A Cohort Study of Incidences and Risk Factors for Thromboembolic Events in Patients with Idiopathic Membranous Nephropathy
Peimei Zou, Hang Li, Jianfang Cai, Zhenjie Chen, Chao Li, Ping Xu,Mingxi Li, Limeng Chen, Xuemei Li, Xuewang Li*
1Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Chinese Academy of Medical Sciences & Peking Union Medical College,Beijing 100730, China
Key words: idiopathic membranous nephropathy; thromboembolic events; arterial; venous
IDIOPATHIC membranous nephropathy (IMN) is the most common cause of nephrotic syndrome in adult, and its incidence shows a rising trend in recent years.1,2Thromboembolic event, a well-recognized complication of nephrotic syndrome,occurs much more frequently in IMN than in other kidney diseases.3Frequency of venous thromboembolic events (VTEs), including deep vein thrombosis (DVT),renal vein thrombosis (RVT), and pulmonary embolism(PE) ranged from 3% to 48% in patients with nephrotic syndrome.3-5Patients with nephrotic syndrome are also believed to have increased risk of atherosclerosis and coronary heart disease (CHD). Most studies about the association between CHD and nephrotic syndrome were case reports or case series studies.6-8Risk of arterial thromboembolic events (ATEs), mainly consisting of acute myocardial ischemic events and infarction,thrombotic ischemic stroke(IS), and peripheral artery disease(PAD), had been firstly described in a retrospective cohort study in European patients with nephrotic syndrome,9but there was in lack of discussion about the risk factors of ATE. Recently, Lee et al reported that patients with IMN were at high risk of cardiovascular events.10These findings raised our attention on thromboembolic events in Asian patients with IMN. We conducted this research aiming to explore the incidence and the characteristics of ATEs and VTEs in patients with IMN in Chinese population, and to identify the predisposing risk factors of the thromboembolic events at this situation.
MATERIALS AND METHODS
Study cohort
This study was approved by the ethics committee of the Peking Union Medical College Hospital, and the informed consents from participants were waived. A total of 1136 consecutive inpatients who were older than 16 years old and pathologically diagnosed as IMN by biopsy from January 2004 to June 2016 were retrospectively reviewed for this study. The follow-up was conducted till December 31, 2016. The Exclusion criteria were: 1,patients with malignant tumor, autoimmune diseases,serious mental diseases, or hematological diseases; 2,patients with comorbidity of other pathological types of glomerular diseases; 3. IMN diagnose was ambiguous;4, insufficient follow-up information. There were 801(70.5%) patients with complete follow-up information.Among them, 35 patients were excluded by a variety of reasons (Fig. 1). Thus 766 patients in total were enrolled in this study.
Information collection and definition of variables
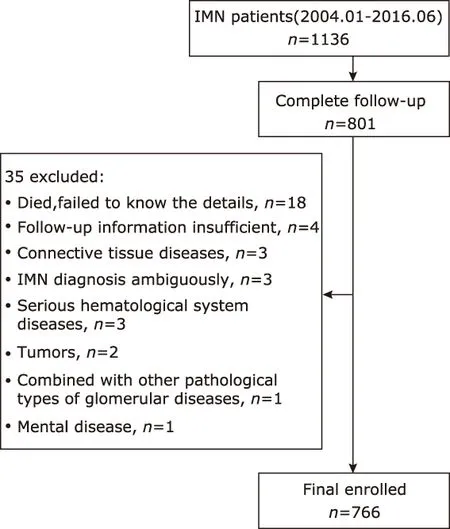
Figure 1. Patient recruitment flowchart of the study cohort.
Clinical information at the time of biopsy were collected as the baseline data, including gender, age, history of smoking, history of diabetes, hypertension, and previous onset of ATEs or VTEs, duration of the disease,laboratory test results of proteinuria, serum albumin,serum creatinine, cholesterol, triglycerides, and low density lipoprotein-cholesterol (LDL-C). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) formula.11Nephrotic syndrome (NS) status was identified as proteinuria ≥3.5 g/24 h and hypoalbuminemia (≤30 g/L). Sub-nephrotic syndrome was defined as proteinuria >0.3 g/24 h and <3.5 g/24 h.The complete remission (CR) of the NS was identified as proteinuria ≤0.3 g/24 h, with normal serum creatinine.12
Thromboembolic events
We identified all symptomatic thromboembolic events.VTEs consisted of DVT, RVT and PE, and ATEs included CHD, IS and PAD. The diagnoses of DVT, RVT and PAD were confirmed by compression sonography and color-Doppler ultrasound. The diagnosis of PE was made by CT pulmonary angiography. CHD was diagnosed if any of myocardial infarction, angina pectoris or silent myocardial ischemia was confirmed clinically by physicians. IS was diagnosed by medical imaging of CT or MRI. Since cerebral transient ischemic attack was hard to assess, we did not take it into assessment.
