Bioprospecting optimal phenology for bioactive molecules in native golden yellow Pleurotus citrinopileatus Singer
Godfrey Nattoh, Erastus Gatebe, Fredrick Musieba, Julius MatharaDepartment of Molecular Biology and Biotechnology, Pan African University, Institute for Basic Sciences,Technology and Innovation, P.O. Box 6000-0000 Nairobi, KenyaResearch Technology and Innovation Department, Kenya Industrial Research and Development Institute,P.O. Box 0650-00100 Nairobi, KenyaDepartment of Chemistry, Jomo Kenyatta University of Agriculture Science and Technology,P.O. Box 6000-0000 Nairobi, KenyaDepartment of Food Science and Technology, Jomo Kenyatta University of Agriculture Science and Technology,P.O. Box 6000-0000 Nairobi, Kenya
?
Bioprospecting optimal phenology for bioactive molecules in native golden yellow Pleurotus citrinopileatus Singer
Godfrey Nattoh1*, Erastus Gatebe2,3*, Fredrick Musieba2, Julius Mathara1,41Department of Molecular Biology and Biotechnology, Pan African University, Institute for Basic Sciences,
Technology and Innovation, P.O. Box 62000-00200 Nairobi, Kenya
2Research Technology and Innovation Department, Kenya Industrial Research and Development Institute,
P.O. Box 30650-00100 Nairobi, Kenya
3Department of Chemistry, Jomo Kenyatta University of Agriculture Science and Technology,
P.O. Box 62000-00200 Nairobi, Kenya
4Department of Food Science and Technology, Jomo Kenyatta University of Agriculture Science and Technology,
P.O. Box 62000-00200 Nairobi, Kenya
Original article http://dx.doi.org/10.1016/j.apjtb.2015.10.012
Tel: +254 722 454 909, +254-20-6003842
Fax: +254 20 6007023
E-mail: erastusgatebe@gmail.com
Godfrey Nattoh, Department of Molecular Biology and Biotechnology, Pan African University–African Union, Institute for Basic Sciences, Technology and Innovation, AICAD BLOCK C, Office no: Room 101, P.O. Box 62000-00200 Nairobi, Kenya.
Tel: +254 725 745 282
E-mail: nattohg@yahoo.com
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation Project: Supported by the African Union (African Union, No. 17/2014).
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 24 Jun 2015
Received in revised form 9 Sep 2015
Accepted 25 Oct 2015
Available online 19 Dec 2015
Keywords:
Bioprospecting
Primordial
Phenology
Bioactive molecules
Liquid chromatography-quadrupole time of flight mass spectrometry
Edible fungi
ABSTRACT
Objective: To bioprospect optimal phenological phases as source of novel molecules from native golden yellow Pleurotus citrinopileatus across four phenologies in both aqueous and ethanol extracts, and identify novel molecules responsible for these activities. Methods: Standard qualitative assay, Folin–Ciocalteu assay; aluminium chloride spectrophotometric, 2, 2-diphenyl-1-picrylhydrazyl, 2, 2'-azinobis (3-ethylbenzothiazoline-6-suslfonic acid, ferricyanide reducing antioxidant power were used to determine total flavonoid, polyphenols, radical scavenging, and reducing power. Spectrophotometric methods were used for lycopene,β-carotene, and total carotenoids, while liquid chromatography quadrupole time of flight mass spectrometry was used for identification and comparative quantitation of polyphenols and flavonoids across the four phenological states. ChemSpider?database was used for the identification of compounds based on their empirical formula, accurate mass and literature review of previously reported compounds in mushroom.
Results: Primordialphasesexhibitedhighercontentsofsecondarymetabolitesthanmature basidiocarps. Polyphenols content differed across physiological phases with primordials exhibitingsignificanthighcontents(P<0.05)[(13.803±0.797)mggallicacidequivalent/g dry weight]. Distribution of total flavonoids was significantly different (P<0.05) across physiological states and ranged from (3.311±0.730) to (14.824±0.890) mg quercetin equivalent g dry weight. Ten polyphenol acids and seven flavonoids compounds identified varied across these phases with primordials exhibiting relatively high peak areas. Total antioxidant activities showed a positive correlation with total polyphenols (r=0.969; P<0.05) and total flavonoids (r=0.960; P<0.05) across these phenologies.
Conclusions: These findings provide evidence that primordials of golden yellow mushroom as opposed to their fruiting bodies are potent sources of bioactive health molecules.
1. Introduction
Edible fungi are delicacies that are rich sources of health promoting molecules like polyphenols,flavonoids with radical scavenging properties [1]. The value added health benefits of these basidiomycetes are being ascertained because they also possess protein, minerals, vitamins, unique taste, and flavour [2,3]. Studies have shown that edible fungi in the genera Pleurotus, Agaricus possess antioxidant properties [4,5]. These have elicited interests to bioprospect for natural pharmacological and nutraceutical antioxidants that can quench free radicals. Previously, Athanasakis et al. [6] reported that natural molecules with potent antioxidants would help in quenching such radicals. These radicals are manifested when the enzymatic systems protecting the body from oxidative stress are overwhelmed due to excessive generation of reactive oxygen species resulting in imbalances [7]. Studies by Barros et al. [8,9] demonstrated that different parts of mushroom have varied health properties. Seemingly, studies have shown that mature fruiting bodies have reduced amounts of bioactive molecules associated with radical scavenging properties [10]. As the fruiting body ages, bioactive molecules declines due to their involvement in defence mechanisms [8,9]. Radical scavenging properties are largely attributed to phytochemicals such as flavonoids and polyphenols, that contribute largely to plant ecophysiology and survival against biotic and abiotic stressors [11]. However, little effort has been done to identify appropriate phenological phases for obtaining the fruiting bodies with optimal quantities of these biomolecules. This elicited interest to elucidate distribution of health molecules across four phenological phases from the unique native golden yellow Pleurotus citrinopileatus (P. citrinopileatus), which was recently collected from the forest and tissue culture developed for domestication [12]. To our knowledge, this is the first study ascertaining both phytochemical, total antioxidative properties, and the phenological distribution of health promoting molecules of the Kenyan native golden yellow P. citrinopileatus Singer using biochemical assays and liquid chromatography-quadrupole time of flight mass spectrometry (LC-QToF-MS).
2. Materials and methods
2.1. Source of Pleurotus specie mushroom cultures
P. citrinopileatus was provided by Dr. Fredrick Musieba of Kenya Industrial Research Development Institute. Starting cultures were obtained through tissue culture techniques and cultures maintained on potato dextrose agar medium at 5°C [13].
