1, MDA and MMPs
There were no significant differences in pro-BNP, hs-CRP, ET-1, MDA, MMP-2 and MMP-9 levels at admission between the two groups. Plasma levels of hs-CRP, ET-1, MDA, MMP-2 and MMP-9 in both groups continuously decreased over time after the treatments. In IT group,the levels of pro-BNP, hs-CRP, ET-1 and MMP-9 were significantly lower compared with that of ST group at 1 week after MI onset (all P<0.05) (Table 2).
Effects of atorvastatin treatment on LV remodeling parameter
The 1 year echocardiographic follow-up showed that the LVEDV and LVEF in both groups significantly increased compared with that of baseline (P<0.01 for all comparisons). The LVESV at 1 year follow-up did not change significantly compared with that of baseline in both groups. NO differences were found between both groups in LVEDV, LVESV and LVEF on admission, at 2 week and at 1 year follow-up. The changes in LVEDV,LVESV and LVEF over the follow up time were not significantly different between the two groups either (Table 3).
DISCUSSION
Since significant inflammatory response as well as oxidative stress occurs soon after AAMI, and the LV remodeling is developed subsequently, in the study, we aimed to explore whether short-time high-dose atorvastatin can prevent LV remodeling via inhabiting inflammation and attenuating oxidative stress. Our study showed that intensive treatment with 40 mg atorvastatin for one week in patients with AAMI could decreased the levels of Pro-BNP, hs-CRP, ET-1 and MMP-9 temporarily, but this beneficial effect did not translate into an improvement of LV remodeling at 1 year follow up.
CRP is a highly sensitive, but nonspecific marker of systemic inflammation. Clinical studies have demonstrated the peak circulating level of CRP after MI is correlated with adverse outcomes such as LV remodeling,
development of heart failure, and death.15,16The deleterious effect of CRP on post-MI LV remodeling may be associated with increased MMP-9 activity in the border zone.16Extracellular Matrix (ECM) breakdown by MMPs play essential roles in LV remodeling of the post-MI,and changes in expression of MMP-2 and MMP-9 and their activities have been identified in LV remodeling.MDA is a stable end product of lipid peroxidation and serves as a reliable marker for the assessment of free radical induced damages to tissue. Our study showed that short-term high-dose atorvastatin could further decreased hs-CRP and MMP-9 but not MMP-2 and MDA levels, which was consistent with study results of Tousoulis,17who demonstrated that short term treatment with 40 mg/day of atorvastatin exerted beneficial impact on MMP-9 level.
