Formation of Stable Lamellar Gel Structure Containing Pseudoceramide and Its Evaluation of Barrier Recovery Function
Dong-Hyuk Jang, Kyungmi Joo, Sung-Il Park, June Whan Park, Eunjoo Kim, Younguk Cho, Byung-Young Kang
Amorepacific Co., R&D Center, Korea
Fan Bo, Jongsuk Lee, Aeho Yeon
Amorepacific (Shanghai) R&I Center Co., Ltd., China
Introduction
Stratum coreum (SC), the uppermost layer of skin,act as epidermal barrier by maintaining skin moisture and protecting against various external stresses.[1~4]Presence of SC is very important that TEWL increase approximately 100-fold when removed. This barrier function of SC is derived from bilayer-forming capacity of SC lipids.[5~6]
Essential components of SC lipids are ceramides,cholesterols, cholesteryl esters and free fatty acids. Selfassembly of this SC lipids leads to form multi-lamellar structure, which provides them water-retention capacity.Among them ceramides consist of about 40% of SC lipids and play important role to stabilize the lamellar structure as well as maintain skin barrier function.[2,7,8]
Although ceramides constitute the major fraction of SC, it is difficult to use them commercially because they exist in nature in a small quantity. Because of this,using artificially synthesized pseudo-ceramides can be a good alternative to replace high-cost natural ceramides.Several cosmetic companies have used synthetic pseudoceramides and which are suggested to be useful as a skin moisturizer.
While the rarity of natural ceramides can be resolved by using artificial ceramides, another obstacle in using ceramides or pseudo-ceramides is stem from their physical property. Due to their low solubility in solvents using in cosmectic such as water or emulsion, only small amount of the ceramides have been included in dosage forms. In addition, they are easy to become γ-crystalline state even though they are initially loaded in the form of lamellar structure. Therefore, for an effective function of ceramides on a skin, it is critical to increase the dosage of ceramides and develop a method to improve the long-term stability of lamellar structures in cosmetic or therapeutic formulations.[9~10]
In the present study, we formulated new skin care cosmetics composed of pseudo-ceramides and amphiphilic lipids, which is SC-lipids-mimic in structure as well as in composition. We used PC104 (Kim, Lee et al. 1998)[11]and PC102 as pseudo-ceramides and fatty acids and cholesterol as other SC-lipid components (Figure 1).These formulations were proved to have lamellar structure and, from an in vivo study, were very effective for the recovery of the barrier function of damaged skin.
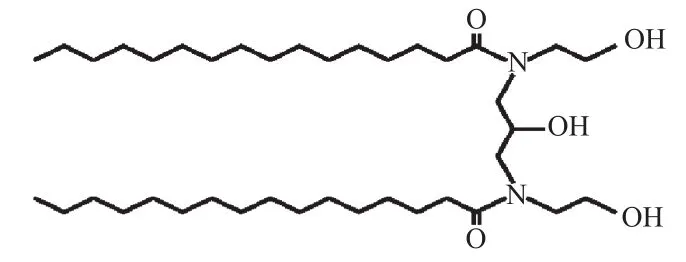
Figure 1. Molecular structure of ceramide PC104. The alkyl chains are lauroyl rather than palmitoyl in case of PC102
Experimental methods
Perperation of pseudo-ceramide containing creams
We used 1,3-bis-(N-(2-hydroxyethyl)-palmitoylamino)-2-hydroxypropane (Figure 1) (PC104, Macrocare Co.,Korea), 1,3-bis-(N-(2-hydroxyethyl)-lauroylamino)-2-hydroxypropane (PC102, Macrocare Co., Korea), fatty acids (Jeongnam Fat and Oil Chemical Co, Korea),cholesterol (Lonza, New Zealand), fatty alcohols and alkyl glucosides (Seppic, France) as a pseudo-ceramide and other lipid components, respectively. Before mixing lipid part was heated to 80 ℃ to melt and water soluble part is dissolved to water heated to 70 ℃. After mixing with high speed homo-mixer, the creams were degassed with vacuum and cooled down to 30 ℃.
Structural analysis
A Bruker D8 Discover GADDS diffractometer was used to identify the structure of formulations. Cu Kα radiation(λ=1.5418) was used in the X-ray diffractometer.