Statistical analysis
Statistical analysis was performed by using SPSS (version 22, IBM, Armonk, NY, U.S.). Continuous variables were presented as mean±SD or median with interquartile ranges (IQR), and the differences were evaluated by two tailed independent sample Student t test,Mann-Whitney U test, Kruskal-Wallis test, or univariate ANOVA, depending on the normality of the data and levels of the outcome variable. Categorical variables were presented as percentages, and the differences were compared using chi-square test or Fisher’s test.
The observation time for each patient was defined as the time period from kidney biopsy to death, or to the time of the last follow-up. The incidences of ATE or VTE at 0.5, 1, 2, 3, and 5 years were defined by the percentage of patients who experienced the events in the total number of patient-years. When calculating the cumulative incidence rates of ATE, we ignored the occurrence of VTE, and vice versa. Kaplan-Meier methods were used for cumulative incidence plots.To assess risk factors for ATEs and VTEs, we used Cox proportional hazards regression models. Baseline variables were incorporated into the models. As some patients experienced at least 1 episode of thromboembolic events during the observation time, when analyzing characteristics of ATE or VTE, we treated the thromboembolic events independently. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Demographic characteristics of the cohort
Of 766 patients enrolled in this study, 58.6% were male and 41.4% were female. The mean age of the cohort was 47.6±14.8 years. Median observation time period was 39.65 (24.5, 62.8) months. Patients in the study cohort presented with a median level of proteinuria 5.49 (3.29, 8.98) g/24 h, a mean serum albumin of 27.2±6.6 g/L, and a mean eGFR of 97.14±21.94 ml/min/1.73 m2. There were 453 out of 766 (59.1%) patients presented with nephrotic syndrome in this cohort.
Clinical manifestations of thromboembolic events
Of 766 enrolled patients, 78 events of ATEs occurred in 71 patients (7 patients experienced 2 episodes of ATEs), 60 events of VTEs occurred in 53 patients (7 patients experienced 2 episodes of VTEs). Besides, 8 patients experienced both episode of ATE and VTE. Of all the ATEs, 45% (35/78) were IS, 50% (39/78) were CAD, and only 5% (4/78) were PAD. DVTs were the majority of VTEs, which accounted for 60%(36/60),while RVT and PE accounted for 13% (8/60) and 27%(16/60) respectively.
Incidences and time distribution of ATE and VTE events
The cumulative incidence rates at 0.5, 1, 2, 3, and 5 years after biopsy were 4.3%, 5.7%, 6.3%, 7.1%, and 8.0% respectively for newly diagnosed ATEs, and 5.9%,6.8%, 6.9%, 7.0%, and 7.1% respectively for newly diagnosed VTEs (Fig. 2). The distribution of events by years during the observing time (Fig. 3) demonstrated that both ATEs and VTEs showed a onset peak in 0.5 year after biopsy. There was as high as 77.4% (41/53)VTEs occurred in 0.5 years early after diagnosis of IMN, while 36.6% (26/71) ATEs occurred within the first 0.5 years, with the rest of ATEs occurred in almost each year during the observation time. The early onset (in 0.5 year) and delayed onset (over 0.5 year)for thromboembolic events distributed significantly different between ATEs and VTEs patients (χ2=20.3,P<0.001).
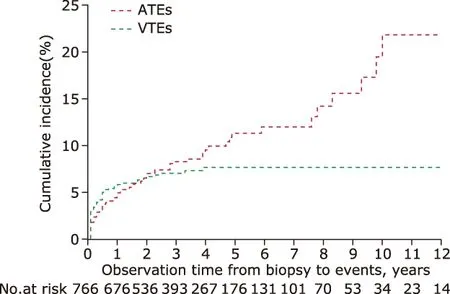
Figure 2. Kaplan-Meier estimates of the probability of VTEs- and ATEs-cumulative incidences. ATEs, arterial thromboembolic events; VTEs, venous thromboembolic events.
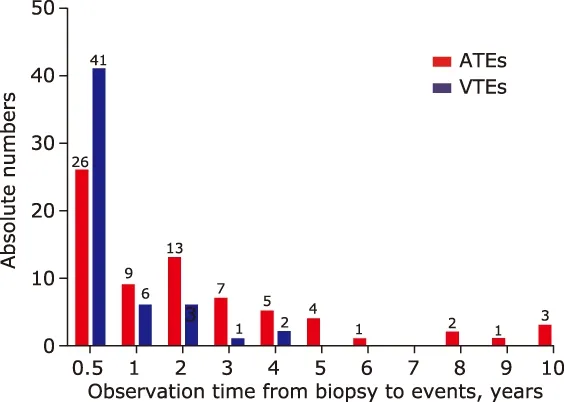
Figure 3. Time distributions of ATEs and VTEs during the observation time.
Comparison of baseline characteristics between patients with ATEs and VTEs
The baseline characteristics of the IMN patients with onset of ATE and VTE were shown and compared in Table 1. The mean age of IMN patients who experienced ATE events was significantly older than those of VTEs; patients who experienced ATE showed a significantly lower level of eGFR (88.65±22.27 vs 97.22±18.86 ml/min/1.73 m2, P=0.03). The percentage of patients with nephrotic syndrome in ATEs group and VTEs group were comparable, with no significant difference. The levels of proteinuria and serum albumin were also similar. There was no difference between two groups in smoking exposure and hypertension.
