Soil microbial characteristics and yield response to partial substitution of chemical fertilizer with organic amendments in greenhouse vegetable production
RONG Qin-lei, Ll Ruo-nan, HUANG Shao-wen, TANG Ji-wei, ZHANG Yan-cai, WANG Li-ying
1 Key Laboratory of Plant Nutrition and Fertilizer, Ministry of Agriculture/Institute of Agricultural Resources and Regional Planning,Chinese Academy of Agricultural Sciences, Beijing 100081, P.R.China
2 Institute of Agricultural Resources and Environment, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang 050051,P.R.China
1. lntroduction
In China, greenhouse vegetable production has undergone rapid development from less than 7 000 hectares in 1980 to almost 3.7 million hectares in 2015 (Huanget al.2016).The amount of inorganic nitrogen (N) fertilizer used on vegetable fields is greater than that used on cereal crops(Juet al.2006; Minet al.2011). This increased application of inorganic N can cause environmental pollution and soil degradation (Shenet al.2010; Rezaei Rashtiet al.2015).To avoid these problems, applying chemical fertilizers that are partially substituted with organic amendments is an economical and effective practice (Syswerdaet al.2012;Aparnaet al.2014). However, as an intensive and unique form of agriculture, greenhouse vegetable production has been characterized by a high cropping index and high agricultural inputs (Liet al.2001; Zhuet al.2011; Yanget al.2014), which is different from open fields in the same region. As a result, nutrient cycling induced by microbial activities undergoes large, relative changes in greenhouse vegetable production (Linet al.2004; Qinet al.2016; Yaoet al.2016). Thus, a better understanding of how partial substitution with organic amendments affects activity and microbial community composition of soil is needed in order to determine the implications for managing greenhouse agroecosystems.
Soil microbes make important contributions to biologicallyand biochemically-mediated processes in soil, e.g., the decomposition of soil organic matter and nutrient retention(Nannipieriet al.2003; K?hl and van der Heijden 2016).Microbial parameters such as microbial biomass, enzyme activities, and activity and composition of the microbial community are sensitive and significant indicators for assessing changes in soil quality (Sharmaet al.2015).Soil microbial biomass, as a living part of soil organic matter, is an early indicator of soil management changes.Several long-term experiments have found that organic fertilization increases microbial biomass because the organic amendment delivers large amounts of external carbon (C) to soil (Kaschuket al.2010; Zhanget al.2015).However, the response of the microbial biomass to organic C input depends upon the application rate and the chemical composition of the organic amendments used (Kallenbach and Grandy 2011). Therefore, more information is needed on the effects of different inputs of organic amendments as substitutes on soil microbial biomass in greenhouse vegetable production.
Phospholipid fatty acid (PLFA) analysis, as an established technique, has been widely used to evaluate the influence of management practices or environmental factors on changes in the composition of microbial communities (Zelles 1999). The relative abundances of Gram-negative bacteria,saprophytic fungi, and actinomycetes were related to soil organic C (SOC) transformation or turnover in the forest soil of the Baotianman Nature Reserve (Youet al.2014).The application of chemical N fertilizer increased the total microbial biomass and fungal abundance but decreased the bacterial abundance in a fluvo-aquic soil in a wheat(Triticum aestivumL.)-maize (Zea maysL.) rotation (Aiet al.2012). The influence of management practices such as cover crops, continuous cropping, and compost on soil microbial community composition has attracted attention in greenhouse vegetable production (Maulet al.2014;Willekenset al.2014). Nonetheless, the effect of inorganic fertilizers with different percentages of organic amendments on soil microbial community composition has not been well documented.
Enzyme activity makes nutrients more available to plants and microorganisms by mineralizing organic C, N, sulphur(S), and phosphorus (P) from soil organic matter (Waringet al.2014). Adding organic material often leads to an overall increase in enzyme activity (Bonanomiet al.2014),but the response depends on changes in soil management and plant cover of soil (Nannipieriet al.2012). Longterm application of horse manure compost to greenhouse vegetable soil enhanced α-galactosidase, β-galactosidase,α-glucosidase, and β-glucosidase activities of soil (Zhanget al.2015). The activities of dehydrogenase, urease, and neutral phosphatase decreased significantly by increasing the rate of N application to a 2-year tomato (Solanum lycopersicumMill.)-cucumber (Cucumis sativusL.) rotation in China’s Yangtze River Delta (Shenet al.2010). At present, most research is focused on enzyme activity related to N and C transformation in greenhouse vegetable production systems. Very few studies have explored the response of soil enzymes involved in the C, N, S, and P biochemical cycles as affected by different percentages of organic amendment substitutes to inorganic fertilizers in greenhouse vegetable production.
The objective of this work was to examine the effect of using different patterns of organic amendments as partial substitutes for chemical fertilizers on microbial biomass carbon (MBC) and nitrogen (MBN), soil microbial community composition, enzyme activity, and vegetable yields in greenhouse conditions. We hypothesized that changes in microbial properties would depend on different organic amendments (pig manure and straw), and these changes would be related to vegetable yield. To test our hypotheses,a 5-year field experiment was conducted with the following aims: (1) to investigate changes in chemical, biochemical,and microbial properties of soil and vegetable yield and to compare these patterns; and (2) to analyze the relationship between soil microbial properties and vegetable yield.
2. Materials and methods
2.1. Site description
The 5-year experiment was conducted at the Dahe Experimental Station belonging to the Hebei Academy of Agriculture and Forestry Sciences, Hebei Province,China (38°08′N, 114°23′E). The study site has a warm,sub-humid continental monsoon climate with an average annual temperature of 11.5°C and annual precipitation of 540 mm.
The solar greenhouse employed in the test measured 8 m× 48 m. The original surface soil was used for building the back wall. A crop rotation of winter-spring cucumber(Bomei 11) and autumn-winter tomato (Jinpeng 11) was used in the experiment from October 2009, and the field was left fallow between the growth periods of the two crops. The soil in the study has a clay loam texture and can be classified as calcareous cinnamon soil (FAO classification). The main soil properties (0–20 cm depth) are as follows: soil bulk density,1.35 g cm–3; electrical conductivity, 185.4 mS cm–1; pH, 8.0;organic matter, 9.1 g kg–1; nitrate N, 18.3 mg kg–1; available P, 6.2 mg kg–1; and available K, 98.2 mg kg–1.
2.2. Experimental design
A randomized block design was used with 3 replications and 5 different treatments as follows: (1) 4/4CN (CN, N in chemical fertilizer); (2) 3/4CN+1/4MN (MN, N in pig manure);(3) 2/4CN+2/4MN; (4) 2/4CN+1/4MN+1/4SN (SN, N in corn straw); and (5) 2/4CN+2/4SN. The amount of nutrient inputs (N, P2O5, and K2O) for the five treatments was the same. The nutrient inputs were determined based on soil tests and nutrient requirements for target yields (150 t ha–1for cucumber and 90 t ha–1for tomato) (Huanget al.2017).The nutrient inputs of N, P2O5, and K2O used in the winterspring cucumber season were 600, 300, and 525 kg ha–1,respectively, whereas those in the autumn-winter tomato season were 450, 225, and 600 kg ha–1, respectively.