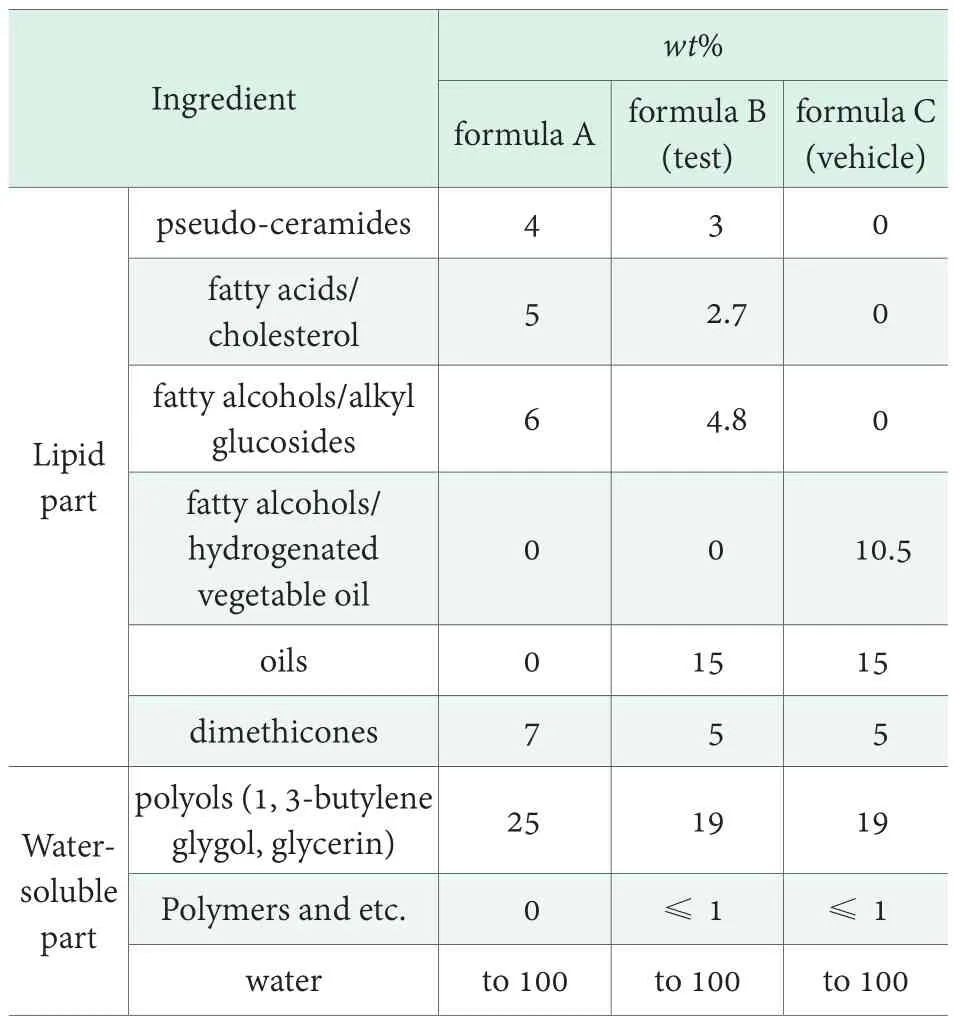
Table 1. Compositions of creams
In vivo skin recovery test
Analysis of lipids. Serial tape stripping (TS) method on the forearms of adult volunteers (10 males and 5 females; age range 20~40) was used to damage the skin barrier function and obtain the SC samples after the treatment with testing creams. Before the TS,subjects cleansed their forearm and were given time to isolated room conditions. The standard adhesive disks(D-Squame, CuDerm, Dallas TX, diameter, 2.2 cm)were placed on the skin under a constant pressure using a cylindrical weight for 2 seconds then were removed.Three sites on the forearms of each volunteer were tape-stripped for twenty times. After that, two types of cream including the cream B of Table 1 and the vehicle cream in which SC-mimic-lipids of the cream B—pseudoceramides, fatty acids and cholesterol—were substituted to fatty alcohols and hydrogenated vegetable oils were applied to each allocated site twice a day; the remaining site was remained as a non-treated control. Ten consecutive disks were collected from each test site 96 hours after the initial tape stripping and were stored at -20 ℃ until analysis.

The stripped tapes were cut into two equal parts and one half of the tape was used to extract lipids. The tapes were immersed into 3 mL of methanol/ethyl acetate(2:1, v/v) with sonication for 10 min. The organic layer was transferred into another tube and evaporated to the dryness using Speedvac (EZ-2 Plus, Genevac, Swiss) at 40℃. The residues were dissolved in 300 μL of methanol/chloroform (2:1, v/v) and vortexed for 5 min, following by centrifuging centrifugation at 14,000 rpm, 4 ℃ for 5 min for analysis by UPLC-MS/MS. Ceramides and cholesterol were quantitated according to the UPLC-MS/MS method previously described.[12]
Protein analysis of the tape strips. The remaining parts of tapes were immersed into 0.1% (w/v) sodium dodecyl sulfate/2% (w/v) propylene glycol in PBS buffer solution and was then sonicated at ambient temperature for 1 h to obtain soluble proteins. The concentration of soluble protein was assayed with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) and through the colorimetric method (a microplate reader, (Bio-Rad Laboratories, Hercules, CA) according to the instruction manual. The residual solution was stored at 4℃ until for the analysis of amino acids and amino acids metabolites.