Association of thromboembolic events with clinical and laboratory characteristics
There were 58 episodes of ATEs and 56 episodes of VTEs available for analysis, because in a few cases thelaboratory examinations at the time of events were left out of the medical records. The detailed clinical and laboratory data at the time of events were statistically analyzed (Table 2). When experienced thromboembolic events, patients of ATEs were significantly older than those of VTEs (mean age 59.0±11.4 vs 50.0±15.4 years, t=3.3, P=0.001), regardless the early or delayed onset. Compared to patients with ATE, patients with VTE had higher level of proteinuria (z=3.1, P=0.002),lower level of serum albumin (t=3.1, P=0.002) and higher level of triglycerides (z=2.0, P=0.04). The mean eGFR level of patients with VTE was relatively higher than those with ATE (t=?2.5, P=0.02). 81.5% (44/54)of patients with VTE were at NS status. In contrast,42.1% (24/57) patients with ATE were at NS status.This difference was statistically significant (χ2=18.1,P<0.001).
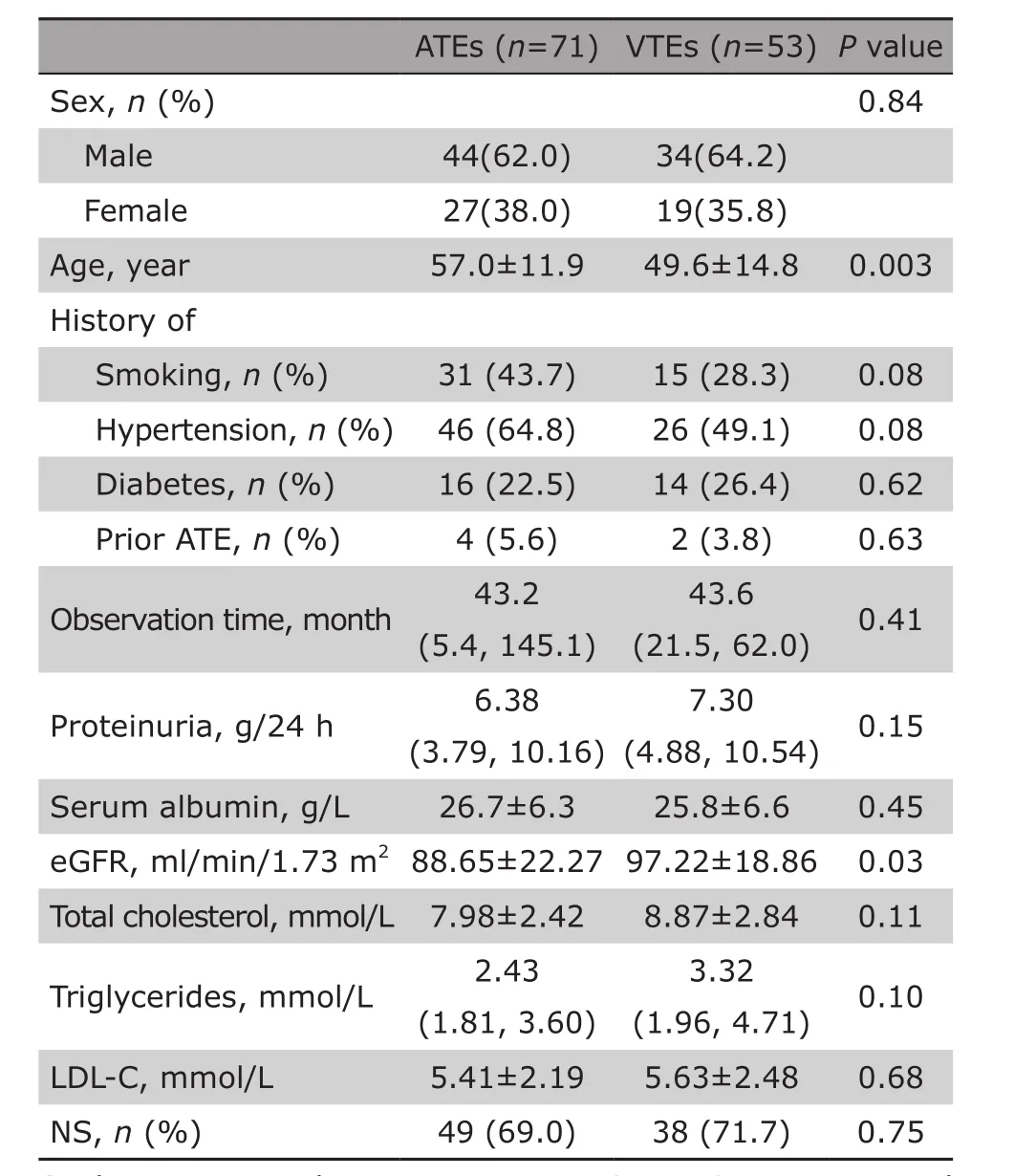
Table 1. Comparison of baseline characteristics between ATE and VTE patients§
Variables related to the early or delayed onset of thromboembolic events
Forty-eight out of 56 patients with VTE and 27 out of 58 patients with ATE had early onset of the events within 0.5 years after biopsied diagnosis. The clinical characteristics of these early onset events were compared with those of delayed onset events that occurred later than 0.5 years. As shown in Table 2, compared to patients with delayed onset of ATE, patients who experienced early onset of ATE had relatively lower serum albumin level (26.8±7.6 g/L, P=0.01), and higher proportion of NS status (59.3% vs 26.7%, P=0.01).Patients experienced the delayed onset of ATE were mainly at sub-NS status or CR status (38.7% and 35.5%, respectively). In patients with VTEs, both early and delayed onset presented with massive proteinuria and obvious hypoalbuminemia, and the majority of both were at NS status (79.2% and 75.0%, respectively). There were no differences of all variables between early and delayed onset of VTEs.