The chemical fertilizers used in the experiment included urea, calcium superphosphate, and potassium chloride. In order to keep the fertilizer treatments comparable with only small interannual changes every year, fresh commercial pig manure was used, which contained (33.2±4.5)% of water,(1.67±0.13)% of N, (1.30±0.12)% of P2O5, (1.04±0.12)%of K2O, and (142.6±6.8) g kg–1of C. The fresh straw used contained ((13.7±1.6)% of water, (0.75±0.08)%of N, (0.20±0.03)% of P2O5, (1.21±0.22)% of K2O, and(324.1±12.3) g kg–1of C. In the winter-spring cucumber season, 9 t ha–1of fresh commercial pig manure was used in the treatments 3/4CN+1/4MN and 2/4CN+1/4MN+1/4SN,of which 25% of the N came from pig manure; 20 t ha–1of fresh straw was used in the 2/4CN+1/4MN+1/4SN treatment,of which 25% of the N came from straw. In the autumnwinter tomato season, 6.75 t ha–1of fresh commercial pig manure was used in the treatments 3/4CN+1/4MN and 2/4CN+1/4MN+1/4SN, of which 25% of the N came from pig manure; and 15 t ha–1of fresh straw was used in the 2/4CN+1/4MN+1/4SN treatment, of which 25% of the N came from straw. When 50% N was substituted, the consumption of pig manure and straw doubled. Inputs of N,P2O5, and K2O for each organic amendment treatment were calculated by each nutrient concentration in pig manure and corn straw and the organic amendment investments. The amounts of N, P2O5, K2O, and C from chemical fertilizer,manure, and straw for each treatment are given in Tables 1 and 2.
All the pig manure and 20% N, 100% P, and 40% K of the chemical fertilizer were evenly broadcast onto and mixed into the soil through rotary tillage. The crop straw was cut into short pieces and scattered within the 20–25 cm soil layer and then was covered with soil. In the winter-spring cucumber season, the remaining 80% of the N and 60%of the K was divided equally into 10 parts and top-dressed according to the nutrient requirements of the crop. In the autumn-winter tomato seasons, the rest of the fertilizer (80%N; 60% K) was divided into four parts and top-dressed at the expanding stage of the first to fourth fruit spikes when the diameter of the fruits reached 3–4 cm.
The experimental plot corresponded to an area of 14.4 m2(2.4 m wide×6 m long). In each plot, 4 rows with 20 plants per row were planted (0.6 m between rows, 0.30 m between plants). Polyvinyl chloride (PVC) plates were embedded into the soil between plots to a depth of 100 cm,which extended above the ground by 5 cm. These were used to prevent lateral and transverse migration of nutrients and water between plots. Furrow irrigation was employed in the experiment. A soil probe (WITU technology Inc.,Shenyang, China) was inserted into the soil to a depth of 20 cm to monitor and control the moisture content at 75–95%of the field capacity.
2.3. Soil sampling and analyses
Sampling protocolSoil samples were collected from each plot at 0–20 cm depth during the uprooting stage of the fifth crop cycle (10 July 2014). Fresh soil samples were immediately transported to the laboratory in an ice-box for further processing. Gravel and residual roots were removed,and the soil samples were sieved (2-mm). A portion of each sample was then stored at 4°C to be analyzed for enzyme activities, soil microbial biomass, and dissolved organic C(DOC) and N (DON). Another part was stored at –70°C for microbiological PLFA analysis. The rest of each sample was air-dried for chemical analysis.
Soil analysesBoth MBC and MBN were determined using the fumigation-extraction technique (Wuet al.1990),while the filtrates were analyzed using a TOC/TN analyzer(Analytik Jena, Multi N/C 3100). Both of the efficiencyconstants required for MBC and MBN calculation,kECandkEN, were taken to be 0.45 (Wuet al.1990; Joergensen 1996). The SOC content was determined by oxidation with potassium dichromate and titration with ferrous ammonium sulfate. The nitrate-N level (NO3–-N) was determined using the dual-wavelength ultraviolet spectrophotometric method(Normanet al.1985). DOC and DON were analyzed as described by Ghaniet al.(2007).
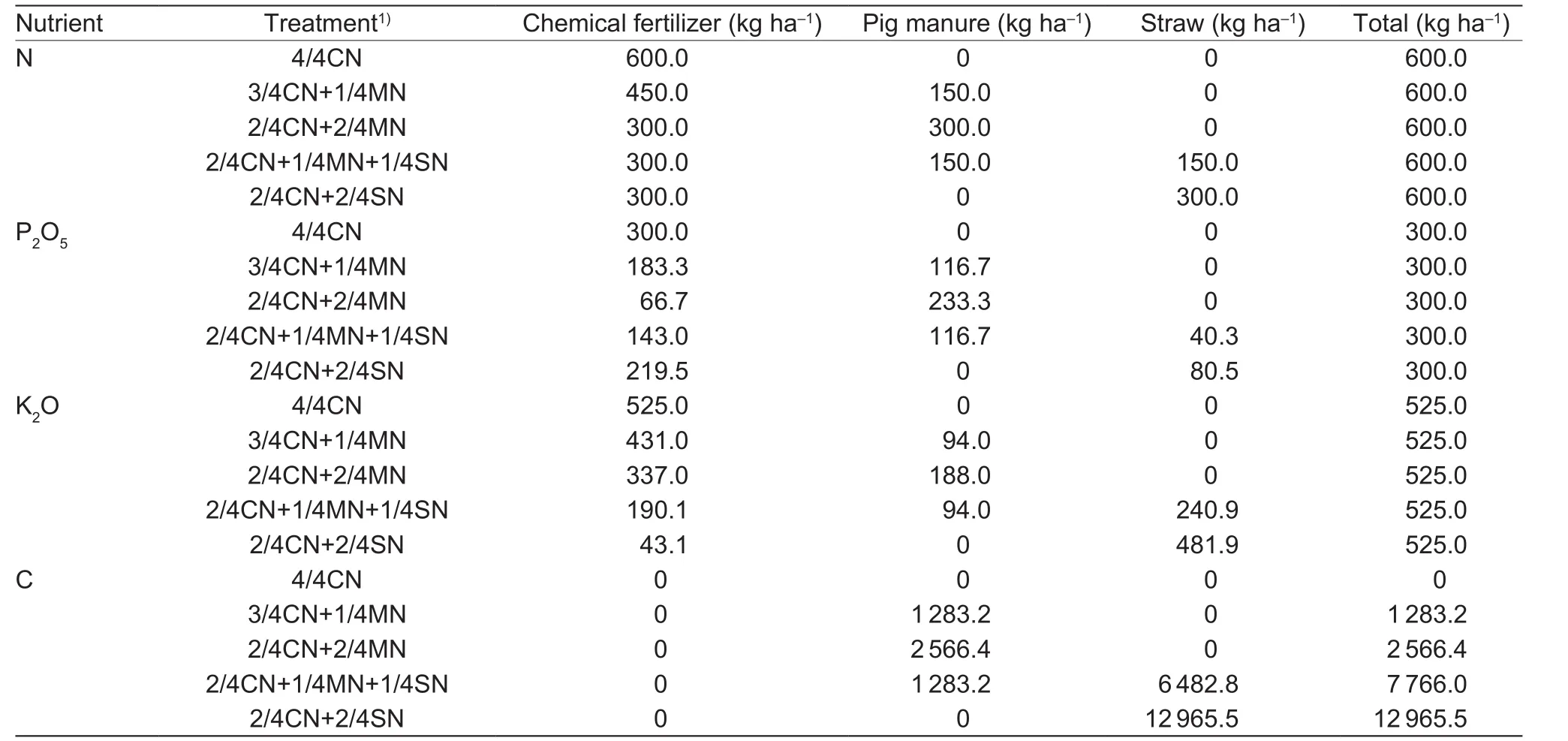