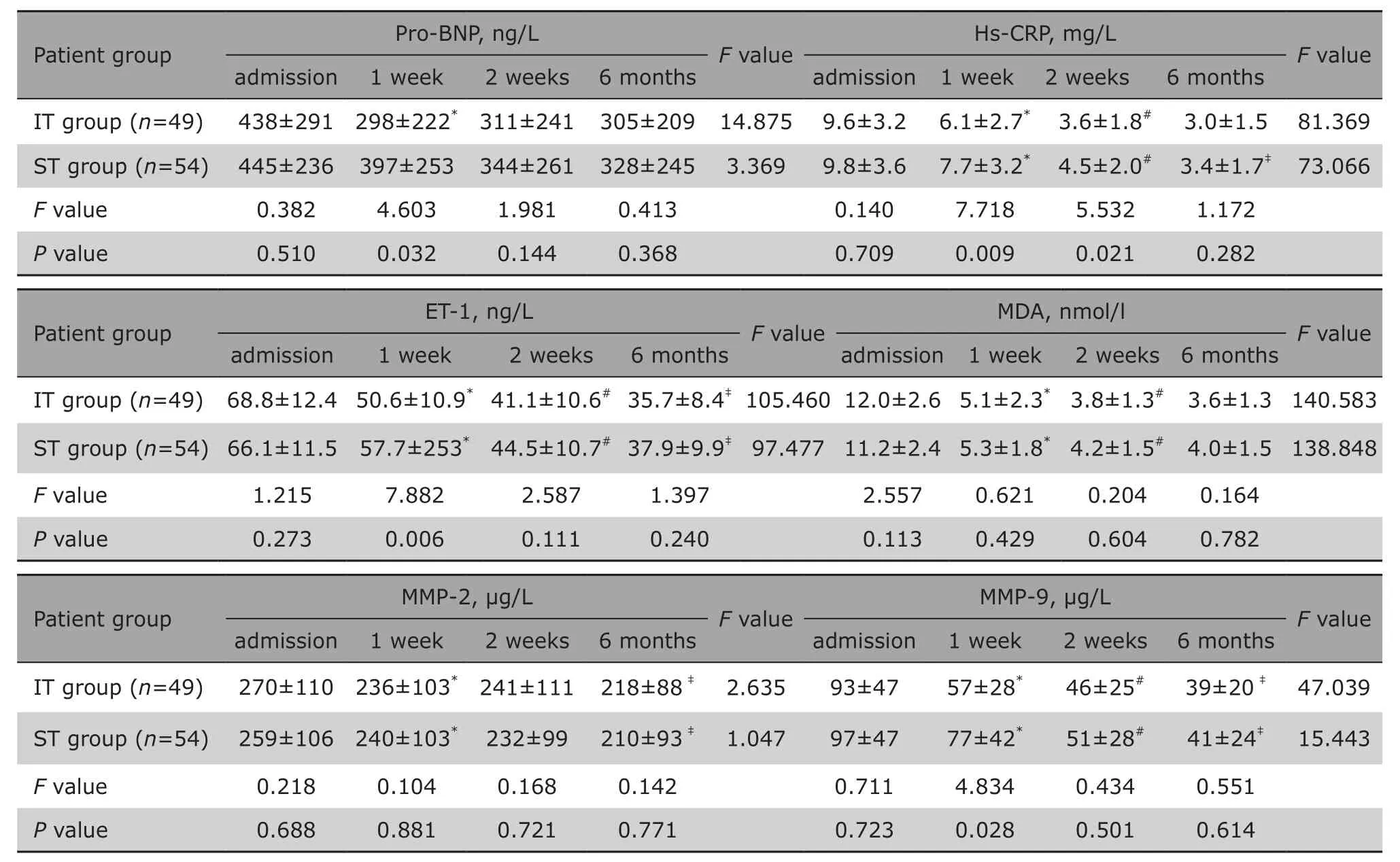
Table 2. Comparison of Pro-BNP and Hs-CRP at different time points and between the IT and ST group§
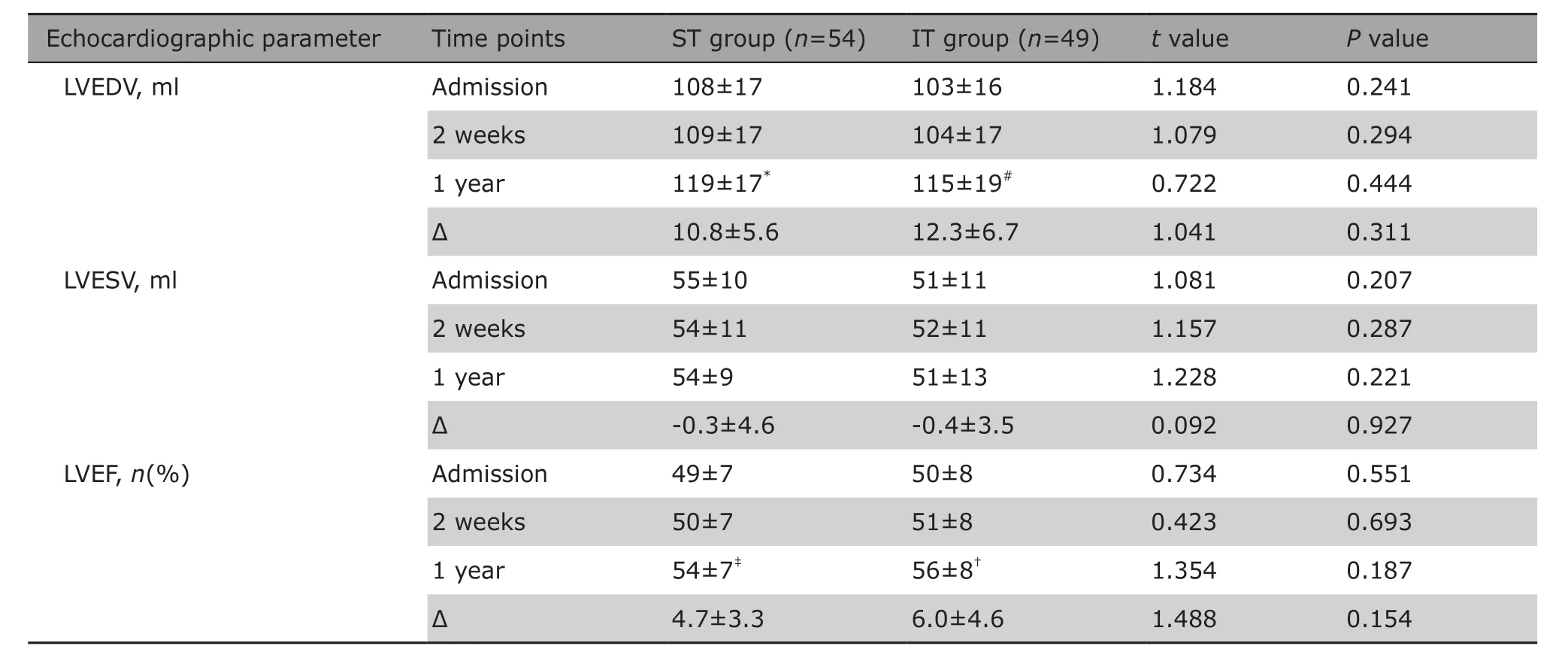
Table 3. Comparison of echocardiographic parameters in left ventricular remodeling between the ST group and the IT group on admission, at 2 weeks and 1 year follow-up§
This study further investigated whether there is an improvement of LV remodeling which was associated to these beneficial effects. We found that among the patients in the short-time high-dose atorvastatin treatment group, LV remodeling parameters at oneyear follow-up were not significantly different compared to patients with standard treatment. There are several possible explanations. Firstly, although shortterm high-dose atorvastatin could significantly inhibit the inflammatory response, this effect may be not strong enough to completely correct the inflammatory damage. Secondly, ECM is remodeled by MMPs,which is a large family of zinc-dependent proteases.Previous studies have showed that MMP-3, MMP-8 and Membrane Type 1-Matrix Metalloproteinase (MT1-MMP)were also involved in LV remodeling besides MMP-9 and MMP-2,.18Inhibiting MMP-9 activity solely may not be enough to prevent ECM degradation and LV remodeling. Thirdly, LV remodeling after MI involves both the infarcted and non-infarcted LV myocardium, therefore,seven days of high-dose atorvastatin may be not sufficient to exert a significant effect on LV remodeling.Finally, LV remodeling is a continuous process that last for months or years, 1 year follow up in this study may not be long enough to appreciate a significant change in LV remodeling.
It has been previously reported that short-term treatment (4 weeks) of atorvastatin at a dose of 40 mg/day in patients with ischemic heart failure exerted beneficial impact on endothelial function, arterial stiffness and LV remodeling.17This trial enrolled patients who had suffered from heart failure and been followed up for 4 weeks, and demonstrated that atorvastatin 40 mg/day resulted in significantly decreased level of serum MMP-9, which was a biomarker of left ventricular remodeling. Ours study also confirmed that shortterm treatment with atorvastatin at a dose of 40 mg/day in patients with AAMI could significantly reduce MMP-9 levels, but it did not result in an improvement of echocardiographic parameters.