Analysis of amino acids (AA). An authentic standard(2.5 μmol amino acid / mL) was serially diluted to obtain concentrations for establishment of a calibration curve standards (0.005 ~ 0.5 μmol / mL). The derivatization of amino acids were performed according to the Waters AccQ·Tagultra method. The chromatographic separation for amino acids was carried out using ACQUITY UPLC system (Waters Co., Milford, MA) equipped with a 2996 photodiode array detector (PDA). The column was AccQ·Tag Ultra C18 column (2.1 mm × 100 mm,1.7 μm). The column temperature and autosampler tray temperature were maintained at 55℃ and 20℃,respectively. The mobile phase consisted of two eluents:(A) AccQ·Tag Ultra eluent A (5% in water, v/v) ; (B)AccQ·Tag Ultra eluent B. Gradient elution was as follows:0 — 0.54 min, 99.9% A — 0.1% B; 3.5 min, 98% A — 2% B; 5.7 min,90% A — 10% B; 7.0 min, 79% A — 21% B; 8 min, 40% A —60% B; 8.5 — 9.0 min, 10% A — 90% B; 10.5 min, 99.9% A —0.1% B. The flow rate was 0.7 mL / min and injection volume was 1 μL. The detection wavelength was set at 260 nm. We controlled the data acquiring and process with Empower 2 software (Waters Co.).
Analysis of AA metabolites (PCA, cis-, trans - UCA,citrulline). Standard stock solutions of PCA, cis-, t-UCA and citrulline were prepared in water at concentration level of 1 mg / mL respectively, stored at -20℃. The mixed working standard solution containing PCA, cis-, t-UCA and citrulline was serially diluted with 30% water in acetonitrile(containinedg 5% phosphate buffered saline) to obtain concentrations for calibration curve standards(0.1~500 ng / mL). Amino acids metabolites were analysed using ACQUITY UPLC-Xevo TQ-S triple quadrupole mass spectrometer (Waters Co., Milford, MA) equipped with ESI source. UPLC-MS/MS was performed according to the method previously described.[13]We controlled the data acquiring and processing with MassLynx Version 4.1(Waters Co.).
Statistics. Data were presented as mean ± SD. Data were analyzed by student t-test to identify the statistically significant difference from the cream B and vehicle or the untreated group (p<0.05). The data were handled with Microsoft EXCEL?.
Results and discussion
Basic system for formulation
In order to formulate stable lamellar structure similar to intercellular stratum corneum lipids, we used “selfbodying action” which occurs when fatty amphiphiles are mixed with more soluble surfactants.[14~15]The fatty amphiphiles we used were mainly fatty alcohols and the lipids for mimicking SC lipids (pseudo-ceramides,fatty acids and cholesterol) themselves also were ones of them. The fatty amphiphiles were with various chain lengths and pseudo-ceramides were two types with different chain lengths (PC104 and PC102). This chain length mismatch and cholesteric effect seems to lower transition temperature of α-to β-or γ-form[15]and prevent solidification of alkyl chains.[16,17]this method can stabilize the resulting creams in wide range of temperature.
Preparation of lamellar gel creams
Based on this system we prepared new skin care cosmetics which were similar in structure to the intercellular lipids in the stratum corneum (Table 1).Firstly, we intended to make a cream mainly with lamella phase (sometimes this is described as hydrosome which is described by Tadros, T., et al. (2006)[18]to mimic structure of SC lipid. We tried to design formula A using only amphiphic lipids as far as possible because they are good to form lamella structure, except small amount of dimethicone as a defoaming agent. Cream A made according to formula A was translucent (Figure 2, left) and showed optical anisotropy when observed on polarized light (Figure 3), demonstrating that lipid molecules comprising the gel cream were highly structured.