2.2. Reagents
Potassium ferricyanide, 1,1-diphenyl-2-picrylhydrazyl (DPPH), hexane, 2,2'-azinobis (3-ethylbenzothiazoline-6-suslfonic acid) (ABTS), gallic acid, quacertin, L-ascorbic acid (L-AA),α-tocopherol (TOC), butylated hydroxytoluene (BHT), Folin–Ciocalteu reagent, sodium carbonate, aluminium chloride, silica gel, petroleum ether, acetone, ethanol, ferric chloride, acetic acid, Mayer's reagent were of analytical grade supplied by Sigma Aldrich (Germany).
2.3. Developing spawn and substrate inoculation
Development of spawn was conducted according to previous method with slight modification[14]. In this case, media bottles and bird millet were utilized instead of spawn bags and wheat grains. Ten-day-old pure spawn with fully colonized mycelium was used in the inoculation of substrate. Sugarcane bagasse (Saccharum officinarum) and wheat straw (Triticum aestivum) substrates were treated according to previous studies [12,15] and spawned at 10% w/w; with each bag carrying 500 g w/w substrate. Sugarcane bagasse (Saccharum officinarum) and wheat straw (Triticum aestivum) substrates, spawn run for efficient substrate colonization took 14 days, and primordials emerged on the second pinning. Fruiting bodies were picked at predetermined phenological stages (Figure 1). These phases were ten-day-old fully-grown spawn mycelium (SPM),first primordials two days after pinning, second primordials three days after pinning, and mature fruiting bodies collected a week after pinning.
2.4. Preparation of samples
Intact SPM, two phases of primordials, and a fruiting body phase of P. citrinopileatus were picked separately (Figure 1) and dried in oven at 42°C for 3 days and milled to powder using electric blender (Kenwood: BL370 400W 1.6L, USA). A total of 10 g of powder was placed in high performance liquid chromatography (HPLC) grade bottles and mixed separately with 100 mL of distilled water and 100 mL of analytical grade ethanol (Sigma Aldrich, Germany) and left in a 24-h shaker set at 150 revolutions per minute at room temperature in darkness. The liquid was decanted and the residue resuspended in 100 mL of the solvent for re-extraction for 24 h. The filtrate was pooled and filtered through Whatman paper No. 1 (12.5 cm) and stored at 4°C awaiting further analysis [16]. The filtrate was concentrated using a vacuum rotary evaporator (40°C). Pooled samples were screened for secondary metabolites whiles samples across phenological states were analysed for total polyphenols, total flavonoids, and total radical scavenging properties using various in vitro assays and LCQToF-MS.
2.5. Screening of phytochemicals of phenological samples
Screening of phytochemicals was carried out according to previously described standardized methods [17]. Briefly, extracted samples were prepared and screened for bioactive secondary metabolites like flavonoids, phenols, terpenoids, saponins, alkaloids, tannins, resins, phytosterols, cardiac glycoside, saponins, and anthraquinones constituents.
2.6. Total carotenoids content
Carotenoids were determined as previously described with a few modifications[18]. Ten gram of sample and 5 g of celite 454was extracted in 40 mL stepwise cold acetone at room temperature in darkness until complete extraction was attained. Cleaning of extracts with distilled water was done carefully to prevent formation of emulsion. This was partitioned through a separation funnel with petroleum ether as the solvent to separate acetone, which was carefully drained. Trapping of water from the layer was done using on a funnel with anhydrous sodium sulphate. Absorbance was measured at 450 nm. Calculation of total carotenoids in the mushroom extract was done using the formula:

Figure 1. Processes of developing pure tissue cultures, spawning, substrate preparation/inoculation and phenological states of basidiomycetes.
Total carotenoids (μg/mL)=(3:856×Absorbance of sample ×Volume (mL)×100)/Weight of sample×1 000
2.7. Determination of β-carotene and lycopene
β-Carotene and lycopene were determined based on protocol previously described with a modification in sample volume[19]. In brief, previously extracted ethanol extracts of mushroom was vigorously shaken in a mixture of acetone and hexane (8:12; 20 mL) for 1 min. This was filtered with Whatman No. 4 and the optical density of the filtrate determined at various wavelengths of 453, 505, and 663 nm. Each test was done in triplicate and results expressed as means±SD. Contents expressed as milligram carotenoid per gram of the extract. Calculations of β-carotene and lycopene contents were determined according to the equations below:
Lycopene(mg/100 mL)=?0:0458A663+0:372A505?0:0806A453β- Carotene(mg/100 mL)=0:216A663?0:304A505+0:452A453
2.8. Total polyphenol content (TPC)
Folin–Ciocalteau calorimetric assay according to Kumar et al. [20] with modification in mushroom stock was used. Sample extract (0.5 mL) from this aliquot was transferred in a 10 mL test tube and diluted with equal volume of their respective extraction solvent. Folin–Ciocalteu reagent, 1.25 mL was added and after 5 min, 6.25 mL of 20% aqueous sodium carbonate added to the mixture. Resulting mixture was vortex mixed and incubated at room temperature in darkness for 40 min. Different concentrations of gallic acid standard were prepared (0.1 mg/mL to 1 mg/mL). Absorbance was measured at 725 nm. Each experiment was done in triplicates to minimize errors. The total amounts of phenols were expressed as milligram gallic acid equivalent (mg GAE) per gram dry weight of mushroom material.
2.9. Total flavonoid content (TFC)
Total flavonoids were determined using spectrophotometric method as previously described [21]. One millilitre of sample extracts previously prepared in different concentrations were aliquoted separately and 4 mL of extraction solvent added, after 5 min 0.3 mL of 5% sodium nitrite (NaNO2) was added. The resulting solution was vortex mixed and allowed to stand at room temperature for 3 min. To this, 0.3 mL of 10% AlCl3(Sigma Aldrich, Germany) was added. After 5 min, 2 mL of 1 mol/L NaOH was added and shaken to react. This was diluted by topping up to 10 mL mark. Each experiment was done in triplicates. Absorbance was measured at 510 nm. Quercetin standard was prepared in the range of 200 mg/L to 1000 mg/L,flavonoids content were expressed as milligram quercetin per gram dry weight (mg QE/g).
2.10. ABTS assay
The potential of edible mushroom antioxidant potential to oxidise peroxidise substrate ABTS free radicals were evaluated as previously described[22]. Samples were analysed in triplicates. Each sample test was mixed with ABTS working solution, centrifuged and optical density of lower phase determined at 734 nm. The underlying principle is the ability of the extract to reduce the preformed ABTS radicals by decolorizing the blue green chromophore. A polar soluble vitamin E analogue, trolox was used as standard for this evaluation. Results were expressed as mg trolox equivalent per gram (mg TE/g) dry weight (Linearity curve range 0.1–0.8 mmol/L).