Elevated level of ET-1 has been shown to be associated with post AMI death.19Previous study has shown that statin therapy could significantly reduce circulating ET-1 concentrations.9We evaluated the effect of short time high-dose atorvastatin on ET-1 level and found that compared with normal dose, high-dose atorvastatin was further associated with reduced ET-1 levels in patients with MI. Restricted by the costs and length of follow up, this study was not able to investigate whether the reduced ET-1 level could further improve endothelial function or reduce cardiovascular mortality.
The limitations of this study also include: first,the inadequate number of patients limit the power of the results in this study, and prospective study with a larger sample size would help to confirm the present observations. Secondly, the one-year follow-up in the current study may not be long enough to appreciate a significant change in LV remodeling. Thirdly, only plasma MMP-2 and MMP-9 activity were measured, while the tissue inhibitors of metalloproteinases (TIMPs)which specifically inhibite MMPs activity in tissue were not measured in this study.
In conclusion, the present study shows that shortterm high-dose atorvastatin treatment in ST-elevation AAMI could significantly decrease hs-CRP, ET-1, MMP-9 and pro-BNP levels. This beneficial effect did not result in improvement of LV remodeling. Our study suggests that large scale clinical trial that observe the long-term effects of high-dose atorvastatin should be carried out to further evaluate the potential benefit of high dose of atorvastatin on LV remodeling.
REFERENCES
1. Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: A review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev 2011;16(1): 13–21. doi: 10.1007/s10741-010-9181-7.
2. Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 2016;67(17): 2050-60. doi: 10.1016/j.jacc.2016.01.073.
3. Nilsson L, Hallén J, Atar D, et al. Early measurements of plasma matrix metalloproteinase-2 predict infarct size and ventricular dysfunction in ST-elevation myocardial infarction. Heart 2012; 98(1): 31-6. doi:10.1136/heartjnl-2011-300079.