Figure 2. Appearances of creams formulated according to Table 1 (Left, cream A; right, cream B)

Figure 3. Optical anisotropy of cream A
To elucidate fine molecular structure of the cream A, we analyzed X-ray scattering pattern. Wide angle x-ray scattering(WAXS) data showed a peak around 2θ=21.5 ℃ which is not as sharp as the peaks observed in α-crystals[15,16](Figure 4). This seem to because α-gel structure is somewhat liquefied by cholesteric effect.[9,16]Small angle x-ray scattering (SAXS)data showed that this gel cream had multi-lamellar structure, which was confirmed by the ratio between the distances of the peaks (Figure 5).[19]
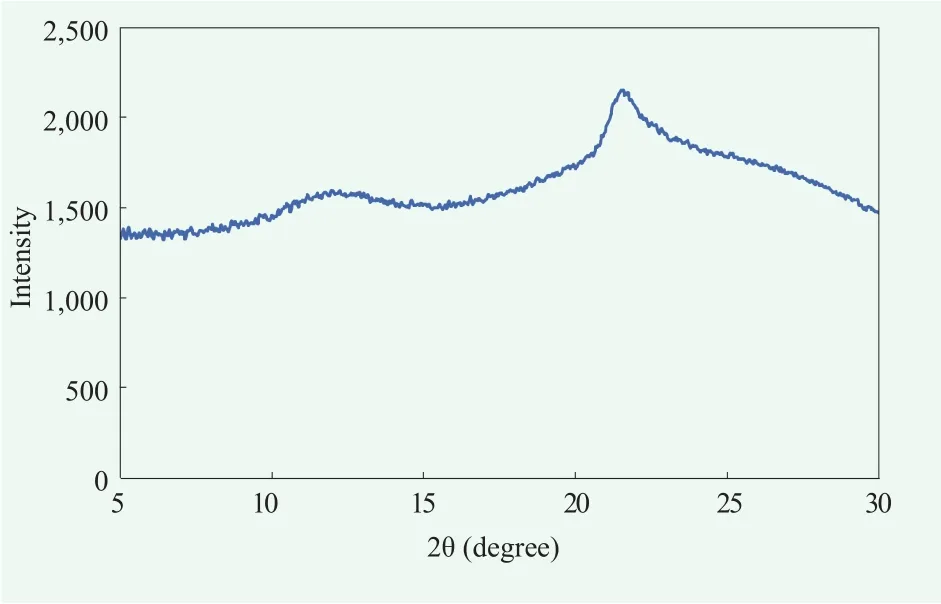
Figure 4. Molecular structure of formula A cream detected by WAXS
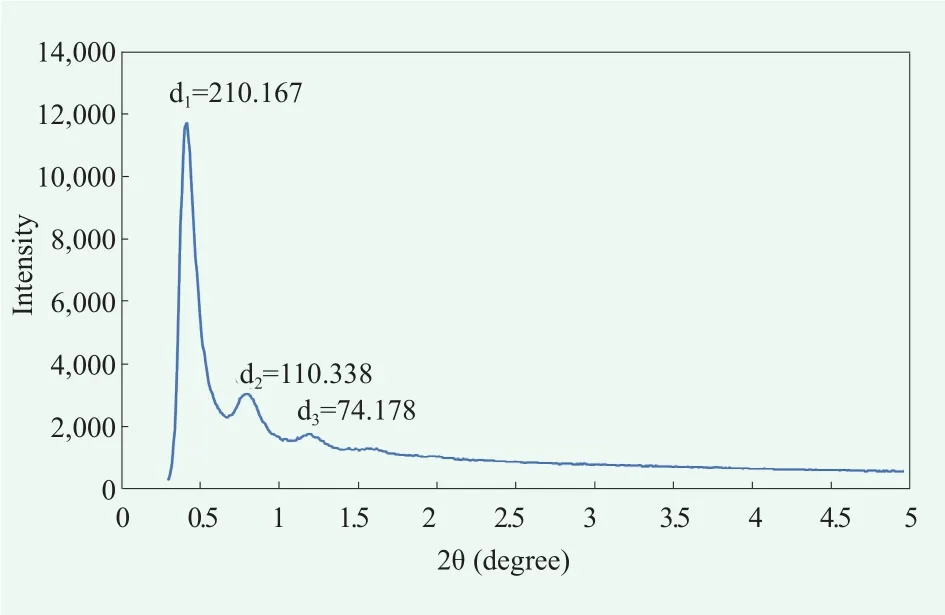
Figure 5. Inter-molecular structure of cream A detected by SAXS
Based on formula A, we designed formula B to have more practical skin-feel and function by adding skinlubricating oils. Small amount of polymers were also added because lamella structure alone could not prevent creaming in high temperature (≥45℃) if high-content of oils were incorporated. Appearance of the cream B was opaque because of oil droplets’ light scattering, and more like a normal skin care cream (Figure 2, right).Because the basic lipid content was very similar to cream A, formation of lamella structure was not affected by adding of oils and polymers as shown by SAXS data(Figure 6), but skin-feel became smoother because of oils.