2.11. Ferricyanide reduction antioxidant power (FRAP) assay
The potential of mushroom reducing power was determined by following the previously described [23]. A concentration gradient for each phenological state was designed. Mushroom aliquot of 2.5 mL was mixed with equal volumes of 200 mmol/L sodium phosphate buffer (pH 6.5, AR) and 2.5 mL of 1% potassium ferricyanide (AR). This mixture was incubated for 20 min at 50°C before adding 2.5 mL of 10% trichloroacetic acid (w/v) and carrying out separation by centrifugation at 1200 r/min for 10 min. Five millilitres of supernatant was pipetted in a new labelled tube and volume doubled with deionised water (5 mL). Immediately, 1 mL of 0.1% ferric chloride (AR) was added and optical density determined at 700 nm. BHT, L-AA and TOC were analysed alongside the samples to serve as positive standards. Samples were analysed in triplicates.
2.12. DPPH free radical assay
Fresh solution of DPPH (Sigma Aldrich, Germany) was always prepared in the solvent initially used for extraction before determining the scavenging activities as previously described [24]. Briefly, 4 mL of previously prepared extract concentration (1.5, 2.5, 5.0, 10.0, 20.0 mg/mL) was used for this analysis by mixing with 1 mL of 0.1 mmol/L DPPH diluted in analytical grade ethanol. Standards L-AA, TOC, and BHT were analysed alongside the samples to determine how samples compare with positive controls. The decrease in absorbance was measured at 517 nm. Each experiment was done in triplicates. Antiradical activity was expressed as percentage inhibition. The percentage inhibition of the radicals due to antioxidant properties of the extract was calculated as below:
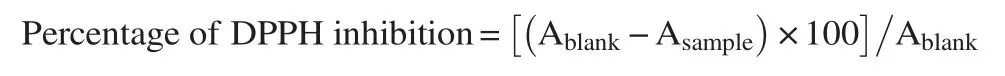
2.13. LC-QToF-MS
Phenologicalsamplesfrom P.citrinopileatus(twoprimordials phases, mature fruiting body, and SPM) were prepared by dissolving 10 g of powdered extracts to 100 mL of ethanol and distilled water (75%:25%) and left overnight in a rotary shaker at 150 r/min in darkness at room temperature for complete extraction.Theresultingextractswerecentrifugedat10000r/min for 20 min at 25°C. The supernatant were filtered with a syringe fittedwith0.22μmfilterandfrozenat?20°Cinvialsbeforebeing subjected to HPLC fractionation before LC-QToF-MS analyses.
LC-QToF-MS (Agilent Technologies, 6120) analyses, the HPLC fractions containing compounds were collected, and 0.5 μL injected into LC-QToF-MS automatically as previously described [25]. Chromatographic separation was achieved on a Waters ACQUITY ultra-performance liquid chromatography (UPLC) I-class system (Waters Corp., Milford, MA, USA)fitted with a Waters ACQUITY UPLC BEH C18 column (2.1 mm×100 mm, 1.7-μm particle size; Waters Corporation, Ireland) that was heated until it attained 40°C, while cooling the auto sampler to 15°C. The acetonitrile (B), and water (A), were used as the mobile phase, each of these with formic acid (0.01%). The process utilised a gradient system and positive mode of full high energy bombardment scan as previously described by Wamalwa et al. [25]. Previously described method by Wamalwa was followed using gradient as follows 0–1.5 min, 10% B; 1.5–2 min, 10%–50% B; 2–6 min, 50%–100% B; 6–9 min, 100% B; 9–10 min, 90%–10%; 10–12 min, 10% B [25]. The flow rate was held constant at 0.4 mL/min. The UPLC system was interfaced by electrospray ionization to a Waters Xevo QToF-MS operated in full scan high energy bombardment in positive mode [25]. Data were acquired in resolution mode over the m/z range 100–1200 with a scan time of 1 s using a capillary voltage of 0.5 kV, sampling cone voltage of 40 V, source temperature 100°C and desolvation temperature of 350°C [25]. The nitrogen desolvation flow rate was 500 L/h. For the high-energy scan function, a collision energy ramp of 25–45 eV was applied in the T-wave collision cell using ultrahigh purity argon (≥99.999%) as the collision gas [25]. A continuous lock spray reference compound (leucine enkephalin; [M+H]+= 556.276 6) was sampled at 10 s intervals for centroid data mass correction. The mass spectrometer was calibrated across the 50- to 1200-Da mass range using a 0.5 mmol/L sodium formate solution prepared in 90:10 2-propanol/water (v/v). MassLynx version 4.1 SCN 712 (Waters Corp., Milford, MA, USA) was used for data acquisition and processing. The elemental composition was generated for every analyte [25]. Potential assignments were calculated using the mono-isotopic masses with specifications of a tolerance of 10-mg/L deviation and both odd- and evenelectron states possible [25]. The number and types of expected atoms was set as follows: carbon≤100; hydrogen≤100; oxygen≤50; nitrogen≤6; sulphur≤6. The empirical formula generated was used to predict structures which were proposed based on the online database (Chemspider database), fragmentation pattern and literature [25].
2.14. Statistical analysis
Data obtained were analysed statistically by SPSS 16 statistical software (SPSS Inc, Chicago, USA). One-way ANOVA and Tukey honest significant difference test were used to ascertain whether there were significant differences between the mean values (P<0.05). Pearson correlation was used to test if the relationship between total phenols, total flavonoids, and radical scavenging activities were significant.
3. Results
3.1. Screening and relative abundance of phytochemicals
Phytoconstituents like tannins, terpenoids,flavonoids, polyphenols, phytosterols, were detected in both ethanol and water extracts for the pooled samples (Table 1). However, relative abundance based on goodness of fit test χ2used in allocating the phytochemicals for the specie showed that these molecules exhibited high concentrations of polyphenols,flavonoids, phytosterols, and terpenoids based on the set critical value (Table 1, Figures 2 and 3). Low levels of saponins and tannins in ethanol and phytosterols in water extracts were detected. Other constituents like cardiac glycosides, resins, alkaloids, anthraquinones, were not detected from both extracts (Table 1, Figures 2 and 3).
3.2. TPCs
Table 2 shows varied distribution of polyphenol content across the four phenological states. The two primordial phases of ethanol extract recorded significant and the highest TPCs of (10.018±0.601) mg GAE/g and (13.803±0.797) mg GAE/g for the 1st and 2nd primordial, respectively (Table 1). Phenolic content from fruiting bodies and 1st primordials were not significant statistically (P>0.05). Values of polyphenols in water extract were not significantly different across the 1st and 2nd primordials. However, SPM recorded significantly higher values than the fruiting bodies (P<0.05) (Table 2).
