Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
Pawitra Pulbutr, Somsak Nualkaew, Sakulrat Rattanakiat, Benjamart Cushnie, Achida JaruchotikamolPharmaceutical Chemistry and Natural Product Research Unit, Faculty of Pharmacy, Mahasarakham University, Maha Sarakham 44150, Thailand
?
Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
Pawitra Pulbutr*, Somsak Nualkaew, Sakulrat Rattanakiat, Benjamart Cushnie, Achida Jaruchotikamol
Pharmaceutical Chemistry and Natural Product Research Unit, Faculty of Pharmacy, Mahasarakham University, Maha Sarakham 44150, Thailand
Original article http://dx.doi.org/10.1016/j.apjtb.2015.10.010
Tel/Fax: +66 43 754 360
E-mail: ppawitra@yahoo.com
Allexperimentalproceduresinvolvinganimalsinthisresearchprojectwereconductedinaccordancetothenationalethicalguidelinesforuseandcareofexperimentalanimals(providedbythe National Research Councilof Thailand,NRCT)andapprovedbytheanimalresearchethicscommittee,Mahasarakham University,Thailand(Referencenumber:009/2556).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation Project: Supported by Mahasarakham University research grant (Grant No. 5805028/2558,fiscal year 2015).
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 15 Sep 2015
Receivedinrevisedform28 Sep2015
Accepted 23 Oct 2015
Available online 19 Dec 2015
Keywords:
Pseuderanthemum palatiferum
Diabetes mellitus
α-Amylase
α-Glucosidase
Postprandial blood glucose
ABSTRACT
Objective: To investigate the effects of the leaf ethanolic extract of Pseuderanthemum palatiferum (PPE) and its isolated phytochemicals, stigmasterol and sitosterol-3-O-β-D-glucopyranoside, against α-amylase and α-glucosidase enzyme activities both in vitro and in vivo.
Methods: A concentration of maltose, which is a product released in α-amylase-catalyzing reaction, was used as an index of in vitro α-amylase activity. Meanwhile, in vitro α-glucosidase enzyme activity was indicated by the amount of liberated p-nitrophenol in α-glucosidase-catalyzing reaction. In vivo α-amylase and α-glucosidase enzyme activities were evaluated in the normal rats by using oral starch tolerance test and oral sucrose tolerance test, respectively.
Results: PPE exerted a concentration-dependent inhibitory action against both αamylase and α-glucosidase in vitro with the IC50values of (11.79±8.10) mg/mL and (1.00±0.11) mg/mL, respectively. Stigmasterol and sitosterol-3-O-β-D-glucopyranoside also exerted an in vitro α-amylase inhibition with the IC50values of (59.41±8.22)μg/mL and (111.19±9.02)μg/mL, respectively. However, these phytochemicals did not produce a concentration-dependent inhibition against in vitro α-glucosidase activity. PPE and its isolated phytochemicals significantly decreased the blood glucose levels at t = 30 min in the oral starch tolerance test. From the sucrose tolerance test, only PPE but not its isolated phytochemicals significantly caused a depletion in the blood glucose levels at t = 30 min
Conclusions: These results indicate an inhibitory action against carbohydrate-digesting enzymes as the anti-diabetic mechanism of action of PPE. Nonetheless, further clinical study is required to justify its role in the treatment of diabetes.
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder, characterized by an elevation of plasma glucose levels as a result of dysfunctions in insulin secretion and/or insulin actions. A prevalence of DM, especially type 2 DM, has continuously raised with the estimated global prevalence of 9% among adults in 2014 [1]. Chronic hyperglycemia evolved from diabetes ultimately leads to a pathogenesis of chronic diabetic complications especially cardiovascular disease, which is a major leading cause of death in diabetic patients [1]. In addition to fasting plasma glucose, postprandial (after-meal) plasma glucose plays a crucial role in the control of diabetes and its complications. Postprandial hyperglycemia contributes to a destruction of pancreatic β-cells and the developments of both micro- and macro-vascular complications [2,3]. A level of postprandial plasma glucose is mainly regulated by the function of carbohydrate-digesting enzymes in the intestine specifically α-amylase and α-glucosidase. Pancreatic α-amylase enzyme catalyzes a breakdown of α(1–4) glycosidic bond of starch, whilst intestinal α-glucosidase is essential for a digestion of oligosaccharides and disaccharides [4]. These two enzymesthus serve as a target for a regulation of postprandial plasma glucose levels.
Pseuderanthemum palatiferum(Nees) Radlk.(P. palatiferum) has been used as a folk medicine for the treatment of diabetes in several Southeast Asian countries. The fasting plasma glucoselowering effect of the plant's leaf ethanolic extract and its isolated phytochemicals were evidenced in the streptozotocininduced diabetic rats [5,6]. Our previous study showed that the leaf ethanolic extract of P. palatiferum (PPE) possessed antilipolytic, antioxidant and in vitro α-amylase inhibitory activities [7]. However, it is not known whether the extract exerts its inhibitory action against carbohydrate-digesting enzymes in vivo. Additionally, the plant extract-derived phytochemical which acts as an enzyme inhibitor has not been documented yet. The current study thus aimed to investigate the effects of PPE and its major isolated phytochemicals against α-amylase and αglucosidase enzyme activities both in vitro and in vivo.
2. Materials and methods
2.1. Preparation of PPE extract
The leaves of P. palatiferum were collected from the plantation in Roi Et Province, Thailand. The authentication of the specimens was performed by the Plant Varieties Protection Division, Department of Agriculture, Ministry of Agriculture and Cooperatives. A voucher is collected at the Herbarium of Pharmaceutical Chemistry and Natural Product Research Unit, the Faculty of Pharmacy, Mahasarakham University, Thailand (code: MSU.PH-ACA-P1). The leaves were dried at 50°C for 45 h in a hot-air oven and subsequently macerated with 80% ethanol in 1:10 ratio for 7 days. The extract was evaporated by using a rotary evaporator (Heidolph Laborota 4000, Germany) followed by a freeze dryer (Christ Alpha 1–4, Germany). PPE with the yield (%) of 12.7% w/w was then achieved. The extract was kept freezing at?20°C until use.