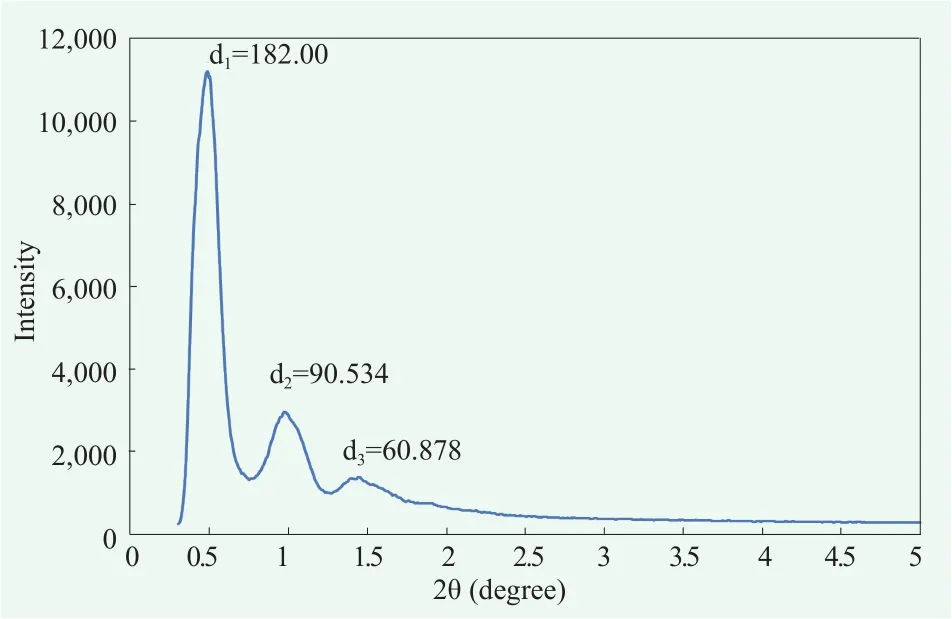
Figure 6. Inter-molecular structure of cream B detected by SAXS
Although both creams had multi-lamellar structures,Maltese cross, which is typical in multi-lamellar emulsion,was found only in very few or none when observed on polarizing microscope (data not shown). This seems to because the lamellar structures of the creams exist mainly in D2phase described by Fukushima and Yamaguchi[15]rather than concentric M phase.
Stability of the two creams was evaluated sensorially as well as by instrumental measurements. There was no separation or transformation of the creams in thermostats set temperature to -20℃, 5℃, 37℃ and 45℃ respectively for 1 month. Overall external shape and hardness of samples stored in room temperature maintained for 12 month.
Barrier recovery function in vivo
We chose cream B for clinical study because it was more suitable for practical use compared to cream A as mentioned above. We evaluated the effects of cream B on the recovery of the skin barrier function in vivo through quantitating NMFs and ceramides in SC after tapestripping-induced barrier damage. As a result, amino acids and amino acid metabolites that represent NMFs in SC were markedly higher on the group applied with cream B although a statistical significance was not obtained (Figure 7A). Amino acid metabolites; PCA, cis-/trans-UCA, and citrulline were significantly higher in the group treated with cream B compared to untreated.Also when compared with vehicle group, statistically significant difference were shown for PCA, and cis-/trans-UCA. In addition, the cream B treated group showed higher content of total amino acid (AA) metabolites than untreated or vehicle-treated groups (Figure 7B). However,the amount of AA and AA metabolites was not reversed to baseline (undamaged state) up to 96 h.