3.3. TFCs
Table 2 illustrates that flavonoids were significantly different across the four phases in ethanol extract with 1st and 2nd phases of primordials recording the highest values (7.831±0.576) and (10.619±0.785) mg QE/g, P<0.05, respectively. Fruiting phase recorded a significantly higher value than the spawn mycelia phase (P<0.05). However flavonoids distribution in water extract across these phenologies were not significantly different (Table 2).

Figure 2. Qualitative abundance of pooled P. citrinopileatus Singer ethanol extract.

Figure 3. Qualitative abundance of pooled P. citrinopileatus Singer water extract.
3.4. Determination of total carotenoids,β-carotene, and lycopene
Contents of total carotenoids,β-carotene and lycopene exhibited considerable variation between the four phenological states. Total carotenoids of 2nd primordial [(5.632±1.808) g/ 100 g] differed significantly from the other three phases. However, total carotenoids of 1st primordial [(2.910±1.007) g/ 100 g] did not differ significantly from mature fruiting bodies [(3.124±0.725) g/100 g] (Table 3). Contents of β-carotene ranged from 0.002 to 0.262 mg/g dry weights while lycopene ranged from 0.001 to 0.026 mg/g dry weights (Table 3). The highest content of β-carotene was detected in the second phase of primordial (0.262±0.005) mg/g dry weight) while the relative highest lycopene was detected in the first primordial phase (0.026±0.003) mg/g dry weight). SPM were deprived of these antioxidant properties. Generally, contents of lycopene were lower than those of β-carotene concentrations. Primordials exhibited higher contents of both lycopene and B carotene in than the fruiting phases.
3.5. ABTS assay
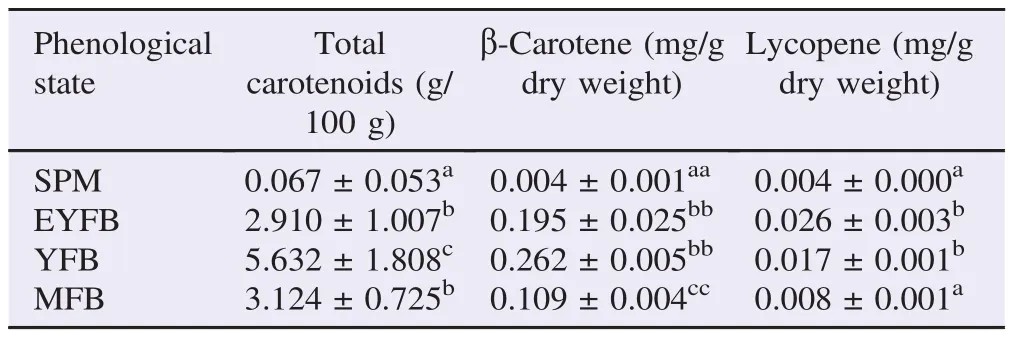
Table 3Contents of total carotenoids,β-carotene and lycopene of coloured edible mushrooms P. citrinopileatus.
The potential of mushrooms to scavenge for ABTS radicals ranged from (0.617±0.008) to (14.075±1.001) mg TE/g dry weight at 20 mg/mL for water extract while ethanol extract reported (0.411±0.103) to (11.825±1.050) mg TE/g dry weight at 20 mg/mL (Figure 4). In both extracts, primordial phases reported significantly higher scavenging properties for ABTS radicals. However, the main observation made is that ABTS scavenging properties were maturity dependent with the 1st and 2nd primordial phases exhibiting significantly higher values than both the fruiting phases and the spawn mycelia phase for both water and ethanol extract. Besides, statistical significant relationship was observed between TEAC,β-carotene, and lycopene; this Pearson correlation points out the strong positive relationship between TEAC and TPC (r>0.9, P<0.9) (Table 4).
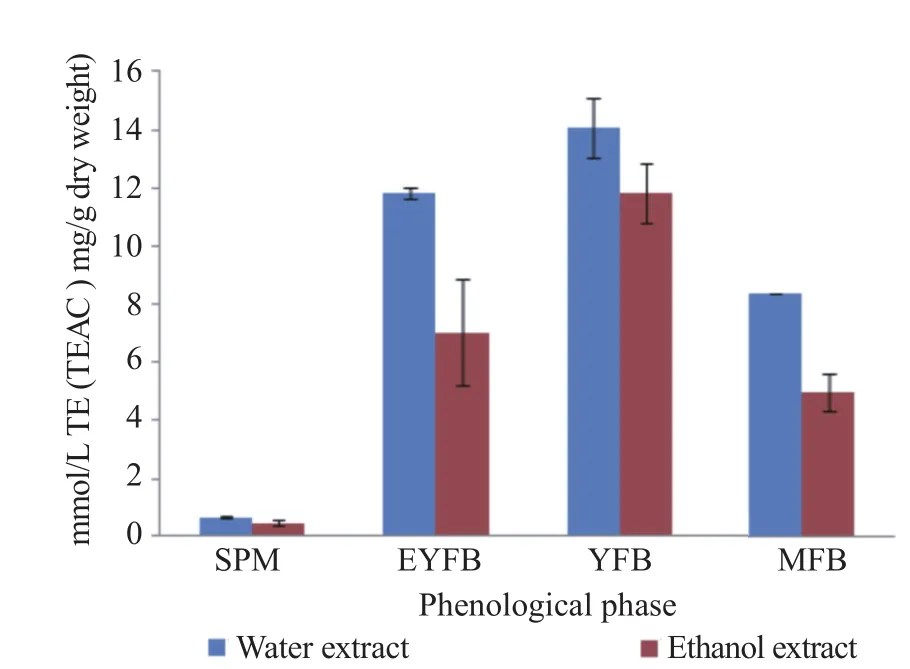
Figure 4. Phenological contents of ABTS assay of coloured edible mushrooms P. citrinopileatus.TEAC: Trolox equivalent antioxidant capacity.

Table 4Pearson correlation between TPC, TFC, lycopene,β-carotene and their total antioxidants at 10 mg/mL (FRAP/TEAC/DPPH).