2.2. Isolation of the phytochemicals from PPE
Thin layer chromatography was conducted using normalphase silica gel 60 F254(Merck, Germany) on precoated aluminium plates. UV light (254 nm) and anisaldehyde-sulphuric acid spray reagent were used for detection. Column chromatography was carried out using silica gel 60 (0.063–0.200 mm; Merck, Germany). The melting point was obtained by using a Buchi melting point meter (Switzerland). UV spectra were obtained from a JASCO V530 UV/Vis spectrophotometer (Japan), while IR spectra were recorded with a Perkin Elmer Fourier transform infrared spectroscopy spectrometer (Germany). The mass spectra were obtained using a Bruker MicroTOF ESI-TOF (USA) spectrometry double-focusing probe at 70 eV, while nuclear magnetic resonance (NMR) spectra (1D and 2D) were measured on a Varian Mercury Plus 400 (USA) at 400 MHz for1H NMR and 100 MHz for13C NMR spectra. Solvents were of analytical grade such as n-hexane (Lab-scan, Ireland), dichloromethane (CH2Cl2), ethyl acetate (EtOAc), methanol (MeOH) (Carlo Erba, Italy), and ethanol (EtOH) (Merck, Germany).
The procedure was similar to that described previously [6]. Concisely, the PPE was suspended in 80% MeOH and partitioned successively with n-hexane extract. The n-hexane extract was chromatographed on a silica gel column and eluted with gradient of n-hexane and EtOAc (100:0, 80:20, 70:30, 50:50, 30:70, 20:80 and 0:100 v/v), each portion of 500 mL. These elutes were collected in a series of test tubes with 20 mL in each fraction. The homogeneity of the eluted was monitored by thin layer chromatography and the identical fractions were combined to afford five fractions. Fraction 1 was rechromatographed on a silica gel column and eluted with n-hexane: EtOAc (8:2 v/v) to afford stigmasterol. Fraction 5 was precipitated with EtOAc to yield sitosterol-3-O-β-D-glucopyranoside.
2.3. In vitro study
2.3.1.α-Amylase enzyme activity assay
The α-amylase enzyme activity assay was performed according to the method of Ali et al. with slight modifications[8]. Briefly, a mixture of 200 μL of freshly prepared porcine pancreatic α-amylase (4 unit/mL) and 40 μL of the sample was prepared and incubated at 25°C for 5 min. Potato starch solution (0.5% w/v, 400 μL) and distilled water (160 μL) were subsequently added into the mixture and incubated at 25°C for further 3 min. A 200 μL volume of the mixture was added into a new microfuge tube containing 100 μL of 3,5-dinitrosalicylic acid color reagent solution (96 mmol/L 3,5-dinitrosalicylic acid and 5.31 mol/L sodium potassium tartrate in 2 mol/L NaOH) and then heated at 85°C for 15 min. The optical density of the mixture was measured at the wavelength of 540 nm. The absorbance of the colored sample was subtracted by the absorbance of the blank in order to obtain the exact absorbance of the mixture. The amount of generated maltose, calculated from the maltose standard curve, was used as an index of α-amylase enzyme activity. Percent reaction was obtained with the following equation: % Reaction=(Mean maltose in sample/Mean maltose in negative control)×100.
Percent inhibition was calculated as 100 - % reaction. The concentration-inhibitory response curve was plotted by using GraphPad Prism software version 6.0.
2.3.2.α-Glucosidase enzyme activity assay
The α-glucosidase enzyme activity was examined following theprotocolofElyaetal.withslightmodifications[9].Thereaction mixturecontaining10μLofsample,250μLofp-nitrophenyl-α-D-glucopyranoside (p-NPG; 5 mmol/L) and 490 μL of phosphate buffer (100 mmol/L, pH 6.8) was prepared and incubated at 37°C for 5 min. A 250 μL volume of α-glucosidase enzyme (0.15 unit/mL) was added into the mixture and then incubated for further 15 min at 37°C. A 250 μL volume of the mixture was then transferred into a new microfuge tube containing 500 μL of Na2CO3. The optical density (OD) of the mixture was measured at the wavelength of 405 nm. The amount of pnitrophenol released from α-glucosidase-catalyzing reaction was used as an index of α-glucosidase enzyme activity. The percentage of inhibition was calculated as the following equation: % Inhibition = [(OD of negative control)?(OD of sample)/(OD of negative control)]×100.
The concentration-inhibitory response curve was plotted by using GraphPad Prism software version 6.0.
2.4. In vivo study
2.4.1. Experimental animals
Male Wistar rats weighing 150–170 g were obtained from the National Laboratory Animal Center, Mahidol University, Thailand and housed at a constant temperature of (25±1)°Cwith a 12 h dark–light cycle. The rats were fed ad libitum with free access to water. The oral carbohydrate tolerance test was performed after 1-week acclimatization. All procedures with the animals were approved by the Animal Research Ethics Committee, Mahasarakham University, Thailand (No. 009/2556).
2.4.2. Oral starch tolerance test
The rats were divided into nine groups (n = 5 per group) and fasted overnight but had free access to water. Groups 1–3 were treated orally with PPE at the doses of 250, 500 and 1000 mg/kg body weight. Stigmasterol and sitosterol-3-O-β-D-glucopyranoside, isolated from PPE, were given to Groups 4–7 at the doses of 5 and 10 mg/kg, respectively. Group 8 was treated with acarbose (a positive control) at the dose of 10 mg/kg body weight, whilst Group 9 was orally fed with 2% Tween 80 (a vehicle control). After treatment with the designated agents for 10 min, all rats were orally given with starch (3 g/kg body weight). The blood glucose levels were measured by using a glucometer (Accu-Chek?Active, Roche Diagnostic, Thailand) at before (t = 0) and at 30, 60 and 120 min after starch feeding.