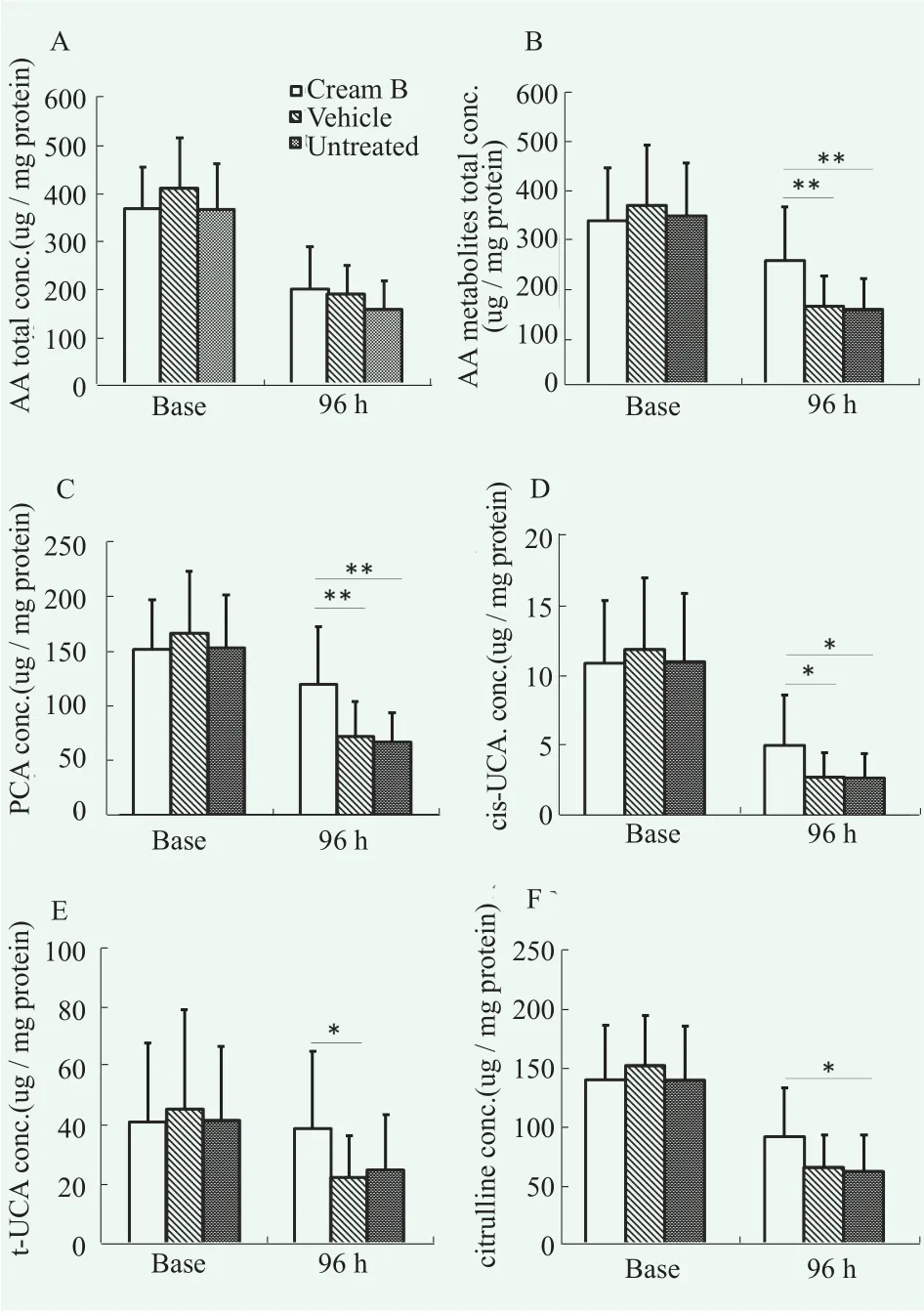
Figure 7. Alteration of AA, AA metabolites levels over time(initial baseline and 96 h after barrier perturbation) after treatment of cream B on the forearm
In the analysis of SC lipids associated with barrier function, the cream B treated group showed higher content of total ceramides (sum of 5 ceramides) at 96 h after tape-stripping than untreated (Figure 8A). Especially,there was a distinctive pattern shown for ceramide NP series (Figure 8B). However, no significant difference was observed when compared with vehicle-treated group.Examination of the chain length profile for major ceramide series like CER-NP and CER-AP revealed an unapparent trend (Figure 8, C and D) but failed to reach statistical significance. Total ceramide contents were not recovered to baseline up to 96 h after tape-stripping (Figure 8A), reflecting that longer or more frequent use of cream might be necessary to manifest functional effects. Cholesterol, another SC lipid, was not changed by tape-stripping (Figure 9).