Table 1 Amounts of nitrogen (N), phosphorus (P2O5), and potassium (K2O) used in each treatment during the winter-spring cucumber season
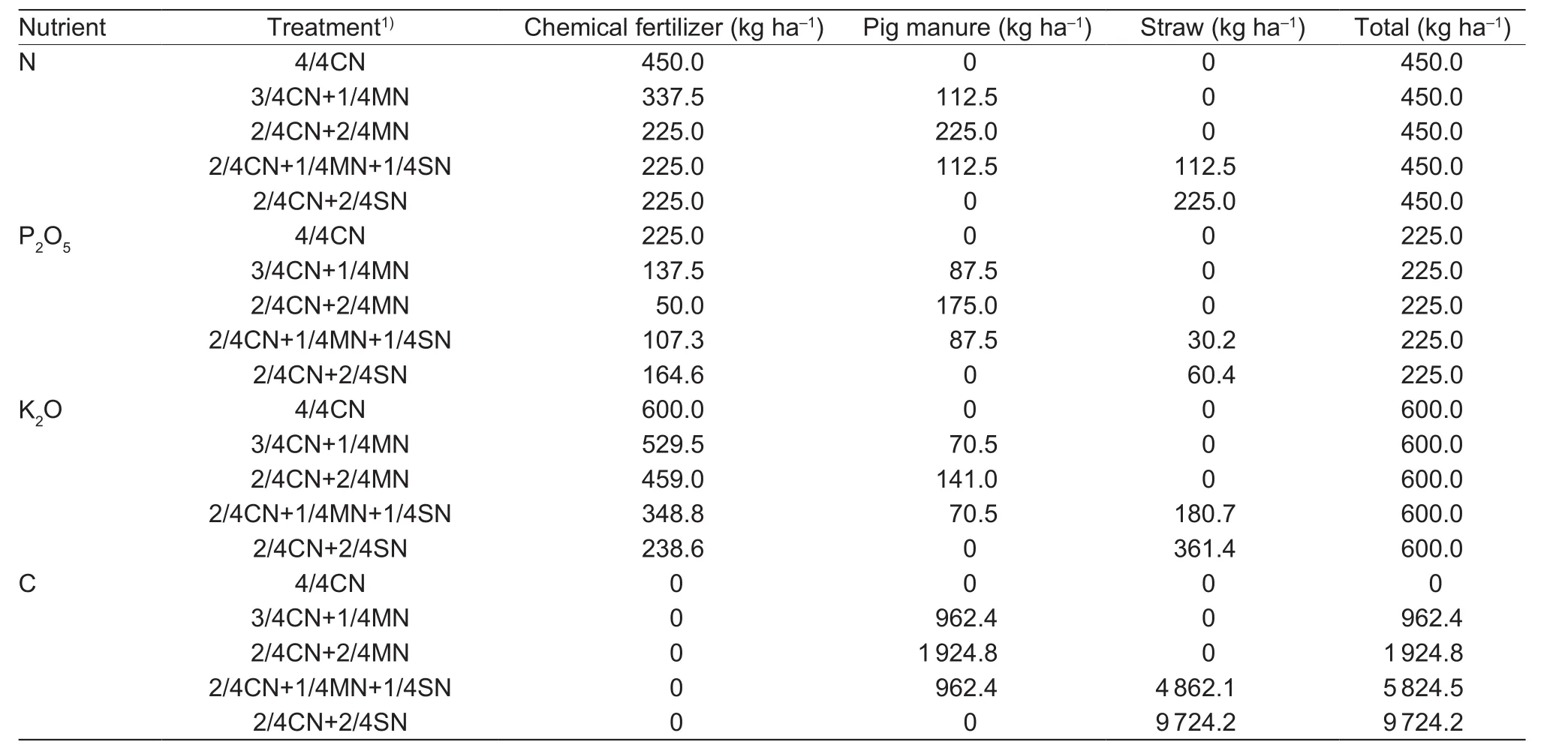
Table 2 Amounts of nitrogen (N), phosphorus (P2O5), and potassium (K2O) used in each treatment during the autumn-winter tomato season
Enzyme activityAll enzyme activities with the exception of urease, phenol oxidase, and peroxidase activities were determined using the microplate fluorometric assay(DeForest 2009; Aiet al.2015). The fluorescence was quantified using a microplate fluorometer (Scientific Fluoroskan Ascent FL, Thermo, USA) with 365 nm excitation and 450 nm emission filters (Saiya-Corket al.2002). The enzyme activities were expressed in nmol h–1g–1. Urease activity was determined using urea as the substrate as described by Kandeler and Gerber (1988) and was expressed as mmol NH4+g–1dry soil h–1.
PLFA analysisThe composition of the soil microbial community was determinedviaPLFA analysis according to the procedure described by Wuet al.(2009). The abundance of individual PLFAs was indicated by its %mole abundance in each sample. PLFAs were divided into various taxonomic groups based on previously published PLFA biomarker data (Aiet al.2012; Moeskopset al.2012).Specifically, we used i14:0, a15:0, i15:0, 16:0, i16:0, 17:0,a17:0, cy17:0, i17:0, and cy19:0ω8c as bacteria biomarkers;i14:0, a15:0, i15:0, i16:0, a17:0, and i17:0 as Gram-positive bacteria biomarkers; and cy17:0 and cy19:0ω8c as Gramnegative bacteria biomarkers. The unsaturated PLFA 18:1ω9c was used as a fungal biomarker. The fatty acids 16:0 (10Me), 17:0 (10Me), and 18:0 (10Me) were used as markers for actinomycetes.
2.4. Statistical analysis
Statistical analysis was carried out using the SAS software package (ver. 9.1). A two-way randomized block ANOVA test was performed to analyze each variable using Fisher’s least significant difference (P=0.05) to compare the treatment means. Pearson correlation analysis was performed to determine the relationship of MBC and MBN, enzyme activity, microbial community composition, and yield (5 different treatments of the fifth year, three replicates each).Principal component analysis (PCA) and redundancy analysis (RDA) with the Monte Carlo permutation test (499 permutations) were performed to determine if soil enzyme activity or community composition was correlated with soil properties, as implemented in Canoco 5.0.
3. Results
3.1. Changes in vegetable yield
The cucumber and tomato yields from 2010 to 2014 are shown in Table 3. The 4/4CN treatment had higher vegetable yield than treatments using organic amendment substitutions in the first three growing seasons. Since the autumn-winter tomato season in 2012,straw treatment (2/4CN+2/4SN) significantly increased tomato yield compared with 4/4CN treatment (except in the autumn-winter tomato season in 2013). For cucumber yield,straw treatment (2/4CN+1/4MN+1/4SN, 2/4CN+2/4SN)induced a slight increase in the fifth growing season in 2012, however, a significant increase was observed in the following winter-spring cucumber seasons in 2013 and 2014.However, there was no significant difference between 2/4CN+1/4MN+1/4SN and 2/4CN+2/4SN.
3.2. Changes in microbial biomass C and N
Both MBC and MBN were significantly affected by the organic amendments in the ninth growing season (except 3/4CN+1/4MN) (Fig. 1). The MBC and MBN values in organic-amended soil (i.e., 3/4CN+1/4MN, 2/4CN+2/4MN,2/4CN+1/4MN+1/4SN, and 2/4CN+2/4SN), were in the ranges of 137.0–290.2 and 36.2–57.2 mg kg–1, respectively.These values are much higher than those in 4/4CN treatment, which were in the ranges of 10.5–134.1 and 28.1–102.1%, respectively (Fig. 1).
Both MBC and MBN also increased with higher amounts of added pig manure or straw (Fig. 1). The MBC and MBN in the 2/4CN+2/4MN treatment were, on average,25.2 and 32.8% higher, respectively, than those in the 3/4CN+1/4MN treatment. Similarly, MBC and MBN in the 2/4CN+2/4SN treatment were, on average, 27.9 and 6.7%higher, respectively, than those in the 2/4CN+1/4MN+1/4SN treatment.