2.4.3. Oral sucrose tolerance test
The rats were divided into nine groups (n = 5 per group) and treated with the similar treatments as those in the oral starch tolerance test. All rats were fed with sucrose (4 g/kg body weight) solution at 10 min after the treatment. The blood glucose levels were measured at before (t = 0) and at 30, 60 and 120 min after sucrose feeding.
2.5. Statistical analysis
All data from the in vitro experiments were expressed as mean±SD,exceptthe IC50valueswereexpressedasmean±SEM. The in vivo data were presented as mean±SEM. The data were statistically analyzed using One-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test. Statistically significant differences were indicated by a P-value of<0.05.
3. Results
3.1. Isolation of stigmasterol and sitosterol-3-O-β-D-glucopyranoside from PPE
Stigmasterol was obtained as white needle crystals, melting point 135–136°C and its molecular formula was assigned to be C29H48O according to it mass spectrum (m/z 412 [M]+). Sitosterol-3-O-β-D-glucopyranoside was obtained as white needle crystals, melting point 297–298°C and its molecular formula was assigned to be C35H60O6according to it mass spectrum (m/z 599 [M+Na]+). The stigmasterol and sitosterol-3-O-β-D-glucopyranoside were identified by comparing their physical and spectroscopic data of IR, NMR and mass spectra with the reported in the literature [6] and analyzing their 2D NMR spectral data.
3.2. Inhibitory effects of PPE against in vitro α-amylase enzyme activity
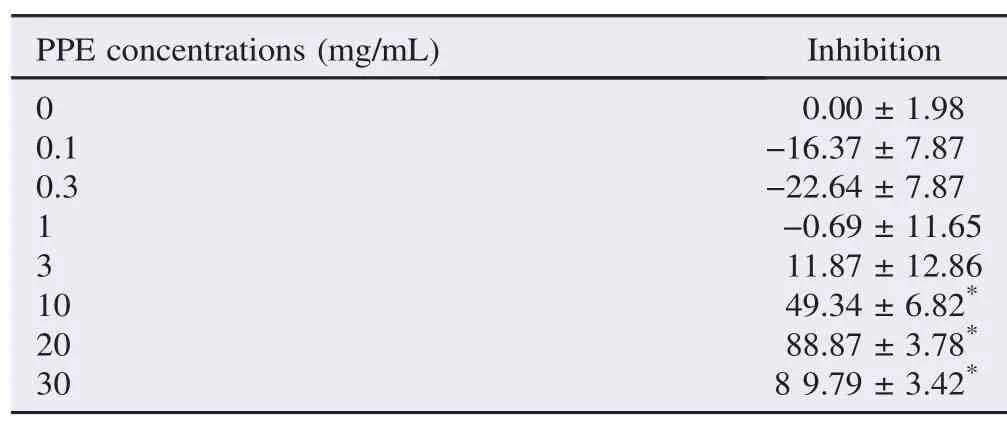
Table 1The percentage of inhibition of PPE against α-amylase enzyme activity. %.
PPEattheconcentrationsof10,20and30mg/mLsignificantly inhibited α-amylase enzyme activity (Table 1). The maximal inhibitionof (89.79±3.42)% was found at the PPE concentration of 30 mg/mL (n = 6, P<0.05). This is similar to the α-amylase inhibitoryeffectof100μg/mLacarbose(85.12±3.47)%.The PPE extract produced a concentration-dependent inhibitory action against α-amylase enzyme activity with an IC50of (11.79±8.10) mg/mL (mean±SEM, n = 6) (Table 2).

Table 2Median inhibitory concentrations (IC50) of PPE and its phytochemicals against α-amylase and α-glucosidase enzyme activities.
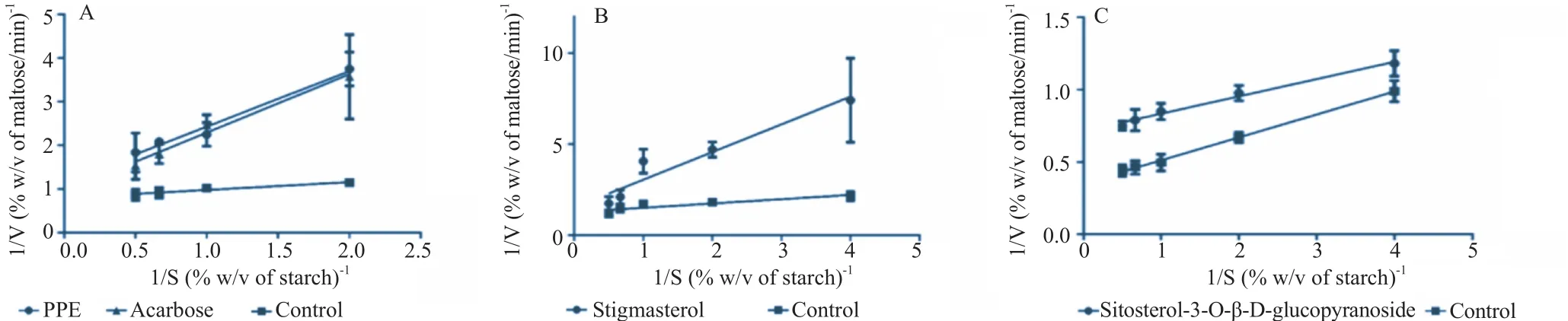
Figure 1. Kinetics analysis of α-amylase inhibition by (A) PPE and acarbose, (B) PPE-derived stigmasterol and (C) PPE-derived sitosterol-3-O-β-D-glucopyranoside.Data are expressed as mean±SEM, n = 6. Lineweaver–Burk plot of the reaction against α-amylase in the presence or absence of the test agents.