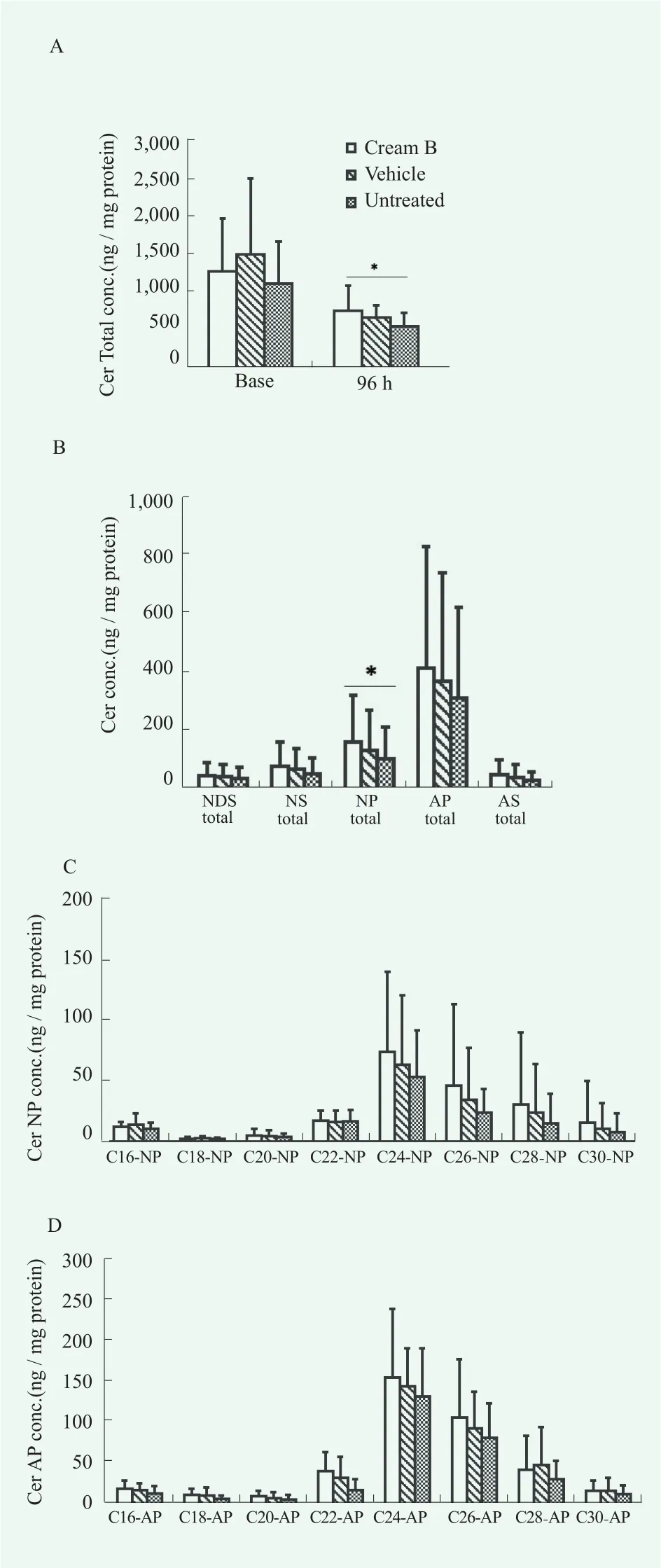
Figure 8. Changes of profiles of ceramides’ contents in SC of cream B treated group compared to vehicle treated group and untreated group
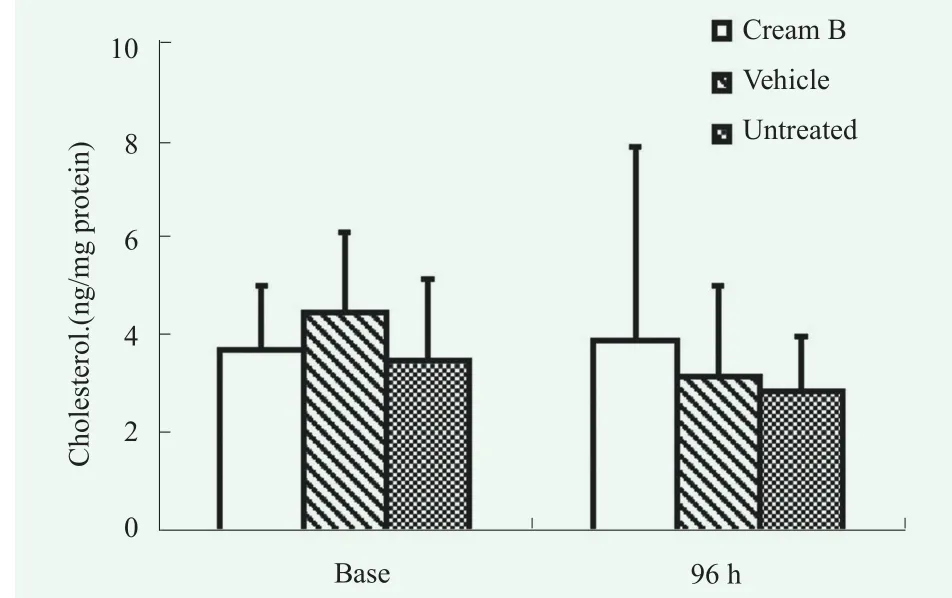
Figure 9. Changes in amount of cholesterol in the SC of cream B treated group compared to vehicle treated group and untreated group
Collectively, we demonstrated that cream B which as SC-lipid-mimic lamellar structure helped to recover skin barrier function of SC as by increasing contents of several ceramides and NMFs in skin damaged by tape-stripping.