Table 3 The effects of different partial replacements of inorganic fertilizer (t ha–1) on the yields of winter-spring cucumber and autumn-winter tomato in the greenhouses from 2010 to 2014
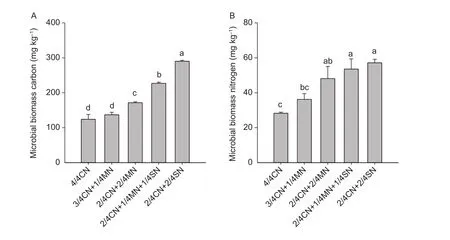
Fig. 1 The effects of different partial organic-amendment replacements on microbial biomass carbon (A) and nitrogen (B) in the soil in the uprooting stage of the 9th growing season (winter-spring cucumber). CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw. Bars indicate standard error, n=3. Different letters indicate significant differences between treatments (at the P< 0.05 level).
3.3. Changes in soil enzyme activity
Enzymes involved in C, N, and P cycling were significantly increased in activity in the organic-amended soil. The largest soil enzyme activities were observed in soils amended with straw (Fig. 2). Compared with 4/4CN, the activities of β-glucosidase, β-cellobiosidase, β-xylosidase,and α-glucosidase (involved in the C cycle) were,respectively, increased by 130.9–477.7, 215.1–968.6,81.6–412.4, and 47.0–183.9% in soil amended with straw(2/4CN+1/4MN+1/4SN, 2/4CN+2/4SN). The activity of N-acetyl-glucosaminidase, L-leucine aminopeptidase, and urease (related to the N cycle) were increased by 58.4–545.2, 0.1–37.8, and 38.8–188.5%, respectively. In contrast,the activity of soil phosphomonoesterase (involved in the P cycle) increased by 8.6–162.9% (Fig. 2). Sulfatase activity in the organic-amended soil was significantly decreased,especially when straw was used. The sulfatase activity fell by 2.1–9.3% compared to 4/4CN, and by 1.2–7.4%compared to pig-manure treated soil (Fig. 2).
Ordination of the fertilization treatmentsviaPCA shows that they are primarily related to the first canonical axis(PC1) (PC1=95.1%). The samples were separated into three distinct groups, each possessing a specific range of soil MBC values (Fig. 3-A). The first group included 4/4CN,3/4CN+1/4MN, and 2/4CN+2/4MN treated soil, i.e., those generally having lower MBC values of 124.0–171.6 mg kg–1(Fig. 3-A). The second group included soil treated with 2/4CN+1/4MN+1/4SN which had an intermediate MBC value of 227.0 mg kg–1. The third group, containing 2/4CN+1/4MN+1/4SN treated soil, had a high MBC value of 290.2 mg kg–1. Indeed, RDA confirmed that soil MBC had a statistically significant effect (F=110,P<0.01) on enzyme activity, and that it accounted for 89.5% of the total enzyme activity variation (Fig. 3-B). In addition, DON was also significantly related to enzyme activity (F=2.3,P=0.03)and accounted for 2.8% of the variation in the total enzyme activity (Fig. 3-B).
3.4. Changes in abundance and composition of the microbial communities
Treatments using different organic-amendment substitutes increased the total PLFA content, though to different degrees, with the increases ranging from 7.0 to 66.1%compared with 4/4CN (Fig. 4-A). In particular, the soils treated with 50% N substituted by straw (2/4CN+2/4SN)had significantly higher PLFA content than that in 4/4CN treatment.
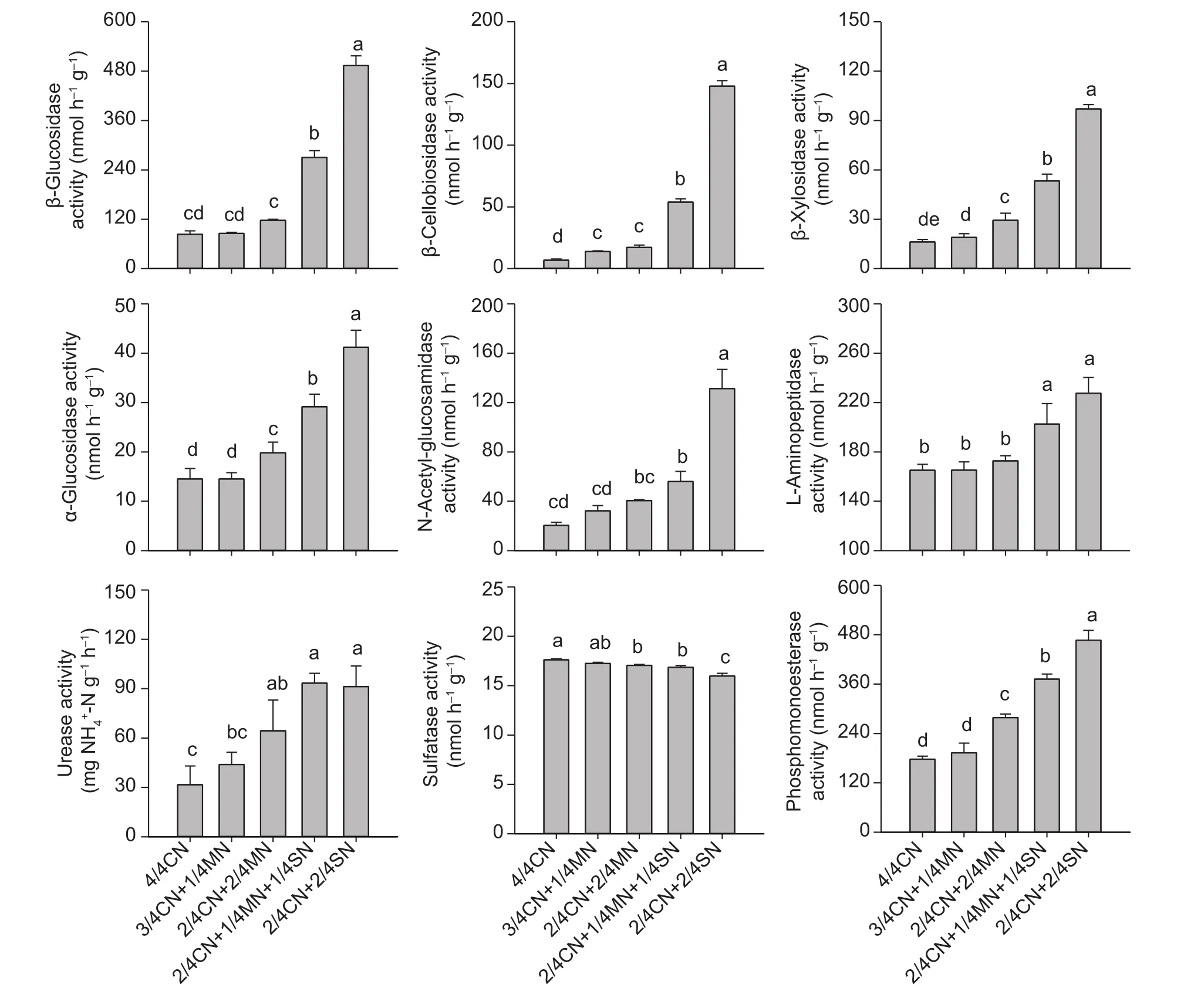
Fig. 2 The response of enzyme activity to different partial organic-amendment replacements in the uprooting stage of the 9th growing season. CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw. Bars indicate standard error, n=3. Different letters indicate significant differences between treatments at P<0.05.
The relative abundance of bacteria was significantly higher in the 2/4CN+2/4SN-treated soil than that in 4/4CN-treated soil, whereas there were no differences between pig manure and straw-treated soils. Abundance of fungi did not show a clear trend in response to organic-amended soil, except for a small but significant difference between 2/4CN+1/4MN+1/4SN and 4/4CN-treated soil. Compared with the 4/4CN-treated soil, the relative abundances of actinomycetes were remarkably increased in the strawtreated soil and the soils treated with 50% N substituted by pig manure. The ratio of Gram-positive to Gram-negative bacteria and bacteria to actinomycetes was significantly decreased in straw-treated soils (2/4CN+1/4MN+1/4SN and 2/4CN+2/4SN) compared to that in 4/4CN-treated soil.However, there were no significant differences in these ratios between pig manure-treated and 4/4CN-treated soil.