The experiment on α-amylase enzyme kinetics was then subsequently studied by varying substrate concentrations at 0.5%, 1%, 1.5% and 2% w/v with the fixed PPE concentration of 10 mg/mL (approximate IC50). Lineweaver–Burk plot was drawn to determine the enzyme kinetics in the presence and the absence of PPE (Figure 1A). PPE (10 mg/mL) caused a decreasein maximal rate of reaction (Vmax) and an increase in Michaelis constant (Km) (Table 3). This mode of changes in enzyme kinetics was similar to that caused by acarbose.
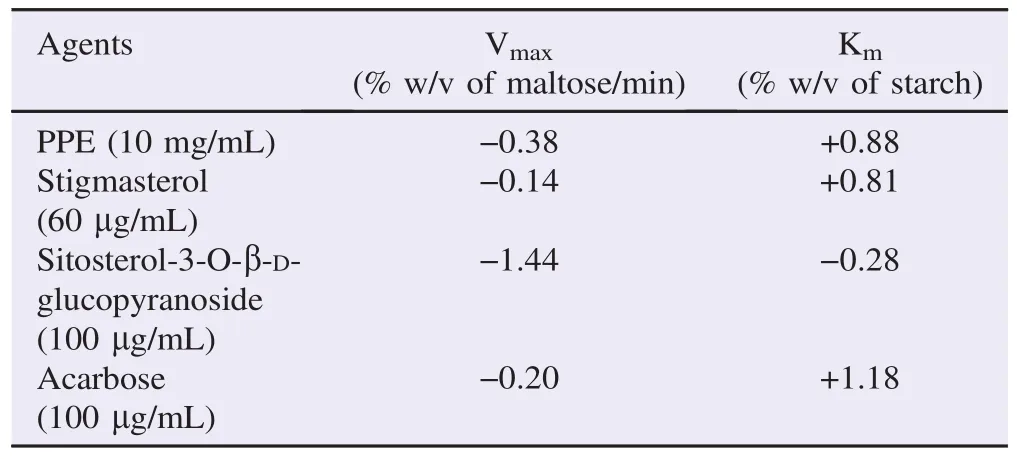
Table 3Changes of Vmaxand Kmof α-amylase enzyme in the presence of the test agents.
3.3. Inhibitory effects of PPE-derived stigmasterol against in vitro α-amylase enzyme activity
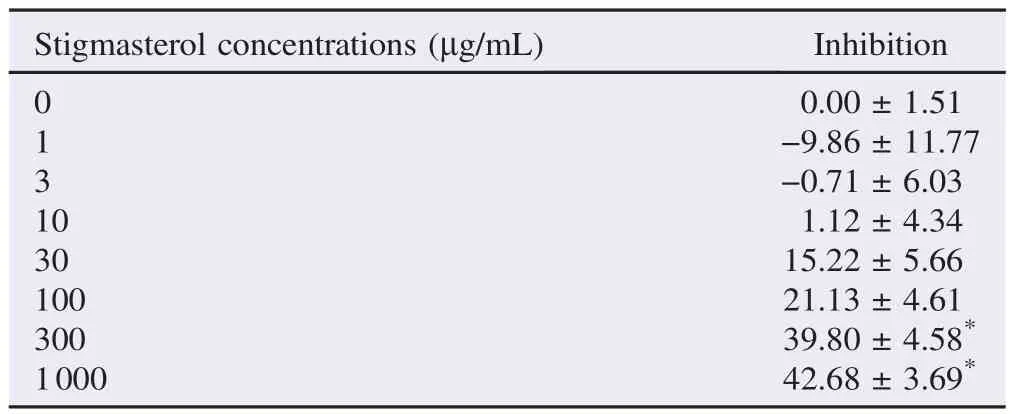
Table 4The percentage of inhibition of PPE-derived stigmasterol against α-amylase enzyme activity. %.
Stigmasterol, isolated from PPE, at the concentrations of 300 and 1000 μg/mL significantly inhibited α-amylase enzyme activity with the maximal inhibition of (42.68±3.69)% (n = 3) achieved at the concentration of 1000 μg/mL (Table 4). IC50of (59.41±8.22)μg/mL (mean±SEM, n = 3) was obtained from a concentration-inhibitory response curve of PPE-derived stigmasterol (Table 2). Lineweaver–Burk plot was drawn from the α-amylase enzyme kinetics study in the absence and the presence of 60 μg/mL stigmasterol (approximate IC50) (Figure 1B). Stigmasterol produced changes in both Vmaxand Kmwith a decrease in Vmaxand an increase in Km(Table 3).
3.4. Inhibitory effects of PPE-derived sitosterol-3-O-β-D-glucopyranoside against in vitro α-amylase enzyme activity
Sitosterol-3-O-β-D-glucopyranoside isolated from PPE at the concentrations of 100, 300, 1000 and 3000 μg/mL produced a significant inhibitory action against α-amylase enzyme activity (n = 3, P<0.05) (Table 5). The maximal inhibitory action (47.53±5.59)% was found at the concentration of 1000 μg/mL. PPE-derived sitosterol-3-O-β-D-glucopyranoside produced a concentration-dependent inhibition against α-amylase enzyme with the IC50of (111.19±9.02)μg/mL (mean±SEM, n = 3) (Table 2). From the Lineweaver–Burk plot (Figure 1C), sitosterol-3-O-β-D-glucopyranoside isolated from PPE at the concentration of 100 μg/mL (estimated IC50) provoked a decrease in both Vmaxand Km(Table 3).