Conclusion
Pseudo-ceramides were successfully incorporated into lamellar gel creams at concentrations of up to 4%. As well as the creams we developed contained high amount of SC-mimic-lipids including pseudo-ceramides, their inter-molecular structures were very similar to SC lipids as shown by SAXS. Application of cream B on damaged skin enhanced barrier recovery by increasing related materials.Further study is needed because 96 h time window is not sufficient to recover to initial state, and cannot discern efficacy of cream B from that of vehicle cream which also has basic skin care function although does not contain SC-mimic-lipids.

[1] Elias P. M. Epidermal lipids, membranes, and keratinization.Int J Dermatol 1981, 20 (1), 1-19.
[2] Elias P. M. Lipids and the epidermal permeability barrier. Arch Dermatol Res 1981, 270 (1), 95-117.
[3] Elias P. M. Epidermal lipids, barrier function, and desquamation.J Invest Dermatol 1983, 80 (1 Suppl), 44s-49s.
[4] Lampe M. A.; et al. Human stratum corneum lipids:characterization and regional variations. J Lipid Res 1983, 24(2), 120-130.
[5] Onken H. D.; C. A. Moyer. The water barrier in human epidermis.Physical and chemical nature. Arch Dermatol 1963, 87, 584-590.
[6] Scheuplein R. J.; I. H. Blank. Permeability of the skin. Physiol Rev 1971, 51(4), 702-747.
[7] Downing D. T.; et al. Skin lipids: an update. J Invest Dermatol 1987, 88 (3 Suppl), 2s-6s.
[8] Wertz P. W.; et al. Composition and morphology of epidermal cyst lipids. J Invest Dermatol 1987, 89 (4), 419-425.
[9] Kim D.H. K.; et al. Fabrication of pseudo-ceramide-based lipid microparticles for recovery of skin barrier function. Colloids and Surfaces B: Biointerfaces 2012, 94, 236—241.
[10] Suzuki T.; et al. Multilamellar emulsion of stratum corneum lipid — Formation mechanism and its skin care effects. A 101,17th IFSCC Congress 1992, 3-28.
[11] Kim D.-H.; et al. The synthesis, physical property, and the biological activity of novel neo-ceramides. 20thIFSCC Congress 1998, 2, 87-95.
[12] Joo K. M.; et al. Metabolomic analysis of amino acids and lipids in human hair altered by dyeing, perming and bleaching.Exp Dermatol 2016, 25 (9), 729-731.
[13] Joo K. M.; et al. Rapid, simultaneous and nanomolar determination of pyroglutamic acid and cis-/trans-urocanic acid in human stratum corneum by hydrophilic interaction liquid chromatography (HILIC)—electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2012, 897, 55-63.
[14] Eccleston G. M. Multiple-phase oil-in-water emulsion. J Soc Cosmet Chem 1990, 41, 1-22.
[15] Fukushima S.; M. Yamaguchi. Physical chemistry of cetyl alcohol: occurrence and function of liquid crystals in OW creams. Surface and Colloid Science 2001, 16, 1-98.
[16] Iwai H.; et al. A liquid crystal application in skin care cosmetics. Int J Cosmet Sci 1998, 20 (2), 87-102.
[17] Mizushima H.; et al. Phase behavior of artificial stratum corneum lipids containing a synthetic pseudo-ceramide: a study of the function of cholesterol. Journal of Lipid Research 1996,37, 361-367.
[18] Tadros T.; et al. Correlating the structure and rheology of liquid crystalline phases in emulsions. Cosmetics & Toiletries 2006, 121 (5), 89-94.
[19] Alexandridis P.; et al. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil). Langmuir 1998, 14, 2627-2638.
 China Detergent & Cosmetics2017年2期
China Detergent & Cosmetics2017年2期
- China Detergent & Cosmetics的其它文章
- Research Progress for Kitchen Cleaner
- Study on the Determination Conditions of Lipase Activity and the Effects of Commercial Detergent Products
- Evaluation for Whitening Efficacy of Cosmetics Containning Extract from Tricholoma Matsutake Sing
- Trend of the Latest Regulation—Efficacy Claims of Cosmetics
- Interpretations on Safety and TSSC for Cosmetics
- China National Standard—Technical Specification for Safety of Soaps and Detergents(GB/T 26396-2011)