PCA ordination analysis showed that the fertilization treatments could be separated into two distinct groups,each possessing a specific range of soil MBC values(Fig. 5-A). The first group (4/4CN, 3/4CN+1/4MN, and 2/4CN+2/4MN treated soil) generally had lower MBC,ranging from 124.0 to 171.6 mg kg–1(Fig. 5-A). The second group (2/4CN+1/4MN+1/4SN and 2/4CN+2/4SN treated soil) had higher MBC, ranging from 227.0 to 290.2 mg kg–1. The difference is significantly related to the change in soil MBC (F=14.3,P<0.01) (Fig. 5-B), which accounted for 52.3% of the total variation in the composition of the microbial community, based on the RDA between microbial community and soil property.
3.5. Correlations between yield and soil microbial properties
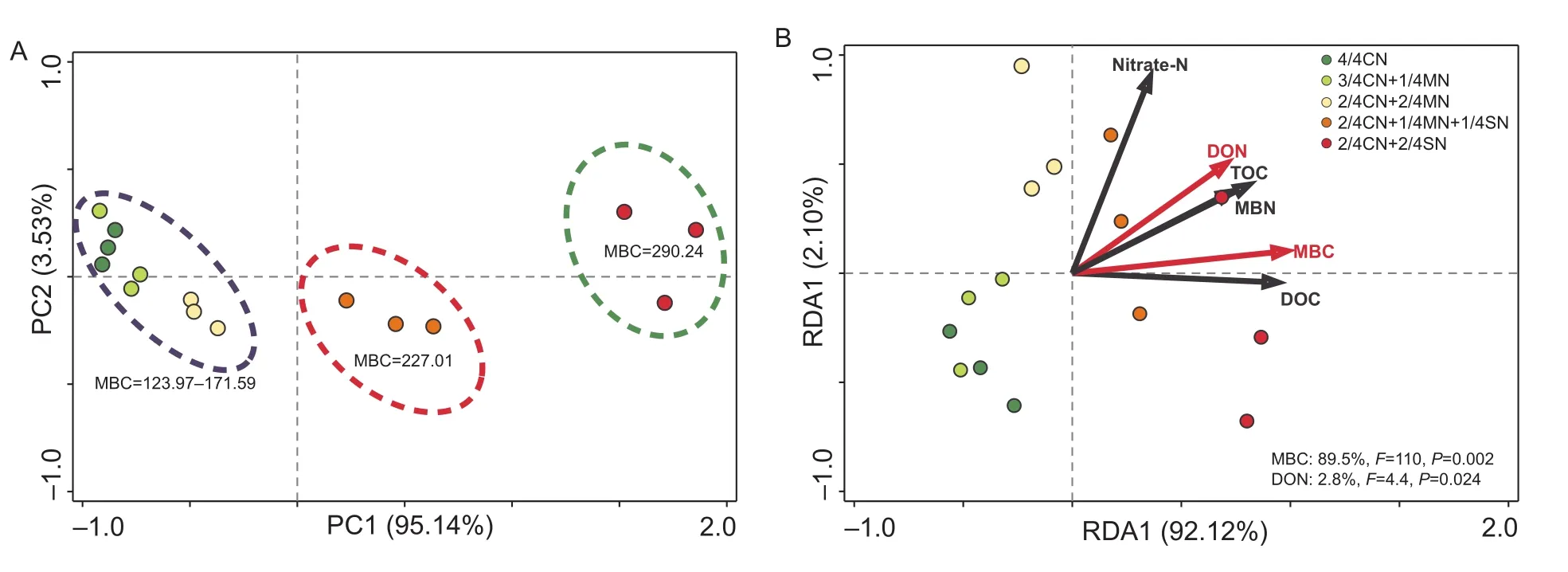
Fig. 3 Results of principal component analysis (PCA) of the enzyme activity in soil subjected to different fertilization treatments(A), and redundancy analysis (RDA) of the correlation between soil parameters and enzyme activity profiles (B). MBC, microbial biomass carbon; nitrate-N, NO3–-N; DON, dissolved organic nitrogen; TOC, total organic carbon; MBN, microbial biomass nitrogen;DOC, dissolved organic carbon. CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw. The data are from the uprooting stage of the ninth growing season (winter-spring cucumber). Red arrows indicate soil parameters that have a strong and significant impact on enzyme activity (P< 0.05). The corresponding proportion of the variation accounted for is shown in the lower right corner.

Fig. 4 A comparison of the total phospholipid fatty acid (PLFA; A), the relative abundance of bacteria (histograms) and the G+ :G– ratio (dotted line; B), the relative abundance of fungi (C) and the relative abundance of actinomycetes (histograms) and bacteria:actinomycetes ratio (dotted line; D). G+, Gram-positive bacteria; G–, Gram-negative bacteria; CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw. Vertical bars represent the standard error (n=3) and lower case letters indicate significant differences between fertilizer treatments at the P< 0.05 level.
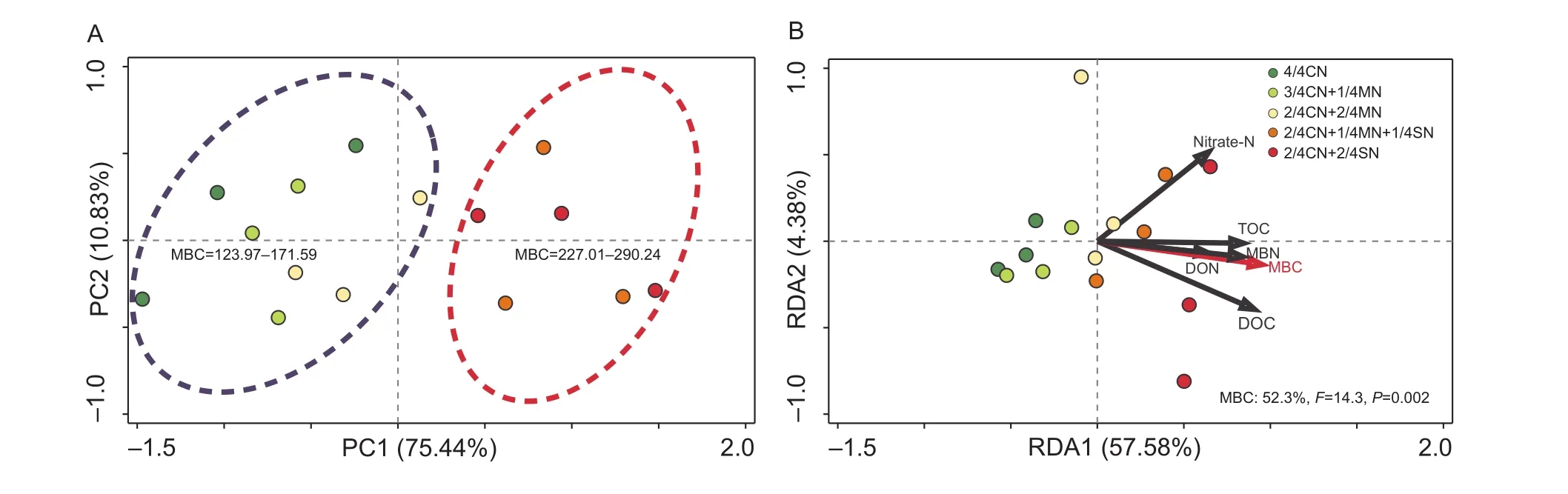
Fig. 5 Results of principal component analysis (PCA) of the microbial community composition in soils receiving different fertilization treatments (A), and redundancy analysis (RDA) of the correlation between soil parameters and microbial community composition (B).MBC, microbial biomass carbon; nitrate-N, NO3–-N; TOC, total organic carbon; MBN, microbial biomass nitrogen; DON, dissolved organic nitrogen; DOC, dissolved organic carbon. CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw. The data are from the uprooting stage of the ninth growing season (winter-spring cucumber). Red arrows indicate soil parameters that have a strong and significant impact on the microbial community composition (P< 0.05). The corresponding proportion of the variation accounted for is shown in the lower right corner.