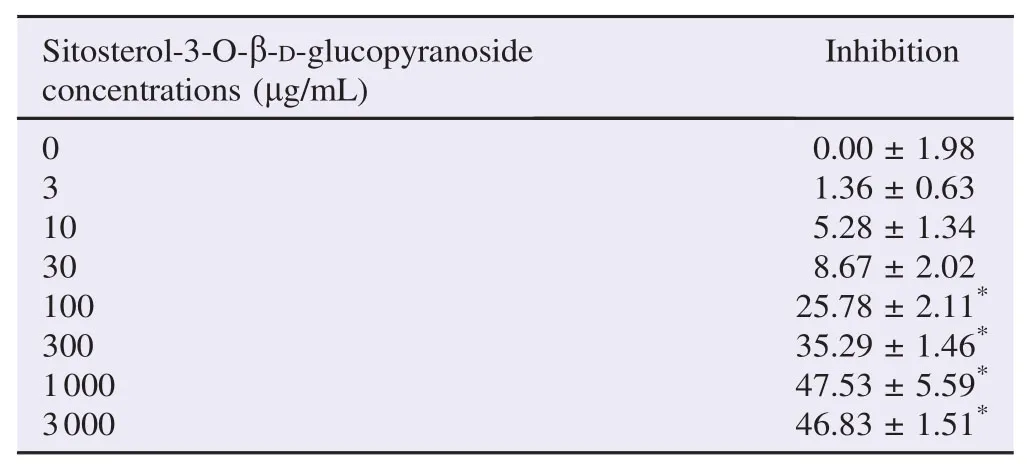
Table 5The percentage of inhibition of PPE-derived sitosterol-3-O-β-D-glucopyranoside against α-amylase enzyme activity. %.
3.5. Inhibitory effects of PPE against in vitro α-glucosidase enzyme activity

Table 6The percentage of inhibition of PPE against α-glucosidase enzyme activity. %.
PPE at every concentration tested produced a significant inhibitory action against α-glucosidase enzyme activity with a maximal inhibition of (47.70±2.13)% reached at the concentration of 3000 μg/mL (Table 6). A similar degree of inhibition of (43.86±1.97)% was found with 200 μg/mL of acarbose. Median inhibitory concentration of (1.00±0.11) mg/mL (mean±SEM, n = 4) was obtained from the concentrationinhibitory response curve of PPE (Table 2).
α-Glucosidase enzyme kinetics study was performed with various concentrations of p-NPG (0.037 5, 0.075, 0.15 and 0.30 IU/mL) in the presence or the absence of 1 mg/mL PPE (approximate IC50). From the Lineweaver–Burk plot, PPE caused a decrease in Vmaxwithout any change in Km. On the contrary, acarbose induced changes in both Vmaxand Km, with a decrease in Vmaxand an increase in Km(Table 7 and Figure 2).
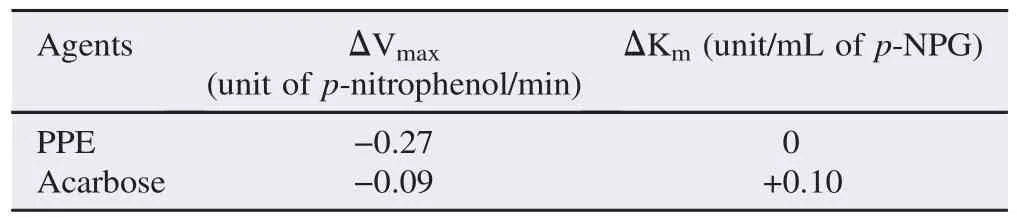
Table 7Changes of Vmaxand Kmof α-glucosidase enzyme in the presence of the test agents.
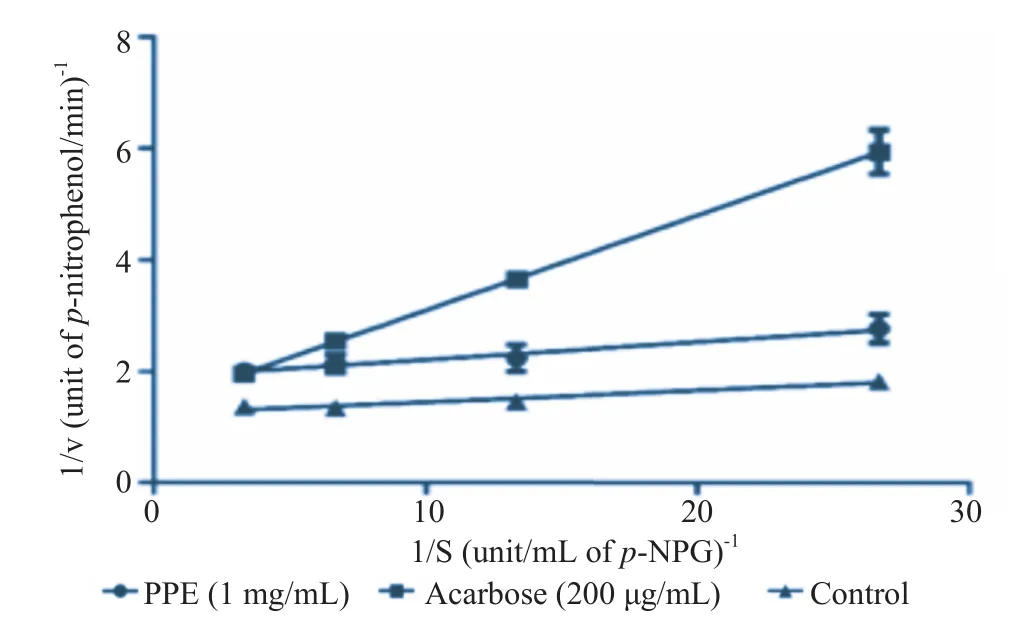
Figure 2. Kinetics analysis of α-glucosidase inhibition by PPE.Data are expressed as mean±SEM, n = 6. Lineweaver–Burk plot of the reaction against α-glucosidase in the presence of PPE (1 mg/mL) or acarbose (200 μg/mL).