4. van Klei WA, Buhre WF. Anti-inflammatory effects of perioperative statin therapy. Can J Anaesth 2012;59(6): 516-21. doi: 10.1007/s12630-012-9703-y.
5. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017; 120(1): 229-43. doi: 10.1161/CIRCRESAHA.116.308537.
6. Mao Y, Koga JI, Tokutome M, et al. Nanoparticle-mediated delivery of pitavastatin to monocytes/macrophages inhibits left ventricular remodeling after acute myocardial infarction by inhibiting monocyte-mediated inflammation. Int Heart J 2017; 58(4): 615-23. doi:10.1536/ihj.16-457.
7. Shirakabe A, Asai K, Hata N, et al. Immediate administration of atorvastatin decreased the serum MMP-2 level and improved the prognosis for acute heart failure. J Cardiol 2012; 59(3): 374-82. doi: 10.1016/j.jjcc.2012.01.009.
8. Ansheles AA, Rvacheva AV, Sergienko IV. Effect of atorvastatin therapy on the level of CD34+CD133+CD309+ endothelial progenitor cells in patients with coronary heart disease. Bull Exp Biol Med 2017;163(1): 133-6. doi: 10.1007/s10517-017-3753-7.
9. Sahebkar A, Kotani K, Serban C, et al. Statin therapy reduces plasma endothelin-1 concentrations:A meta-analysis of 15 randomized controlled trials.Atherosclerosis 2015; 241(2): 433-42. doi: 10.1016/j.atherosclerosis.2015.05.022.
10. Ishida K, Geshi T, Nakano A, et al. Beneficial effects of statin treatment on coronary microvascular dysfunction and left ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol 2012;155(3): 442-7. doi: 10.1016/j.ijcard.2011.11.015.
11. Rebic D, Rasic S, Rebic V. Influence of endothelin-1 and nitric oxide on left ventricular remodeling in patients on peritoneal dialysis. Ren Fail 2014; 36(2):232-6. doi: 10.3109/0886022X.2013.836935.
12. Lee BK, Koo BK, Nam CW, et al. Does Pre-treatment with high dose atorvastatin prevent microvascular dysfunction after percutaneous coronary intervention in patients with acute coronary syndrome? Korean Circ J 2016; 46(4): 472-80. doi: 10.4070/kcj.2016. 46.4.472.
13. Masci PG, Ganame J, Francone M, et al. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J 2011; 32(13): 1640-8. doi: 10.1093/eurheartj/ehr064.
14. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2(5):358–67.doi:10.1016/s0894-7317(89)80014-8.
15. Casas JP, Shah T, Hingorani AD, et al. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008; 264(4): 295-314. doi: 10.1111/j.1365-2796.2008.02015.x.
16. Takahashi T, Anzai T, Kaneko H, et al. Increased C-reactive protein expression exacerbates left ventricular dysfunction and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 2010; 299(6):H1795-1804. doi: 10.1152/ajpheart.00001.2010.
17. Tousoulis D, Oikonomou E, Siasos G, et al. Dose-dependent effects of short term atorvastatin treatment on arterial wall properties and on indices of left ventricular remodeling in ischemic heart failure. Atherosclerosis 2013; 227(2): 367-72. doi: 10.1016/j.atherosclerosis.2013.01.015.
18. DeLeon-Pennell KY, Meschiari CA, Jung M, et al. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog Mol Biol Transl Sci 2017; 147: 75-100.doi: 10.1016/bs.pmbts.2017.02.001.
19. Wang J, Tan GJ, Han LN, et al. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol 2017;14(2): 135-50. doi: 10.11909/j.issn.1671-5411.2017.02.008.
 Chinese Medical Sciences Journal2018年2期
Chinese Medical Sciences Journal2018年2期
- Chinese Medical Sciences Journal的其它文章
- Early Diagnosis of Recurrent Optic Neuritis Using Contrast-Enhanced T2 Fluid-attenuated Inversion Recovery Imaging: a Case Report
- Effect of Red Ginseng Extract on the Pharmacokinetics of Aspirin Metabolite in Sprague Dawley Rats
- Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study
- A Cohort Study of Incidences and Risk Factors for Thromboembolic Events in Patients with Idiopathic Membranous Nephropathy
- Irrationality of Allogeneic Red Blood Cell Transfusion in Intraoperative Cell Salvage Patients: a Retrospective Analysis
- Midterm Follow-up of Coronary Artery Bypass Grafting with 64-Slice Multi-detector Computed Tomography:Identification of Risk Factors Affecting Graft Patency