Overall, the trends found in the total PLFA content,composition of the microbial communities (bacteria,actinomycetes, Gram-positive bacteria, Gram-negative bacteria), and soil enzyme activity showed that these properties generally increased with increasing MBC and MBN (Table 4). A correlation analysis revealed that the activities of most of the enzymes (phosphomonoesterase,β-glucosidase, β-cellobiosidase, N-acetylglucosaminidase,β-xylosidase, α-glucosidase, L-leucine aminopeptidase,and urease) were significantly positively correlated with soil MBC and MBN. However, sulfatase was significantly negatively correlated with MBC and MBN. MBC was significantly positively correlated with the total PLFA content, bacteria, actinomycetes, Gram-positive and Gram-negative bacteria. The analysis also showed that there were significantly positive correlations between enzyme activities (phosphomonoesterase, β-glucosidase,β-cellobiosidase, N-acetylglucosaminidase, β-xylosidase,α-glucosidase, L-leucine aminopeptidase, and urease)and the composition of the microbial communities(bacteria, actinomycetes, Gram-positive bacteria, Gramnegative bacteria). Furthermore, MBC, enzyme activity(phosphomonoesterase, L-leucine aminopeptidase, and urease), and composition of the microbial communities (total PLFA content, bacteria, fungi, actinomycetes, and Gramnegative bacteria) were significantly positively correlated with yield (Table 4).
4. Discussion
Many studies on grain cropland and vegetable fields have shown that the use of organic amendments, e.g., manure,compost, straw, can help to increase crop yield and improve soil quality (Bowleset al.2014; Agegnehuet al.2016). In this study, however, we found that crops grown in 4/4CN-treated soil produces the best cucumber and tomato yields in the first three growing seasons. This is mainly because the topsoil was removed when the greenhouse was newly built, and the organic amendments release nutrients more slowly compared to chemical fertilizer (Tianet al.1992). In our study, the highest yield was achieved using the strawsubstituted treatments after three consecutive fertilization management seasons. Thus, successive applications of the organic amendments were required to effectively improve cucumber and tomato yield and soil quality in the new greenhouse. Productivity in agricultural ecosystems relates to the size and activity of the microbial biomass (Anandet al.2015). Soil MBC was found to be significantly correlated with yield in our study. This result confirms previous findings that there is a close relationship between crop yield and microbial biomass in the soil, under both greenhouse (Chenet al.2000) and open field conditions (Anandet al.2015).
We also found that cucumber and tomato yields were significantly correlated with soil enzymes associated with N (L-leucine aminopeptidase and urease) and P cycling(phosphomonoesterase). This may be because large amounts of organic C are brought into the soil by the manure and straw and because a portion of the N and P absorbed from the soil by microbes and plants will lead to microbes regulating extracellular enzyme production to acquire the limited nutrients (Allisonet al.2007; Bowleset al.2014).Interestingly, indicator PLFAs for Gram-negative bacteria,fungi, and actinomycetes were also significantly and positively correlated with yield. Daiet al.(2013) previously reported that peanut (Arachis hypogaeaLinn.) growth and yield are promoted by an increase in Gram-negative bacteria. A higher proportion of Gram-negative bacteria in the soil is usually interpreted as a shift from oligotrophic to more copiotrophic conditions(Borgaet al.1994; Saetre and B??th 2000).Additionally, actinobacteria can produce a wide variety of antimicrobial metabolites (Basilioet al.2003), which are beneficial to crop growth.These results indicate that manipulating these MBC-mediated microbial properties may be an important method of improving the yield in the greenhouse-vegetable field. Improved soil biological function has been revealed by noting general trends pointing to positive effects on soil enzyme activities and soil respiration in vegetables grown in plastic tunnels (Bonanomiet al.2014). This suggests that soil MBC is a good indicator to assess the change in biological activity of greenhouse-vegetable soil caused by alternatives to inorganic fertilizers.
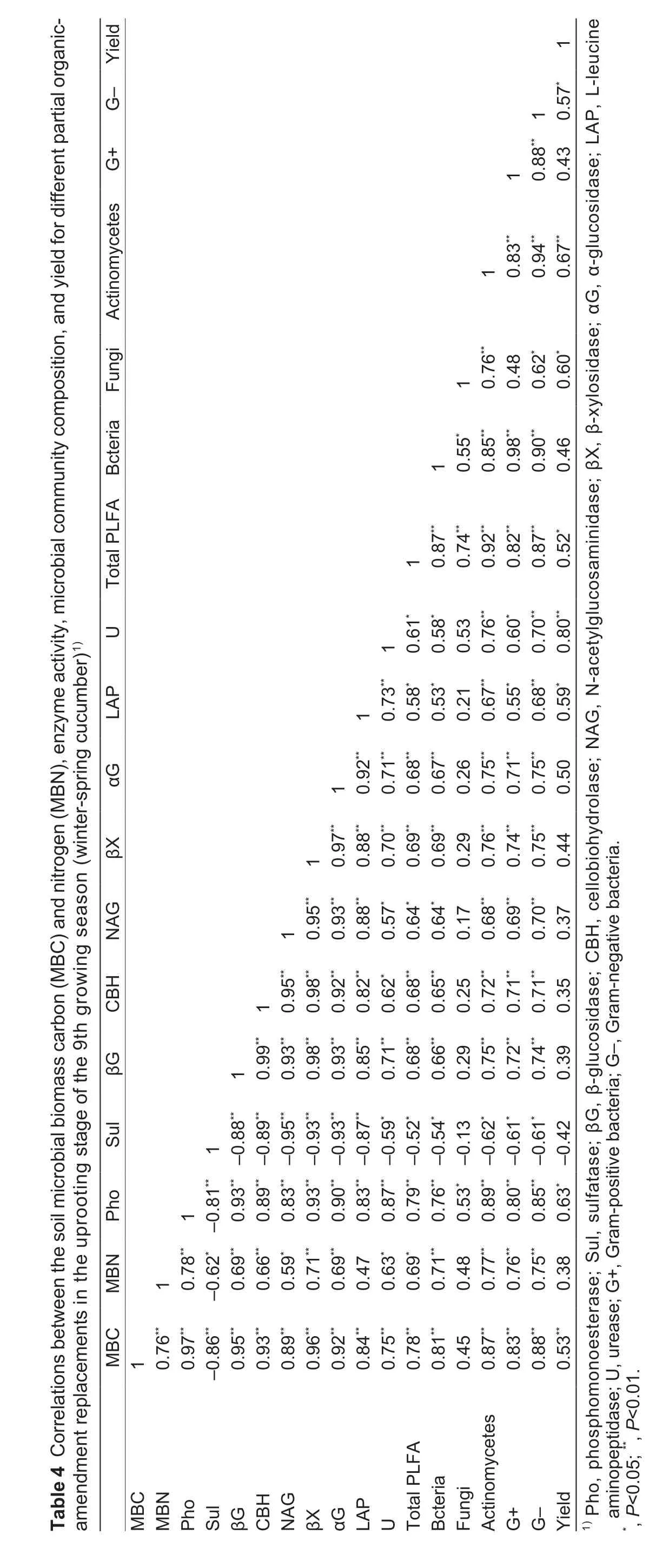
Several studies have shown that the composition of the soil microbial community is changed by organic amendments and that these changes are related to the soil C content(Lazcanoet al.2013; Willekenset al.2014).Interestingly, we found that the composition of the microbial community was more strongly affected by straw than pig manure. This may be because the input of new organic matter significantly stimulated the growth of microorganisms in the soil (Shiet al.2015), and because straw provided more organic C than the pig manure(Table 5). It has also been reported that straw may improve the physical properties of the soil by providing nutrients to directly promote microbial growth and enhance the SOC pool (Luet al.2015).It is generally recognized that soil MBC is used to indicate the size of the microbial community(Bastidaet al.2008). However, enhancement of the soil microbial biomass usually occurs through specific groups of microbial communities(e.g., bacteria, actinomycetes) (Donget al.2014). The results acquired in this study show that the relative abundances of bacteria, Grampositive bacteria, Gram-negative bacteria, and actinomycetes were significantly and positively correlated with soil MBC. We also found that the soil microbial community composition (except for fungi) is significantly correlated with all of the soil enzyme activities involved in the C, N,P, and S cycles, indicating a close relationship between microbial community composition and soil enzyme activity (Allisonet al.2007; Burnset al.2013).