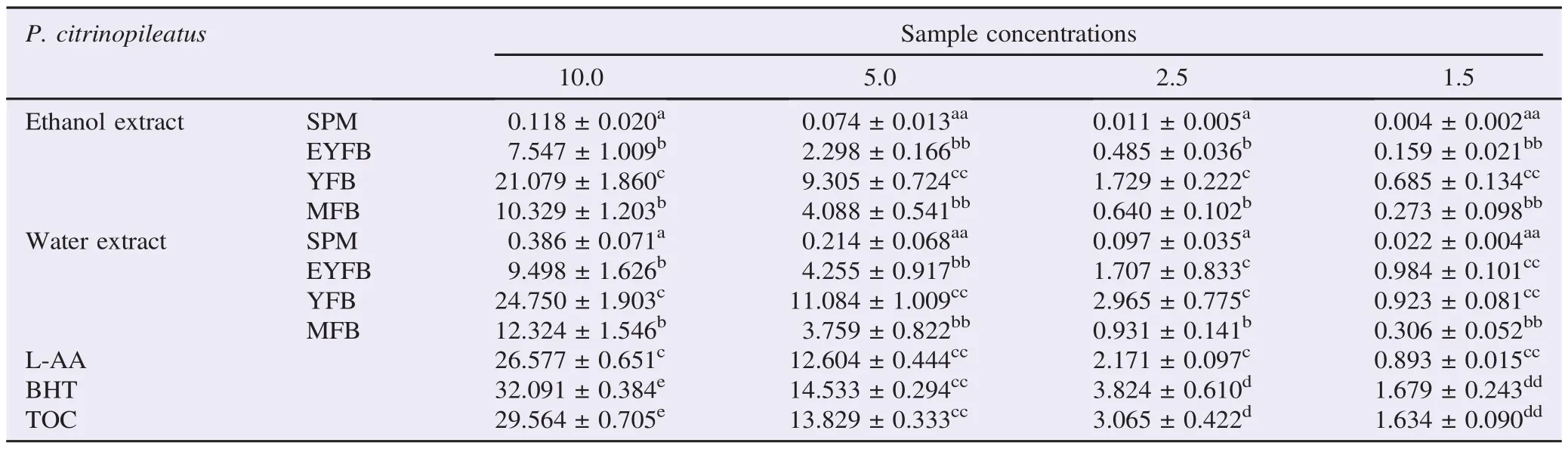
Table 5FRAP assay of golden yellow edible mushrooms P. citrinopileatus. mg/mL.
3.6. Ferricyanide reducing power assay
This study demonstrates the existing phenological differences in the specie to reduce ferric ions. Generally, the 2nd primordial phase exhibited comparable reduction power with L-AA, BHT, and TOC in both water and ethanol extracts (Table 5). There was significance difference in the ability of each phenology to reduce ferricyanide in both solvents. Spawn mycelia phases were deprived of these properties. Pearson correlation shows positive and strong relation of reducing power (10 mg/mL) with TPC, TFC, lycopene, and β-carotene (r>0.9; P<0.001) for both extracts as represented in (Table 4).
3.7. DPPH radical scavenging properties
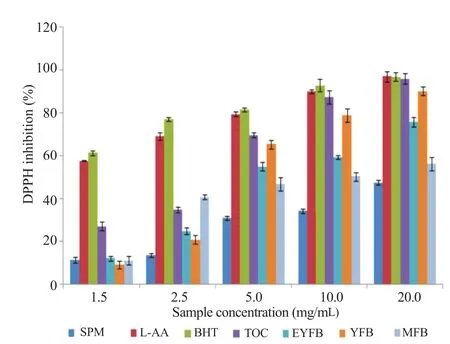
Figure 5. DPPH radical scavenging activity of various phenological states of P. citrinopileatus ethanol extract, L-AA, TOC, BHT.
DPPH radical scavenging properties showed a concentration dependency for all phenological phases (Figures 5 and 6). Percentage inhibition increases with sample concentration with the highest percentage inhibition recorded at 20 mg/mL for all the four growth levels in both water and ethanol extracts (Figures 5 and 6). Radical scavenging properties were significantly different between primordials of the basidiocarps (EYFB, YFB) and the mature basidiocarps MFB (P<0.05). Spawn mycelia phase and the fruiting phase showed a reduced capacity, which were significantly lower (P<0.05) compared with positive controls BHT, L-AA, and TOC. Second primordial (YFB) exhibited relatively higher radical scavenging values that were not significantly different from positive controls (Figures 5 and 6).
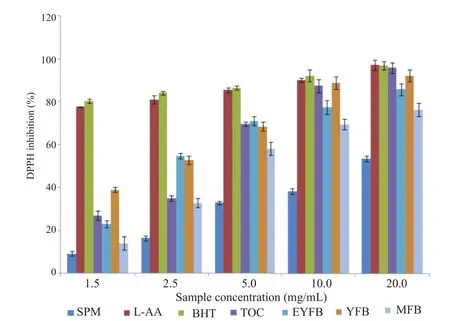
Figure 6. DPPH radical scavenging activity of various phenological states of Pleurotus citrinopileatus water extract compared with L-AA, TOC, and BHT.
3.8. Profile and distribution of metabolites across four phenologies
Profiling of compounds across phenologies for the specie exhibited comparative variation as shown by peak areas (Figure 7 and Table 6). Elemental composition of each peak was determined to acquire accurate mass, percentage confidence, peak area, and empirical formula. Peak areas were used to determine quantitative comparison of identified compounds while accurate mass and empirical formula was used to identify the compound against those listed in Chemspider?database and searched against compounds previously reported in mushrooms. The main flavonoids identified were resveratrol, formononetin, roseoside, dinoprostone, loganate, kaemferol, hesperetin, biochanin A, and naringin, while polyphenol acids identified included ferulic acid, 4-coumaric acid, homogentisic acid, gallic acid, protocatechic acid, 4-phenylbutyric acid, and caffeic acid (Table 6).

Figure 7. Accurate mass of compounds across four phonologies in P. citrinopileatus.GB1: SPM; GB2: Early young basidiocarps; GB3: Young basidiocarps; GB4: fruiting bodies.