Risk factors identification for VTE and ATE in IMN patients
Associations of baseline variables with the onset of ATEs and VTEs were shown in Table 3. In the univariable risk prediction models, patients with a history of smoking, hypertension and ATEs were at higher risk of ATEs (HR=1.7, 95% CI:1.0-2.7; HR=1.8, 95% CI:1.1-3.0; and HR=2.4, 95% CI:1.1-5.2, respextively).A higher incidence of ATEs was coincidence with aging,as the hazard ratio increased to 5.1 in patients of 35-54 years old, and reached to 13.0 (95% CI:3.1-53.3)in patients aged above 55 years old. The higher level of proteinuria was significantly associated with ATEs,with a hazard ratio of 2.2 (95% CI:1.1-4.4) when urine protein reached over 8.0 g/24 h. Severe renal dysfunction (decreased eGFR) at baseline was also associated with increased likelihood of ATEs, although the difference had no significance (P=0.10). VTEs were obviously associated with hypoalbuminemia and massive proteinuria (P=0.01 and P=0.03). The multivariable survival analysis model revealed that the age at the time of diagnosis of IMN independently predicted ATEs (P=0.001), and serum albumin level was the independent predicting factor for VTEs(P=0.03) (Table 4).
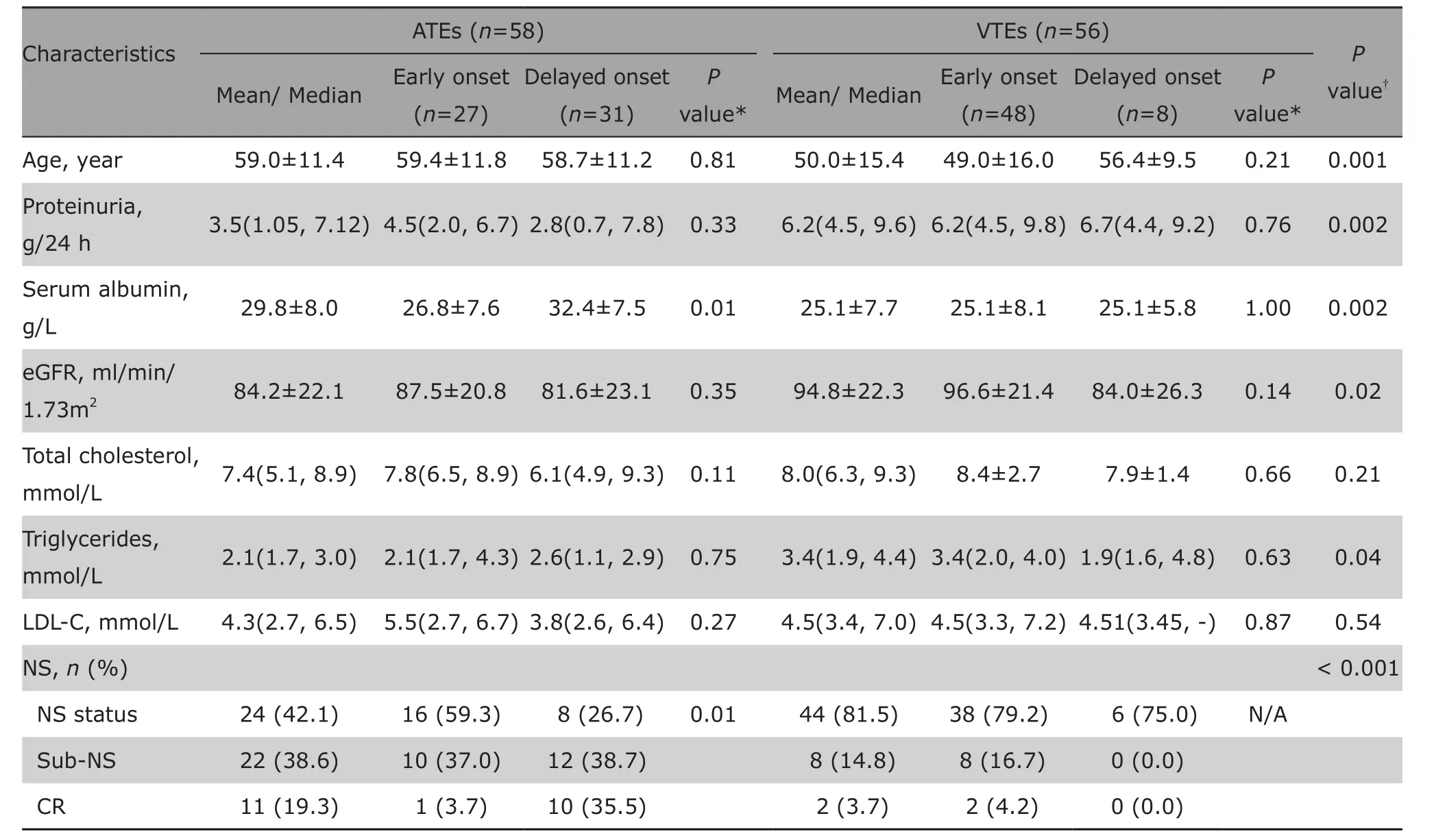
Table 2. Comparison of the clinical characteristics at the time of events between ATEs and VTEs§
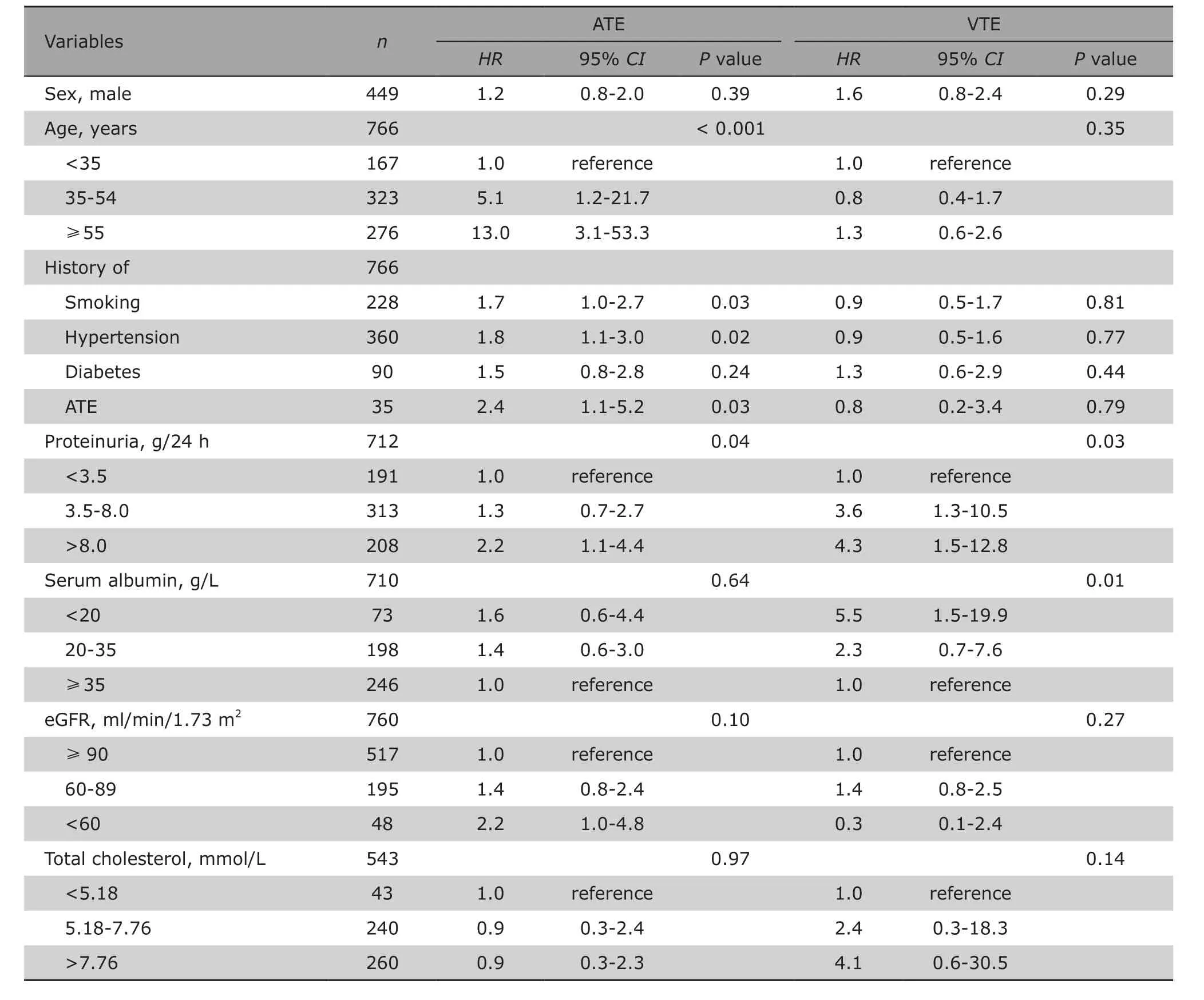
Table 3. Univariable model proportional-hazards analysis on association of clinical characteristics with VTE and ATE
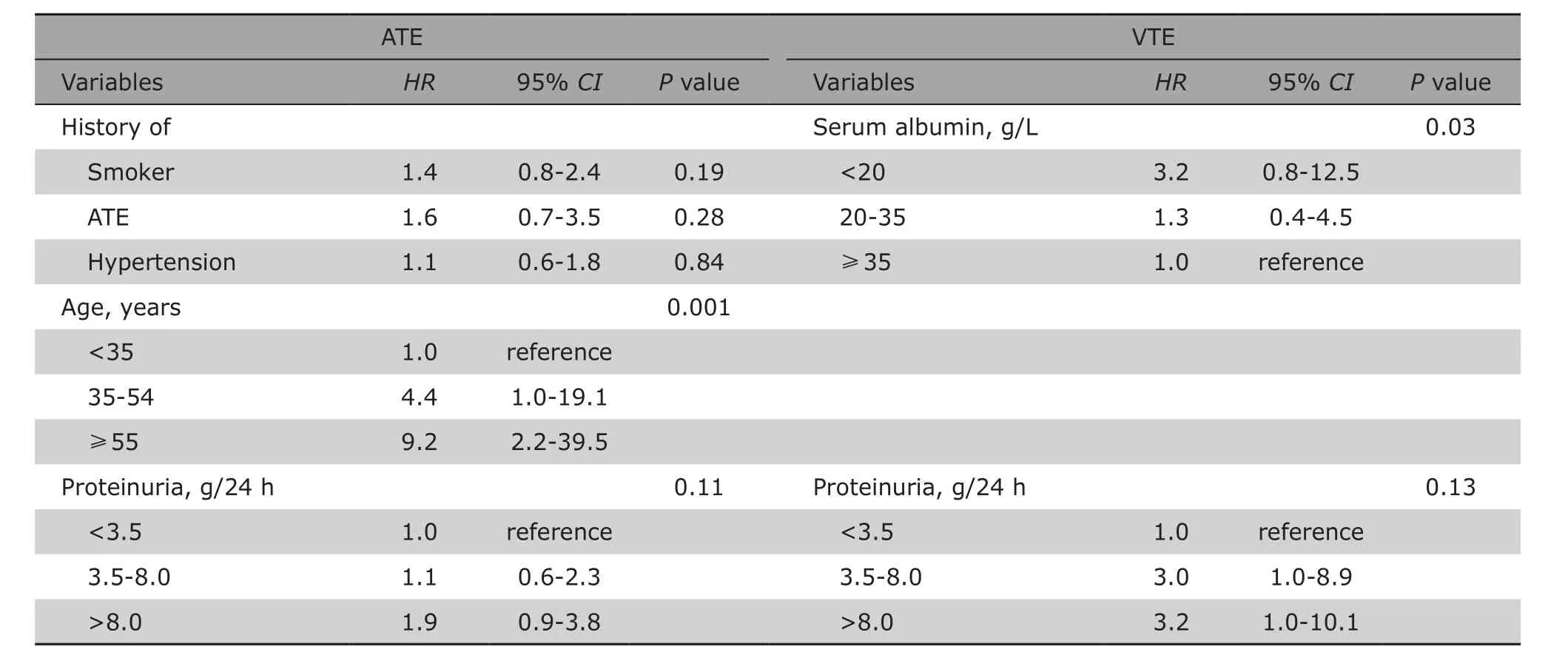
Table 4. Multivariable model proportional-hazards analysis on risk factors of VTE and ATE
DISCUSSION
To our knowledge, this study is the largest cohort that investigate the arterial and venous thromboembolic events among Chinese patients with IMN. The findings in this study demonstrate that patients with IMN have high incidences of thromboembolic events compared to the general population, particularly within the first 6 months after pathological diagnosis of the disease. In this Study, we systematic exhibited the cumulative incidence rates of ATEs and VTEs, provided the detail elements of each events, and further investigated the risk factors.
Nephrotic syndrome is characterized by hyperlipidemia, with elevated levels of total cholesterol and LDL-C.13It is assumed that nephrotic syndrome related hyperlipidemia can accelerate atherosclerosis, thus increase the risk of cardiovascular diseases in patients with NS.14This hypothesis had been verified by some studies with small specimen,6-9but the knowledge about cardiovascular diseases risk among patients with IMN are limited.10,15-17Lee et al demonstrated that patients with IMN were at high risk of cardiovascular events (CVEs).10In their cohort, the cumulative incidences of CVEs were 4.4%, 5.4%, 8.2%, and 8.8%at 1, 2, 3, and 5 years respectively. Severe renal dysfunction at baseline and severity of nephrotic syndrome were statistically associated with an increased occurrence of CVEs. In our cohort, the cumulative incidences of ATEs were relatively lower compared to those in the Lee’s study.10This difference might due to there were more patients with severe nephrosis and renal dysfunction in their cohort (proteinuria: 8.7 ±6.2 g/d, serum albumin: 25 ± 8.0 g/L, eGFR: 68.9 ±33.5 ml/min/1.73m2) than in ours. Low eGFR was also associated with ATEs, although this association did not reach statistical significance (P=0.10).