3.6. Inhibitory effects of PPE-derived phytochemicals against in vitro α-glucosidase enzyme activity
Stigmasterol and sitosterol-3-O-β-D-glucopyranoside, isolated from PPE, produced a concentration-independent inhibition against α-glucosidase enzyme activity with a small inhibition of approximately 10% found at the concentrations of 0.1 and 1 μg/mL (Table 8).
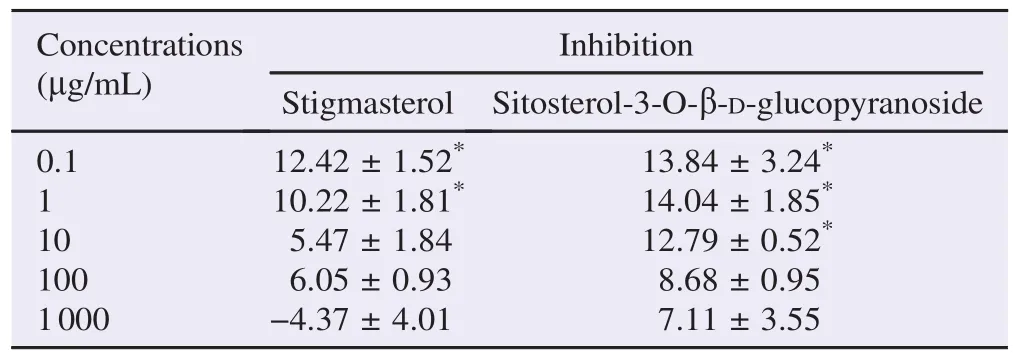
Table 8The percentage of inhibition of PPE-derived stigmasterol and sitosterol-3-O-β-D-glucopyranoside against α-glucosidase enzyme activity. %.
3.7. Oral starch tolerance test
When PPE at the doses of 250, 500 and 1000 mg/kg body weight were given to the rats prior to oral starch feeding, a significant decrease in the blood glucose concentrations was found at 30 min after oral starch feeding (3 g/kg body weight) (Figure 3A). PPE at the doses of 250 and 1000 mg/kg body weight also significantly decreased the blood glucose levels at 60 min after oral starch feeding. However, PPE at any doses tested did not cause any significant change at 120 min after oral starch feeding. Stigmasterol and sitosterol-3-O-β-D-glucopyranoside, isolated from PPE, at the doses of 5 and 10 mg/kg also caused a significant decrease in blood glucose at t = 30 min (Figure 3B). However, these two phytochemicals did not affect the blood glucose levels at 60 and 120 min after oral starch feeding. Acarbose (a positive control) at the dose of 10 mg/kg body weight caused a significant decrease in the blood glucose at 30 and 60 min after oral starch feeding (Figure 3). Acarbose did not cause a significant reduction in the blood glucose levels at t = 120 min.
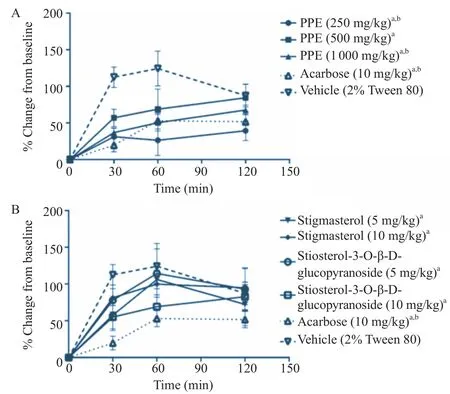
Figure 3. Effects of PPE (A) and its isolated phytochemicals (B) on oral starch tolerance test.The levels of blood glucose at t = 0, 30, 60 and 120 min after oral starch feeding were expressed as mean±SEM (n = 5).aP<0.05 when compared to vehicle control at t = 30 min;bP<0.05 when compared to vehicle control at t = 60 min.
3.8. Oral sucrose tolerance test
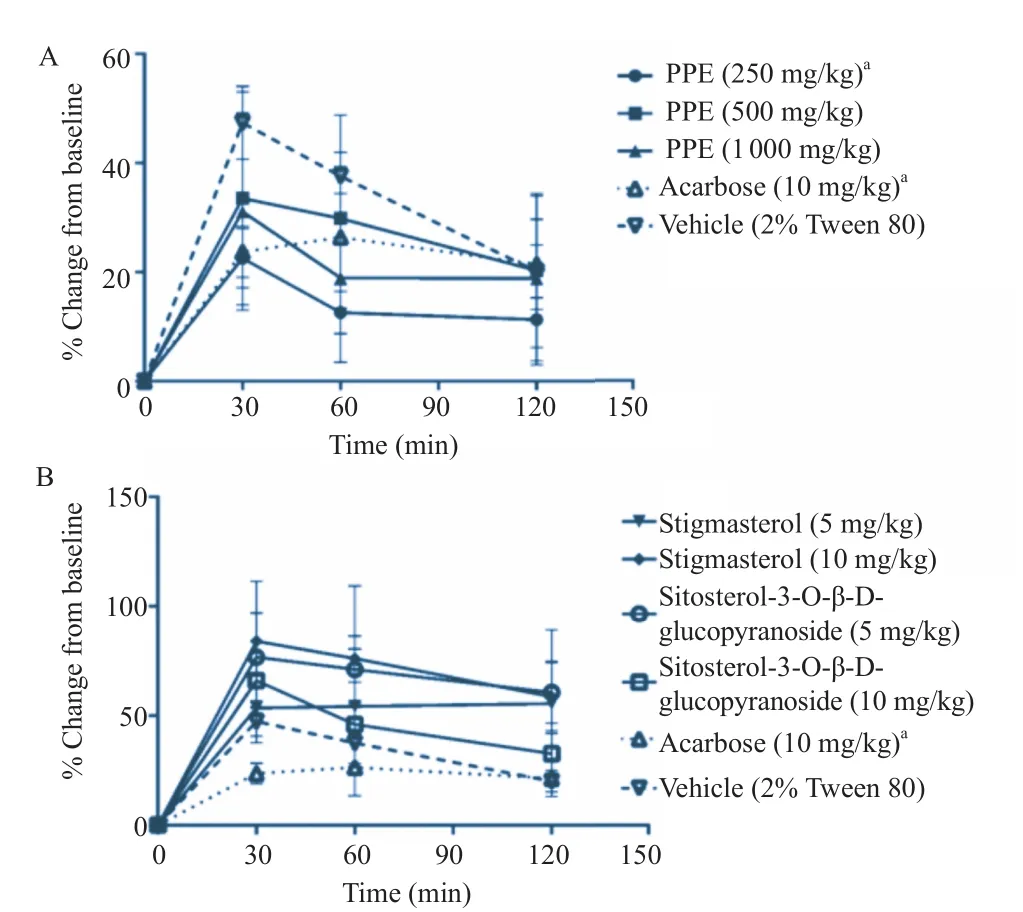
Figure 4. Effects of PPE (A) and its isolated phytochemicals (B) on oral sucrose tolerance test.The levels of blood glucose at t = 0, 30, 60 and 120 min after oral starch feeding were expressed as mean±SEM (n = 5).aP<0.05 when compared to vehicle control at t = 30 min.