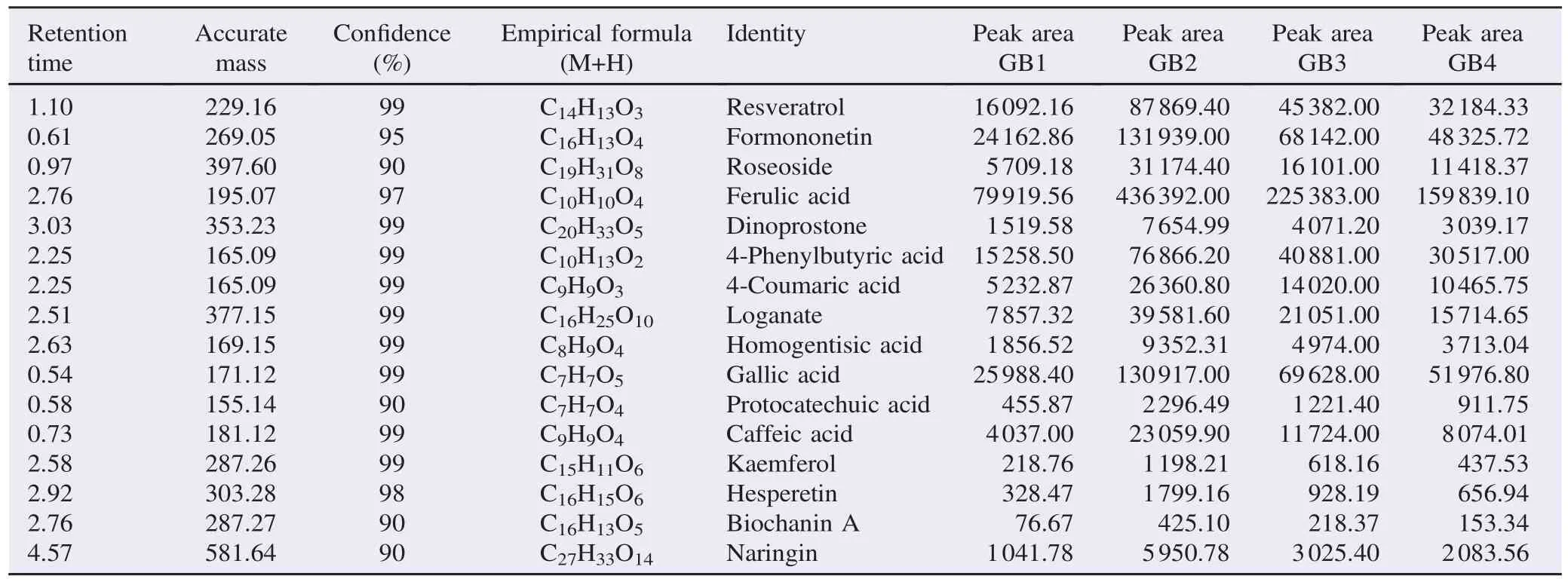
Table 6Identity of flavonoids and polyphenol compounds based on accurate mass and empirical formulas of P. citrinopileatus.
4. Discussion
Previous studies documents bioactivity of lyophilized, fresh and dried mushrooms samples without contextualizing the distribution of bioactive molecules across phenological and physiological phases [16,3]. This study documents essential health promoting bioactive molecules across phenological phases of a native unique basidiomycete. We demonstrated that golden yellow P. citrinopileatus is a rich source of phytochemicals like flavonoids, polyphenols with immense health properties. These findings are in agreement with those from previous studies on phytochemical of various native Kenyan edible mushrooms collected from tropical forests [10]. Previous studies on chemical composition of various maturity stages revealed that immature spores (herein referred to as primordials) have high content of bioactive molecules, which agrees with our finding [8]. However, in the present study, phytochemicals like cardiac glycosides, and anthraquinones were not detected. These finding provide leads to mycologists interested in biotechnological processes of solid states fermentation to tap these molecules at an appropriate phenological phase for either pharmaceutical or nutraceutical functions. Most of these bioactive molecules in other plants have been documented structurally and pharmacologically as containing pharmacological properties like anti-diabetic, antiinflamatory[26]and anticancer[27]. This highlights the need for a paradigm shift in collection of health promoting molecules from the primordials of basidiocarps.
Our findings show that phenolic compounds exhibited phenological variation. Primordial phases of P. citrinopileatus recorded the highest values of polyphenols. Previous studies recommended immature spores [9] as the main source of these bioactive molecules, other studies [28] suggested that spores, mycelium, and fruiting bodies parts of mushrooms possess differential but significance differences. Our study collaborates previous finding by others who used button mushrooms [16] reported variation in chemical and bioactive compounds with mature fruiting bodies presenting lower antimicrobial and bioactive activities as opposed to immature spores (primordials). Besides, our study used whole mushroom unlike other studies that majored on different parts of mushroom that can only be obtained in fruiting bodies after differentiation [29]. Primordial stages provided a better source for bioactive molecules. High phenolic acid contents in the primordial phases contribute partially to their high antioxidant properties as postulated in how the value correlates with in vitro antioxidant assays. Polyphenol content increases with the maturity of the basidiomycete, so that young golden yellow primordial showed higher polyphenol contents as opposed to fruiting bodies.
There was significant difference in the TFC in the four phenological levels (P<0.05) for P. citrinopileatus (Table 1). Primordial reported the highest flavonoid values (Table 1). This observation agrees with those obtained from wild mushrooms [16]. TFCs of P. citrinopileatus recorded in all the growth levels were significantly different (P<0.05). This denotes that as the mushroom grows the contents of flavonoids increases to a certain level before declining sharply, a similar trend with polyphenol contents. Similarly, other studies have shown that bioactive molecules in Ganoderma mushrooms changes with maturity so that different stage determines quantities of specific bioactive molecules [28]. Although previous studies were not phenologically designed, they demonstrated that immature mushroom (primordials) from Lactarius piperatus have high contents of phenol compound than their mature part (fruiting bodies), which agrees with our finding [16]. The existing explanation for the low values in the mature stages is the possibility of involvement of these molecules in defensive processes against ageing stressors [1]. However, others demonstrated that secondary metabolites vary depending on the solvent used for their extraction, which is true as well based on the solvents used in the present study [30]. Ethanol extracts and water extract may not give a complete profile hence our values could not be effectively compared with other studies that used non-food grade solvents like methanol and chloroform in studying these molecules.
Pigment molecules like β-carotene, carotenoids, and lycopene have been reported previously in edible fungi and their potential roles in antioxidant properties studied[16,31]. Our work showed varied concentrations of these molecules across the four phenological states. Spawn mycelia phases reported low values, which denotes that they are deprived of these essential phytochemicals. However, 1st and 2nd primordials phases exhibited highest contents of β-carotene, total carotenoids, and lycopene. Total carotenoid was significantly high in each phenology compared to both β-carotene and lycopene. Previous studies reported low quantities of these bioactive molecules in their respective studies on mushrooms [9,31]. Their finding agrees with values obtained from mature fruiting bodies, which were much lower than values in primordials perhaps because their studies were not phenologically designed. This depicts that chances of missing on optimal quantities of these molecules is high if primordials are not taken into account. However, increased values in our study could be attributed to the species pigmentation (golden yellow mushroom) as opposed to white Agaricus specie used in previous studies. These molecules have pharmacological value because supplementation of diet with β-carotene has shown increased plasma antioxidant properties [6], while lycopene has protective value in preventing oxidative modification of lymphocyte DNA [31]. Pharmacological benefits of these molecules highlight the need to consider primordial phases as alternative source of potent molecules that can be targeted for pharmaceutical or nutraceutical health benefits. This is because primordial phases of P. citrinopileatus offers better outlet of these health-promoting molecules.