In our cohort, IS accounted for 45% of the ATEs,which was obviously distinguished from the results in almost all the studies from western countries, where the majority of the ATEs in their cohorts were CHD.This finding should raise attention on IS as a frequent thromboembolic event in Chinese patients with IMN.
ATEs presented a peak onset early in the 0.5 years after biopsy, with an increasing accumulate incidence over time. It seemed that the occurrence of ATEs had two types: ATE early onset (occurred within 0.5 years) and ATE delayed onset (occurred after 0.5 years). At the time of ATE events, 59.3% of patients experienced early onset of ATE were at nephrotic syndrome status, and presented very low serum albumin levels, while those experienced delayed onset of ATE were mainly at sub-nephrotic syndrome (38.7%) or in remission (35.5%). The severity of nephrotic syndrome at the time of the event was closely related to the risk of early onset ATEs. These findings were in accordance with studies of Lee et al10and Mahmoodi et al.9Furthermore, univariable survival analysis showed ATEs were associated with proteinuria, age, smoking,history of hypertension and prior ATE, while multivariable survival analysis model indicated that age independently predict ATEs. All these findings above in this study support the hypothesis that early onset of ATE is probably due to the thrombophilic state of NS, and delayed onset of ATEs may be caused by the accelerated atherosclerosis associated with hyperlipidemia.10
VTE is a common complication in patients with nephrotic syndrome, especially in MN. In our cohort,VTE occurred in 6.9% of IMN patients. The underlying mechanisms are incompletely understood. In nephrotic syndrome, the decreased levels of serum proteins,such as antithrombin Ⅲ, and increased production of fibrinogen in the liver due to urinary loss of pro- and anticoagulant proteins or hypoalbuminemia were considered to be involved in the hypercoagulable state,which results in the increased risk of VTEs.18Many researchers reported RVT as the most common venous thromboembolism in patients with MN,18-20however,there is still controversial in whether DVT or RVT occurs more frequently in this situation.5,21This study revealed that DVTs accounted for 60% of all VTE events in our cohort.
We found that VTEs were mainly occurred within the first 0.5 years after diagnosis, and the accumulative incidence went stable after 2 years. At the time of VTEs events, majority of patients were at NS status (81.5%), with no difference between the early and the delayed onset (79.2% and 75.0%).Previous studies demonstrated that proteinuria, hypoalbuminemia, and the ratio of proteinuria to serum albumin were risk factors for the onset of VTE.4,19,20,22Recently, Lionaki et al reported that each 10 g/L reduction in serum albumin was associated with a 2.13 fold increased risk of VTE; an albumin level of 28 g/L was the threshold below which the risk for a venous thromboembolic event was the greatest. The authors concluded that hypoalbuminemia at diagnosis of IMN independently predicted a venous thromboem-bolic event.20Our study found that the hazard ratio reached to 5.5 for VTE when serum albumin <20 g/L,which further confirmed that hypoalbuminemia was the dominant independent risk factor for VTE. However, proteinuria was not shown to independently predict VTE by multivariable analysis. As most patients can reach remission after efficient therapy in 2 years,23patients achieved remission in our cohort were about 70%, thus might explain the stable accumulate incidence of VTE after 2 years of diagnosis.
At the time of events, 78.6% of IMN patients who experienced VTEs were at NS status, compared to 41.4% of IMN patients with ATE were at NS status. This finding suggested that severe nephrotic syndrome status may be prone to the onset of VTE.Besides, history of ATE suggested a high risk of a repeated ATE, but had no association with the VTEs.
There are several limitations in our study. As a retrospective study, some baseline laboratory data and follow-up laboratory data were not gainable in all patients. Patients with asymptomatic VTEs did not routinely followed up, and patients with mild symptoms of ATEs were ignored. The absence of these data could lead to the underestimate of the incidences. Although it is the largest cohort that investigate thromboembolic events in Asian patients with IMN by far, the wide confidence intervals of hazard ratios weaken the power of the results. Besides, we failed to further analyze the influence of medications in the cohort, such as glucocorticoids, immunosuppressors,aspirin, and warfarin. Whether prophylactic anticoagulation therapy could lower the risks of events is still unclear and need to be elucidated by further investigations.