Table 5 Total organic carbon (TOC), dissolved organic carbon (DOC) and nitrogen (DON), and soil nitrate-nitrogen for different partial organic-amendment replacements in the uprooting stage of the 9th growing season (winter-spring cucumber)

Table 5 Total organic carbon (TOC), dissolved organic carbon (DOC) and nitrogen (DON), and soil nitrate-nitrogen for different partial organic-amendment replacements in the uprooting stage of the 9th growing season (winter-spring cucumber)
1) CN, nitrogen in chemical fertilizer; MN, nitrogen in pig manure; SN, nitrogen in corn straw.Data are the mean±SE, n=3. Different letters indicate significant differences among treatments at the P<0.05 level.
–-N (mg kg–1)4/4CN 7.57±0.44 d 50.2±2.1 c 24.5±0.4 c 68.1±10.1 c 3/4CN+1/4MN 9.55±0.10 c 58.3±6.8 c 29.1±0.4 b 81.8±13.2 bc 2/4CN+2/4MN 12.86±0.30 b 68.7±9.3 bc 32.8±1.4 a 111.3±1.1 a 2/4CN+1/4MN+1/4SN 13.30±0.42 b 90.9±5.2 b 34.0±1.5 a 100.9±21.0 ab 2/4CN+2/4SN 14.43±0.52 a 123.4±9.1 a 34.7±2.9 a 90.8±17.6 ab Fertilizer treatments1) TOC (g kg–1) DOC (mg kg–1) DON (mg kg–1) NO3
5. Conclusion
This study demonstrates the changes occurring in soil microbial biomass, enzyme activity, microbial community composition, and cucumber and tomato yield as a result of organic amendment substitution in greenhouse production.The development of indicators of soil ecological functions in the greenhouse vegetable soil may help farmers to evaluate and discover an optimal fertilization management strategy to improve soil quality and increase vegetable yield. We have further presented evidence that MBC-induced changes in soil enzyme activity and microbial community composition might be an important mechanism by which vegetable yield may be improved. In particular, straw-substituted treatments can support high and sustainable yields in greenhousevegetable production systems. In summary, considering all of the effects of different proportions of manure and/or straw substitution on microbial characteristics and vegetable yield(and practical feasibility), combined application of chemical fertilizer, manure, and straw (that is, the combination 2/4CN+1/4MN+1/4SN) appears to be a superior fertilization pattern to use for high, sustainable yields in greenhousevegetable production systems.
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2016YFD0201001),the earmarked fund for China Agriculture Research System(CARS-23-B02), and the Key Research and Development Program of Shandong Province, China (2017CXGC0206).
Agegnehu G, Bass A M, Nelson P N, Bird M I. 2016. Benefits of biochar, compost and biochar-compost for soil quality,maize yield and greenhouse gas emissions in a tropical agricultural soil.Science of the Total Environment,543,295–306.
Ai C, Liang G, Sun J, He P, Tang S, Yang S, Zhou W, Wang X.2015. The alleviation of acid soil stress in rice by inorganic or organic ameliorants is associated with changes in soil enzyme activity and microbial community composition.Biology and Fertility of Soils,51, 465–477.
Ai C, Liang G, Sun J, Wang X, Zhou W. 2012. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil.Geoderma,173, 330–338.
Allison V, Condron L, Peltzer D, Richardson S, Turner B. 2007.Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand.Soil Biology and Biochemistry,39, 1770–1781.
Anand K G V, Kubavat D, Trivedi K, Agarwal P K, Wheeler C,Ghosh A. 2015. Long-term application ofJatrophapress cake promotes seed yield by enhanced soil organic carbon accumulation, microbial biomass and enzymatic activities in soils of semi-arid tropical wastelands.European Journal of Soil Biology,69, 57–65.
Aparna K, Pasha M A, Rao D L N, Krishnaraj P U. 2014.Organic amendments as ecosystem engineers: Microbial,biochemical and genomic evidence of soil health improvement in a tropical arid zone field site.Ecological Engineering,71, 268–277.
Basilio A, Gonzalez I, Vicente M, Gorrochategui J, Cabello A,Gonzalez A, Genilloud O. 2003. Patterns of antimicrobial activities from soil actinomycetes isolated under different conditions of pH and salinity.Journal of Applied Microbiology,95, 814–823.
Bastida F, Kandeler E, Moreno J, Ros M, García C, Hernández T. 2008. Application of fresh and composted organic wastes modifies structure, size and activity of soil microbial community under semiarid climate.Applied Soil Ecology,40, 318–329.
Bonanomi G, D’Ascoli R, Scotti R, Gaglione S A, Caceres M G, Sultana S, Scelza R, Rao M A, Zoina A. 2014. Soil quality recovery and crop yield enhancement by combined application of compost and wood to vegetables grown under plastic tunnels.Agriculture,Ecosystems & Environment,192, 1–7.
Borga P, Nilsson M, Tunlid A. 1994. Bacterial communities in peat in relation to botanical composition as revealed by phospholipid fatty acid analysis.Soil Biology and Biochemistry,26, 841–848.
Bowles T M, Acosta-Martínez V, Calderón F, Jackson L E.2014. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape.Soil Biology and Biochemistry,68, 252–262.
Burns R G, DeForest J L, Marxsen J, Sinsabaugh R L,Stromberger M E, Wallenstein M D, Weintraub M N,Zoppini A. 2013. Soil enzymes in a changing environment:Current knowledge and future directions.Soil Biology and Biochemistry,58, 216–234.
Chen G, He Z, Huang C. 2000. Microbial biomass phosphorus and its significance in predicting phosphorus availability in red soils.Communications in Soil Science & Plant Analysis,31, 655–667.
Dai C C, Chen Y, Wang X X, Li P D. 2013. Effects of intercropping of peanut with the medicinal plantAtractylodes lanceaon soil microecology and peanut yield in subtropical China.Agroforestry Systems,87, 417–426.
DeForest J L. 2009. The influence of time, storage temperature,and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA.Soil Biology and Biochemistry,41, 1180–1186.
Dong W, Zhang X, Dai X, Fu X, Yang F, Liu X, Sun X, Wen X,Schaeffer S. 2014. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China.Applied Soil Ecology,84, 140–147.
Ghani A, Dexter M, Carran R, Theobald P. 2007. Dissolved organic nitrogen and carbon in pastoral soils: The New Zealand experience.European Journal of Soil Science,58, 832–843.