Differential potential of phenological phases of P. citrinopileatus to inhibit lipid peroxidation determined using ABTS+assay with trolox was reported. Primordials of both water and ethanol extracts showed a better activity comparable to SPM and mature fruiting bodies, this difference could be attributed to ageing during which basidiocarps are involved in defence mechanisms [9,16]. Edible fungi have been shown to possess analogous defensive systems comparable to those found in other plants to help fight biotic and abiotic factors [32]. The potency of primordials in discolouration of ABTS radicals could be attributed to high phenolic and flavonoid contents reported in both water and extract, which ascribes to previous studies [31]. These features have been exploited for pharmaceutical and nutraceutical properties [6,32]. Our study strengthens the importance of considering targeted phenological phase to maximize on these potential. This specie is unique because it not only occurs in golden yellow colouration but also has varied distribution of bioactive molecules across their developmental phases. This warrants the need to obtain and use basidiocarps from appropriate phenological phase to optimize on their value addition.
Several in vitro assays have been validated in the determination of antioxidant properties of compounds. These methods scavenge or quench free radicals, inhibit peroxidation of lipids or chelates for metal ions [31]. Reduction power of these samples was compared with BHT, L-AA, and TOC as positive controls. Our finding revels that reducing power varies across phenological phases, with primordials exhibiting significant potential to reduce potassium ferricyanide than both the spawn mycelia and the fruiting phase in water and ethanol extracts. High reduction power of this basidiomycete can be attributed to potent antioxidant property in the primordial phases of basidiocarps. This is because previous studies have associated reduction power to antioxidant properties [16,31]. These in vitro assays justified the existing differential distribution of antioxidant properties along phenological phases in P. citrinopileatus.
Primordial phases of P. citrinopileatus exhibited significant high antioxidant properties than the mature fruiting bodies. These finding highlights the potential of primordials as potent sources of bioactive molecules for antioxidant activities. These finding corroborates previous studies showing a similar trend in Kenyan mushrooms of 0.58 mg/mL to 4.58 mg/mL[10]and from other regions like Brazil, with 0.76–17 mg/mL[9]. Besides, this shows that P. citrinopileatus has a better ability to scavenge for free radicals at both primordial phases. Other than maturity,these properties could also be speculated to depend on a number of other factors like parts of mushroom utilized and the type of species as earlier demonstrated [29]. Our study used whole mushroom basidiocarps to minimize on such demerits. Any discrepancy in our finding may be attributed to other factors like substrate type, solvent used, which may possibly affect the quantity of phytochemicals extracted [30]. Generally, our study depicts that SPM can be an alternative source of important bioactive molecules to substitute mature fruiting bodies, which reported reduced values.
There is a strong positive (r>0.8) and significant correlation (P<0.001) between TPC, TFC,β-carotene, lycopene and various in vitro antioxidant assays, DPPH, ABTS, and FRAP for P. citrinopileatus (Table 4). It is therefore feasible to allude that flavonoids, polyphenols,β-carotene, and lycopene may play a significant role in conferring total antioxidant properties of the extracts. Reported work show that flavonoids and polyphenols could have additive effects on antioxidant properties[33]. Given that our samples were uniquely coloured, unlike most previous findings that did not use such species [8,9,16], which could explain existence of high total carotenoids,β-carotene, and lycopene in our finding. However, some studies did not find any significant correlation between flavonoids and radical scavenging activity properties [34], which contradicts our finding. The significance of this study is attributed to high phenolic and flavonoid compounds in primordial stages of basidiocarps. Interestingly, this study documents spawn mycelia as a potential alternative target for these value added health properties. These flavonoids, polyphenols, carotenoids, lycopene, and β-carotene have positive and significant relationship with various in vitro antioxidant activities. Such molecules could be optimized through biotechnological processes using liquid state fermentation through a bioreactor. Barros et al. [16] reported that phenolic compound and other secondary metabolites like carotenoids, tocopherols, and ascorbic acids contributed to radical scavenging activity. This is the first study justifying variations of health promoting molecules across phenological phases.
Distribution of identified compounds across the four phenological phases for the species was different. A total of 17 compounds (consisting of 10 polyphenol acids and 7 flavonoids) were identified. These compounds included resveratrol, formononetin, roseoside, ferulic acid, dinoprostone, 4-phenylbutyric acid, 4-coumaric acid, loganate, homogentisic acid, gallic acid, caffeic acid, kaemferol, hesperetin, biochanin, and naringin. These compounds varied across the four phenologies as shown in the result section (Table 6). This is the first study to profile and identify these bioactive molecules in golden yellow mushroom using LC-QToF-MS protocol. Previous studies identified compounds like p-coumaric acid, vanillic acid, protocatechuic and p-hydroxybenzoic from edible wild mushrooms like Agaricus specie and Cantharellus cibanus using known commercial standards[9], they however, did not identify any flavonoids. To-date, no existing literature describes profiling of these bioactive compounds using untargeted approach like LC-QToF-MS in mushroom species like P. citrinopileatus. These compounds could be incriminated to confer health benefits associated to traditional knowledge of using mushrooms in ethnomedicine. A synergistic activity of these compounds could have potentiated the total antioxidant properties.
To conclude, this study demonstrates that primordial phases of basidiocarps are potent sources of secondary metabolites that could be utilized in formulating pharmaceutical and nutraceuticals products. There is a direct relationship between the fruit body phenology, the total polyphenols content, TFC, and their total antioxidant properties (DPPH, TEAC, FRAP,β-carotene, and lycopene). SPM could be used an alternative source of important bioactive molecules instead of mature fruiting bodies. Selection of primordial on the 16th day and 17th day after substrate inoculation or 2nd day and 3rd day after pinning is most appropriate for the golden yellow species. Selection of these primordials for elucidation of additional information on additional biological functions and in silico studies will map out unreported pharmaceutical prospects. Future studies will attempt to purify molecules responsible for value addition.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The first author wish to express gratitude to the African Union for providing funds (African Union, No. 17/2014) and the postgraduate scholarship, the Board of Directors and Management of the Kenya Industrial Research and Development Institute (KIRDI) and the Kenya Agricultural Productivity and Agribusiness Project all for providing the experimental facilities and personnel to assist in undertaking this work. We are greatly indebted to all the scientific, technical and support staff at KIRDI, International Centre for Insect Physiology and Ecology (ICIPE-Nairobi), and Jomo Kenyatta University of Agriculture and Technology for their assistance during the study.
References
[1] Pereira E, Barros L, Martins A, Ferreira ICFR. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem 2012; 130: 394-403.
[2] Reis FS, Barros L, Martins A, Ferreira ICFR. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an interspecies comparative study. Food Chem Toxicol 2012; 50: 191-7.