In summary, this study reveals that patients with IMN have increased incidences of ATEs and VTEs, especially within the first 6 months of the disease. The majority of ATEs were cardiovascular diseases and thrombotic ischemic stroke, and for VTEs were deep vein thrombosis. IS accounted for 45%of ATEs, which should raise attention for the clinical management of patients with IMN in Chinese population. Proteinuria, aging, history of smoking, hypertension and prior ATE were associated with ATEs. Hypoalbuminemia was the dominant independent risk factor for VTE. Both ATEs and VTEs occured frequently at the status of nephrotic syndrome, but VTEs tend to be more likely to occur than ATEs among patients with nephrotic syndrome. Clinical management to prevent thromboembolic events in the early stage of IMN should gain particular attention in patients who are at the status of nephrotic syndrome.
REFERENCES
1. Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol 2005; 16(5): 1188-94. doi: 10.1681/ASN.2005010028.
2. Pan X, Xu J, Ren H, et al. Changing spectrum of biopsy-proven primary glomerular diseases over the past 15 years: a single-center study in China. Contrib Nephrol 2013; 181: 22-30. doi: 10.1159/000348638.
3. Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2): 190-5. doi: 10.1038/ki.2011.312.
4. Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol 2007; 18(8): 2221-5. doi: 10.1681/ASN.2006111300.
5. Kayali F, Najjar R, Aswad F, et al. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med 2008; 121(3): 226-30. doi:10.1016/j.amjmed.2007.08.042.
6. Gilboa N. Letter: Incidence of coronary heart disease associated with nephrotic syndrome. Med J Aust 1976; 1(7): 207-8.
7. Ordo?ez JD, Hiatt RA, Killebrew EJ, et al. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 1993; 44(3): 638-42.doi:10.1038/ki.1993.292.
8. Wass VJ, Jarrett RJ, Chilvers C, et al. Does the nephrotic syndrome increase the risk of cardiovascular disease? Lancet 1979; 2(8144): 664-7. doi:10.1016/s0140-6736(79)92067-1.
9. Mahmoodi BK, ten Kate MK, Waanders F, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 2008; 117(2): 224-30. doi:10.1161/CIRCULATIONAHA.107.716951.
10. Lee T, Derebail VK, Kshirsagar AV, et al. Patients with primary membranous nephropathy are at high risk of cardiovascular events. Kidney Int 2016; 89(5): 1111-8. doi: 10.1016/j.kint.2015.12.041.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604-12.
12. Troyanov S, Wall CA, Scholey J W, et al. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int 2004; 66(3): 1199-205. doi: 10.1111/j.1523-1755.2004.00873.x
13. Radhakrishnan J, Appel AS, Valeri A, et al. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis 1993; 22(1): 135-42. doi:10.1016/s0272-6386(12)70179-8.
14. Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int 2016; 90(1): 41-52. doi: 10.1016/j.kint.2016.02.026.
15. Nandwani A, Pathania D, Jha PK, et al. Renal artery thrombosis with renal infarction: a rare cause of acute abdomen. Indian J Nephrol 2017; 27(4): 313-5. doi:10.4103/0971-4065.183581.
16. Parag KB, Somers SR, Seedat YK, et al. Arterial thrombosis in nephrotic syndrome. Am J Kidney Dis 1990;15(2): 176-7. doi:10.1016/s0272-6386(12) 80516-6.
17. Sasaki Y, Raita Y, Uehara G, et al. Carotid thromboembolism associated with nephrotic syndrome treated with dabigatran. Case Rep Nephrol Urol 2014; 4(1):42-52. doi: 10.1159/000362162.
18. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res 2006; 118(3): 397-407. doi: 10.1016/j.thromres.2005.03.030.
19. Li SJ, Guo JZ, Zuo K, et al. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome-a prospective study. Thromb Res 2012; 130(3):501-5. doi: 10.1016/j.thromres.2012. 04.015.
20. Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1): 43-51.doi: 10.2215/CJN.04250511.
21. Llach F. Hypercoagulability, renal vein thrombosis,and other thrombotic complications of nephrotic syndrome. Kidney Int 1985; 28(3): 429-39. doi:10.1038/ki.1985.149.
22. Bellomo R, Atkins RC. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron 1993; 63(3): 249-54. doi:10.1159/000187205.
23. Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy-a modern view. Clin J Am Soc Nephrol 2014; 9(3): 609-16. doi: 10.2215/CJN.04160413.
 Chinese Medical Sciences Journal2018年2期
Chinese Medical Sciences Journal2018年2期
- Chinese Medical Sciences Journal的其它文章
- Early Diagnosis of Recurrent Optic Neuritis Using Contrast-Enhanced T2 Fluid-attenuated Inversion Recovery Imaging: a Case Report
- Effect of Red Ginseng Extract on the Pharmacokinetics of Aspirin Metabolite in Sprague Dawley Rats
- Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study
- Effects of Short-term High Dose Atorvastatin on Left Ventricular Remodeling in Patients with First Time Attack of Anterior Acute Myocardial Infarction
- Irrationality of Allogeneic Red Blood Cell Transfusion in Intraoperative Cell Salvage Patients: a Retrospective Analysis
- Midterm Follow-up of Coronary Artery Bypass Grafting with 64-Slice Multi-detector Computed Tomography:Identification of Risk Factors Affecting Graft Patency