Huang S, Tang J, Li C. 2016. Status of heavy metals in vegetable soils under different patterns of land use.Plant Nutrition and Fertilizer Science,3, 707–718. (in Chinese)
Huang S, Tang J, Zhang H, Yuan S, Wang Y. 2017. Drip fertigation technology of greenhouse cucumber based on management strategy at different growth stages.China Fruit& Vegetable,37, 82–84. (in Chinese)
Joergensen R G. 1996. The fumigation-extraction method to estimate soil microbial biomass: Calibration of thekECvalue.Soil Biology and Biochemistry,28, 25–31.
Ju X T, Kou C L, Zhang F S, Christie P. 2006. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain.Environmental Pollution,143, 117–125.
Kallenbach C, Grandy A S. 2011. Controls over soil microbial biomass responses to carbon amendments in agricultural systems: A meta-analysis.Agriculture,Ecosystems &Environment,144, 241–252.
Kandeler E, Gerber H. 1988. Short-term assay of soil urease activity using colorimetric determination of ammonium.Biology and Fertility of Soils,6, 68–72.
Kaschuk G, Alberton O, Hungria M. 2010. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability.Soil Biology and Biochemistry,42, 1–13.
K?hl L, van der Heijden M G A. 2016. Arbuscular mycorrhizal fungal species differ in their effect on nutrient leaching.Soil Biology and Biochemistry,94, 191–199.
Lazcano C, Gómez-Brandón M, Revilla P, Domínguez J. 2013.Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function.Biology and Fertility of Soils,49, 723–733.
Li W, Zhang M, Zee S V D. 2001. Salt contents in soils under plastic greenhouse gardening in China.Pedosphere,11,359–367.
Lin X G, Yin R, Zhang H Y, Huang J F, Chen R R, Cao Z H.2004. Changes of soil microbiological properties caused by land use changing from rice-wheat rotation to vegetable cultivation.Environmental Geochemistry and Health,26,119–128.
Lu P, Lin Y, Yang Z, Xu Y, Tan F, Jia X, Wang M, Xu D, Wang X. 2015. Effects of application of corn straw on soil microbial community structure during the maize growing season.Journal of Basic Microbiology,55, 22–32.
Maul J E, Buyer J S, Lehman R M, Culman S, Blackwood C B, Roberts D P, Zasada I A, Teasdale J R. 2014. Microbial community structure and abundance in the rhizosphere and bulk soil of a tomato cropping system that includes cover crops.Applied Soil Ecology,77, 42–50.
Min J, Zhao X, Shi W M, Xing G X, Zhu Z L. 2011. Nitrogen balance and loss in a greenhouse vegetable system in southeastern China.Pedosphere,21, 464–472.
Moeskops B, Buchan D, Sukristiyonubowo De Neve S, De Gusseme B, Widowati L R, Setyorini D, Sleutel S. 2012. Soil quality indicators for intensive vegetable production systems in Java, Indonesia.Ecological Indicators,18, 218–226.
Nannipieri P, Ascher J, Ceccherini M, Landi L, Pietramellara G, Renella G. 2003. Microbial diversity and soil functions.European Journal of Soil Science,54, 655–670.
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B,Masciandaro G, Fornasier F, Moscatelli M C, Marinari S. 2012. Soil enzymology: Classical and molecular approaches.Biology and Fertility of Soils,48, 743–762.
Norman R J, Edberg J C, Stucki J W. 1985. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry.Soil Science Society of America Journal,49, 1182–1185.
Qin H L, Zhang Z X, Lu J, Zhu Y J, Webster R, Liu X L, Yuan H Z, Hou H J, Chen C L, Wei W X. 2016. Change from paddy rice to vegetable growing changes nitrogen-cycling microbial communities and their variation with depth in the soil.European Journal of Soil Science,67, 650–658.
Rezaei Rashti M, Wang W, Moody P, Chen C, Ghadiri H. 2015.Fertiliser-induced nitrous oxide emissions from vegetable production in the world and the regulating factors: A review.Atmospheric Environment,112, 225–233.
Saetre P, B??th E. 2000. Spatial variation and patterns of soil microbial community structure in a mixed spruce–birch stand.Soil Biology and Biochemistry,32, 909–917.
Saiya-Cork K, Sinsabaugh R, Zak D. 2002. The effects of long term nitrogen deposition on extracellular enzyme activity in anAcer saccharumforest soil.Soil Biology and Biochemistry,34, 1309–1315.
Sharma S D, Kumar P, Bhardwaj S K, Chandel A. 2015.Agronomic performance, nutrient cycling and microbial biomass in soil as affected by pomegranate based multiple crop sequencing.Scientia Horticulturae,197, 504–515.
Shen W, Lin X, Shi W, Min J, Gao N, Zhang H, Yin R, He X. 2010. Higher rates of nitrogen fertilization decrease soil enzyme activities, microbial functional diversity and nitrification capacity in a Chinese polytunnel greenhouse vegetable land.Plant and Soil,337, 137–150.
Shi P, Wang S, Jia S, Gao Q. 2015. Effect of 25-year fertilization on soil microbial biomass and community structure in a continuous corn cropping system.Archives of Agronomy and Soil Science,61, 1303–1317.
Syswerda S P, Basso B, Hamilton S K, Tausig J B, Robertson G P. 2012. Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA.Agriculture,Ecosystems & Environment,149, 10–19.
Tian G, Kang B, Brussaard L. 1992. Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions-decomposition and nutrient release.Soil Biology and Biochemistry,24, 1051–1060.
Waring B G, Weintraub S R, Sinsabaugh R L. 2014.Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils.Biogeochemistry,117, 101–113.
Willekens K, Vandecasteele B, Buchan D, De Neve S. 2014.Soil quality is positively affected by reduced tillage and compost in an intensive vegetable cropping system.Applied Soil Ecology,82, 61–71.
Wu J, Joergensen R, Pommerening B, Chaussod R, Brookes P. 1990. Measurement of soil microbial biomass C by fumigation-extraction - an automated procedure.Soil Biology and Biochemistry,22, 1167–1169.
Wu Y, Ding N, Wang G, Xu J, Wu J, Brookes P C. 2009.Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses.Geoderma,150, 171–178.
Yang L, Huang B, Hu W, Chen Y, Mao M, Yao L. 2014. The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China.Chemosphere,103, 121–130.
Yao Z, Xing J, Gu H, Wang H, Wu J, Xu J, Brookes P C. 2016.Development of microbial community structure in vegetablegrowing soils from open-field to plastic-greenhouse cultivation based on the PLFA analysis.Journal of Soils and Sediments,16, 2041–2049.
You Y, Wang J, Huang X, Tang Z, Liu S, Sun O J. 2014. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover.Ecology &Evolution,4, 633–647.
Zelles L. 1999. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review.Biology and Fertility of Soils,29, 111–129.
Zhang L, Chen W, Burger M, Yang L, Gong P, Wu Z. 2015.Changes in soil carbon and enzyme activity as a result of different long-term fertilization regimes in a greenhouse field.PLoS ONE,10, e0118371.
Zhu T, Zhang J, Cai Z. 2011. The contribution of nitrogen transformation processes to total N2O emissions from soils used for intensive vegetable cultivation.Plant and Soil,343, 313–327.
 Journal of Integrative Agriculture2018年6期
Journal of Integrative Agriculture2018年6期
- Journal of Integrative Agriculture的其它文章
- Improve access to the EU market by identifying French consumer preference for fresh fruit from China
- Management and prevention of mastitis: A multifactorial approach with a focus on milking, bedding and data-management
- Elimination of ceftiofur hydrochloride residue in postpartum cows’milk after intramammary infusing at dry-off
- Evaluation of a new qPCR test to identify the organisms causing high total bacterial count in bulk tank milk
- Prevalence and characteristics of extended spectrum β-lactamaseproducing Escherichia coli from bovine mastitis cases in China
- Evolutionary analysis of plant jacalin-related lectins (JRLs) family and expression of rice JRLs in response to Magnaporthe oryzae