[3] Reis FS, Heleno SA, Barros L, Sousa MJ, Martins A, Santos-Buelga C, et al. Toward the antioxidant and chemical characterization of mycorrhizal mushrooms from Northeast Portugal. J Food Sci 2011; 76(6): C824-30.
[4] Mohamed IM, Mohamed MRM, Abdul BJ, Asarudeen A. Determination of total phenol,flavonoid and antioxidant activity of edible mushrooms. Pleurotus florida and Pleurotus eous. Int Food Res J 2011; 18: 579-82.
[5] Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012; 12: 221.
[6] Athanasakis G, Aligiannis N, Gonou-Zagou Z, Skaltsounis A, Fokialakis N. Antioxidant properties of the wild edible mushroom Lactarius salmonicolor. J Med Food 2013; 16(8): 760-4.
[7] Fernando CD, Soysa P. Total phenolic,flavonoid contents, in-vitro antioxidant activities and hepatoprotective effect of aqueous leaf extract of Atalantia ceylanica. BMC Complement Altern Med 2014; 14: 395.
[8] Barros L, Baptista P, Estevinho LM, Ferreira IC. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactariu ssp. mushrooms. J Agric Food Chem 2007; 55(21): 8766-71.
[9] Barros L, Baptista P, Ferreira ICFR. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measuredby several biochemical assays. Food Chem Toxicol 2007; 45(9): 1731-7.
[10] Wandati TW, Kenji GM, Onguso JM. Phytochemicals in edible wild mushrooms from selected areas in Kenya. J Food Res 2013; 2(3): 137-44.
[11] Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 2011; 6(11): 1720-31.
[12] Musieba F, Okoth S, Mibey RK, Wanjiku S, Moraa K. Suitability of locally available substrates for cultivation of the Kenyan indigenous golden oyster mushroom (Pleurotus citrinopileatus Singer). Agric J 2012; http://dx.doi.org/10.3923/ aj.2012.240.244.
[13] Sanchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 2010; 85(5): 1321-37.
[14] Liang ZC, Wu KJ, Wang JC, Lin CH, Wu CY. Cultivation of the culinary-medicinal lung oyster mushroom, Pleurotus pulmonarius (Fr.) Qu′el. (Agaricomycetideae) on grass plants in Taiwan. Int J Med Mushrooms 2011; 13(2): 193-9.
[15] Mintesnot B, Ayalew A, Kebede A. Evaluation of biomass of some invasive weed species as substrate for oyster mushroom (Pleurotus spp.) cultivation. Pak J Biol Sci 2014; 17(2): 213-9.
[16] Barros L, Ferreira MJ, Queiros B, Ferreira ICFR, Baptista P. Total phenols, ascorbic acid,β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem 2007; 103(2): 413-9.
[17] Gupta A, Raj H, Sharma B, Upmanyu N. Phytochemical comparison between pet ether and ethanolic extracts of Bacopa monnieri, Evolvulus alsinoides and Tinospora cordifolia. Pak J Biol Sci 2014; 17(4): 590-3.
[18] Marinas?IC, Chifiriuc C, Oprea E, Lazar V. Antimicrobial and antioxidant activities of alcoholic extracts obtained from vegetative organs of A. retroflexus. Roum Arch Microbiol Immunol 2014; 73(1–2): 35-42.
[19] Robaszkiewicz A, Bartosz G,?awrynowicz M, Soszynski M. The role of polyphenols,β-carotene, and lycopene in the antioxidative action of the extracts of dried edible mushroom. J Nutr Metab 2010; http://dx.doi.org/10.1155/2010/173274.
[20] Kumar S, Sandhir R, Ojha S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res Notes 2014; 7: 560.
[21] Radzki W, S?awinska A, Jab?onska-Rys E, Gustaw W. Antioxidant capacity and polyphenolic content of dried wild edible mushrooms from Poland. Int J Med Mushrooms 2014; 16(1): 65-75.
[22] Yim HS, Chye FY, Lee MY, Matanjun P, How SE, Ho CW. Comparative study of antioxidant activities and total phenolic content of selected edible wild mushrooms. Int J Med Mushrooms 2011; 13(3): 245-55.
[23] Pal J, Ganguly S, Tahsin KS, Acharya K. In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus (Mont.) Singer. Indian J Exp Biol 2010; 48(12): 1210-8.
[24] Subedi L, Timalsena S, Duwadi P, Thapa R, Paudel A, Parajuli K. Antioxidant activity and phenol and flavonoid contents of eight medicinal plants from Western Nepal. J Tradit Chin Med 2014; 34(5): 584-90.
[25] Wamalwa LN, Cheseto X, Ouna E, Kaplan F, Maniania NK, Machuka J, et al. Toxic ipomeamarone accumulation in healthy parts of sweet potato (Ipomoea batatas L. Lam) storage roots upon infection by Rhizopus stolonifer. J Agric Food Chem 2015; 63(1): 335-42.
[26] Jedinak A, Dudhgaonkar S, Wu QL, Simon J, Sliva D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-kB and AP-1 signaling. Nutr J 2011; 10: 52.
[27] Xu W, Huang JJ, Cheung PC. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-induced PI3K/AKT signaling pathway. PLoS One 2012; 7(3): e34406.
[28] Smina TP, Mathew J, Janardhanan KK, Devasagayam TP. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ Toxicol Pharmacol 2011; 32(3): 438-46.
[29] Vamanu E, Nita S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed Res Int 2013; http://dx.doi.org/10.1155/2013/313905.
[30] Babbar N, Oberoi HS, Sandhu SK, Bhargav VK. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol 2014; 51(10): 2568-75.
[31] Ozen T, Darcan C, Aktop O, Turkekul I. Screening of antioxidant, antimicrobial activities and chemical contents of edible mushrooms wildly grown in the black sea region of Turkey. Comb Chem High Throughput Screen 2011; 14(2): 72-84.
[32] Vaz JA, Barros L, Martins A, Morais JS, Vasconcelos MH, Ferreira ICFR. Phenolic profile of seventeen Portuguese wild mushrooms. LWT- Food Sci Technol 2011; 44(1): 343-6.
[33] Obodai M, Ferreira IC, Fernandes A, Barros L, Mensah DL, Dzomeku M, et al. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 2014; 19(12): 19532-48.
[34] Gan CH, Nurul Amira B, Asmah R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). Int Food Res J 2013; 20(3): 1095-102.
*Corresponding author:Prof. Erastus Gatebe, PhD., Chief Research Scientist at Kenya Industrial Research and Development Institute (Chemical Engineering Department), P.O. Box 30650-00100 Nairobi, Kenya; Professor at Jomo Kenyatta University of Agriculture and Technology (Chemistry Department), P.O. Box 62000-00200 Nairobi, Kenya.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