Treatment with either 250 mg/kg body weight PPE or 10 mg/ kg body weight acarbose before oral sucrose feeding (4 g/kg body weight) significantly decreased the blood glucose levels at t = 30 min (Figure 4A). However, these treatments did not affectthe blood glucose levels at both t = 60 min and t = 120 min. Other treatments (500 and 1000 mg/kg body weight of PPE; 5 and 10 mg/kg body weight of stigmasterol; 5 and 10 mg/kg body weight of sitosterol-3-O-β-D-glucopyranoside) did not cause any significant decrease in the oral sucrose tolerance test (Figure 4).
4. Discussion
4.1. Inhibitory effects of PPE and its phytochemicals against in vitro and in vivo α-amylase enzyme activity
PPE exhibited a concentration-dependent inhibitory action against in vitro α-amylase enzyme activity with an IC50of (11.79±8.10) mg/mL (mean±SEM, n = 6). The maximal inhibitory action of (89.79±3.42)% was found at the concentration of 30 mg/mL. This is in accordance with our previous preliminary study in which a single IC50of 5 mg/mL was reported [7]. The experiment on α-amylase enzyme kinetics showed that PPE (10 mg/mL) caused a decrease in Vmaxand an increase in Km. Thus, PPE inhibited α-amylase enzyme in a mixed inhibition manner. This suggests that PPE can bind to either enzyme or enzyme–substrate complex to form enzyme-PPE complex or enzyme-substrate-PPE complex, respectively. Following these complex formations,α-amylase enzyme is thus no longer available for catalyzing starch digestion. This mode of enzyme inhibition is similar to that of acarbose (Figure 1A). A mixed inhibitory action of acarbose against α-amylase was also reported by Al Kazaz et al.[10]and Desseaux et al.[11]. It should be noted that 10 mg/mL PPE (approximate IC50) and 100 μg/mL acarbose produced changes in both Vmaxand Kmat the comparable degrees (Table 3).
Stigmasterol derived from PPE possessed a concentrationdependent inhibitory action against α-amylase enzyme with the IC50of (59.41±8.22)μg/mL (mean±SEM, n = 3). The maximal inhibition of (42.68±3.69)% (n = 3) was achieved at the concentration of 1000 μg/mL. Stigmasterol isolated from leaves of Dillenia indica (D. indica) at the concentration of 50 μg/mL inhibited α-amylase enzyme activity by 48% [12]. From the study of Kumar et al. [12], starch azure was used as a substrate and α-amylase enzyme activity was indicated by the levels of its released product, Remazol Brilliant Blue R. The difference in methods used may partly explain the discrepancy of stigmasterol potency found between studies.
Sitosterol-3-O-β-D-glucopyranoside isolated from PPE possessed a concentration-dependent inhibitory action against in vitro α-amylase enzyme activity with an IC50of (111.19±9.02)μg/mL (mean±SEM, n = 3). Nkobole et al. reported a slightly more potent α-amylase inhibitory action of β-sitosterol isolated from Terminalia sericea bark, with the IC50of 216 μmol/L (89.57 μg/mL)[13]. It was also shown that β-sitosterol derived from leaves of D. indica at the concentration of 50 μg/mL inhibited α-amylase enzyme activity by 45% [12]. However, sitosterol-3-O-β-D-glucopyranoside at the concentration of 30 μg/mL exhibited a relatively low level of enzyme inhibition (8.67±2.02)% in this study. These thus suggest that a sugar moiety presented in the chemical structure of isolated sitosterol-3-O-β-D-glucopyranoside possibly impedes its inhibitory action against α-amylase enzyme.
The results from enzyme kinetics study indicated that stigmasterol exhibited its inhibitory action against α-amylase in a mixed inhibition manner, whilst sitosterol-3-O-β-D-glucopyranoside exerted its enzyme inhibition in an uncompetitive fashion. Since PPE demonstrated a mixed inhibitory action against α-amylase enzyme, stigmasterol is thus likely to be a primary phytochemical which plays a role in the α-amylase inhibitory action of PPE. Nonetheless, further experiment should be done to explore whether these phytochemicals can produce either synergistic or antagonistic effect when they coexist. A mixed inhibitory mode of action of PPE proposes its advantage in the clinical use. A decrease in Vmaximplies that the maximal rate of reaction would be lessened, regardless to the amount of ingested starch. Additionally, an increase in Kmsuggests that a substantially lower rate of reaction arises if a similar amount of starch is consumed.
The oral starch tolerance test is essential to prove whether the agents provoke a certain α-amylase inhibitory action in vivo. PPE (250, 500 and 1000 mg/kg body weight) and its two isolated phytochemicals (stigmasterol and sitosterol-3-O-β-D-glucopyranoside, at 5 and 10 mg/kg body weight) caused a significant decrease in the blood glucose levels at t = 30 min. Thus, the inhibitory action of PPE against starch digestion in vivo potentially arises from the actions of these two phytochemicals. At t = 60 min, PPE at the doses of 250 and 1000 mg/kg body weight significantly decreased the blood glucose levels. However, neither stigmasterol nor sitosterol-3-O-β-D-glucopyranoside lowered the blood glucose levels at t = 60 min. It is possible that the given doses of these two phytochemicals were not sufficient to inhibit in vivo α-amylase enzyme activity at t = 60 min, when an adaptive increase of αamylase enzyme secretion may develop in vivo. PPE and its phytochemicals did not produce a substantial change in the blood glucose level at t = 120 min. This suggests that PPE exerted its action in a reversible manner, which may reduce a risk of adverse drug reactions caused by an indigested carbohydrate in the intestine. Acarbose also showed the blood glucose-lowering action only at t = 30 min and t = 60 min. Thus, both PPE and acarbose exhibited their blood glucoselowering action at a similar period of time. To our knowledge, this is the first report of the in vivo α-amylase inhibitory action of PPE and its isolated phytochemicals.
4.2. Inhibitory effects of PPE and its phytochemicals against in vitro and in vivo α-glucosidase enzyme activity
PPE significantly inhibited in vitro α-glucosidase enzyme activity with the maximal inhibition of (47.70±2.13)% and the IC50of (1.00±0.11) mg/mL. The methanolic extract of P. palatiferum leaves (1 mg/mL) was reported to have a slight αglucosidase inhibitory action of 7% [14]. This is different from our results in which 1 mg/mL of PPE exhibited a considerable α-glucosidase inhibition of approximately 40%. The difference in the solvent used in the preparation of the extract may partly be the reason for this discrepancy. Enzyme kinetics study revealed that PPE exhibited a non-competitive inhibition against α-glucosidase enzyme, whilst acarbose inhibited the enzyme in a mixed inhibition manner.
Stigmasterol and sitosterol-3-O-β-D-glucopyranoside extracted from PPE possessed a weak and non-concentrationdependent α-glucosidase inhibition. Stigmasterol and β-sitosterol (50 μg/mL) isolated from D. indica leaves was shown to inhibit α-glucosidase enzyme by 34.2% and 52.5%, respectively[12]. The differences between the studies cannot be justified yet. However, a purification process of the isolated phytochemicals may be involved.
From the oral sucrose tolerance test, PPE only at the dose of 250 mg/kg caused a significant depletion of the blood glucose at t = 30 min. However, neither stigmasterol nor sitosterol-3-O-β-D-glucopyranoside at the tested doses caused a significant decrease in the blood glucose at any time examined. It is possible that other unknown phytochemicals may be responsible for the in vivo α-glucosidase inhibition of PPE. The other phytochemicals found in the PPE include flavonoids and its derivatives such as kaempferol-3-methyl ether-7-O-β-glucoside and apigenin-7-O-β-glucoside [15]. Both kaempferol and apigenin were shown to have in vitro α-glucosidase inhibitory action [16]. Nonetheless, it cannot be excluded that the doses of phytochemicals used in this study are possibly insufficient to inhibit the enzyme activity in vivo.
PPEanditsphytochemicalswereshowntodecreasethefasting plasma glucose levels in the diabetic rats [5,6]. However, their mechanism of anti-diabetic action has not been established. Recently, anti-lipolytic and antioxidant activities of PPE were described to be linked with its anti-diabetic efficacy [7]. The in vitro and in vivo α-amylase and α-glucosidase inhibitions of PPE addressed herein further steadily explain its mechanism of anti-diabetic action. The current in vitro and in vivo experiments suggest that stigmasterol and sitosterol-3-O-β-D-glucopyranoside are likely to be the active phytochemicals acting as α-amylase enzyme inhibitors. However, the inhibitory action of these isolated phytochemicals against α-glucosidase enzyme is scanty.
Postprandial hyperglycemia is implicated in chronic diabetic complications and pancreatic beta-cell destruction [2,17,18]. A restriction of intestinal carbohydrate digestion thus likely conduces to a better control of diabetes. Both α-amylase and αglucosidase enzymes play a crucial role in an intestinal digestion of complex carbohydrate, which is commonly found in a diet. The dual inhibitory actions of PPE against these enzymes suggest its advantage over a sole inhibition on each enzyme alone. Although the current in vitro and in vivo experiments suggest the potential therapeutic benefit of PPE, further clinical study is still required to confirm its efficacy and safety.
In conclusion, PPE and its isolated phytochemicals, stigmasterol and sitosterol-3-O-β-D-glucopyranoside significantly inhibited both in vitro and in vivo α-amylase enzyme activity. PPE also produced an appreciable inhibitory action against αglucosidase enzyme both in vitro and in vivo. However, the two extracted phytochemicals are unlikely to act as an active compound against α-glucosidase enzyme. These results thus provide a scientific support for a traditional use of P. palatiferum leaves in the treatment of diabetes. Nonetheless, further clinical study is necessary to verify its role in the clinical management of diabetes.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was funded by Mahasarakham University research grant (Grant No. 5805028/2558,fiscal year 2015).
References
[1] World Health Organization. Diabetes. Geneva: World Health Organization; 2015. [Online] Available from: http://www.who. int/mediacentre/factsheets/fs312/en/ [Accessed on 19th October, 2015]
[2] Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1-7.
[3] Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother 2015; 16(13): 1959-81.
[4] Nolte Kennedy MS. Pancreatic hormones & antidiabetic drugs. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic & clinical pharmacology. 12th ed. New York: McGraw-Hill; 2012, p. 743-68.
[5] Padee P, Nualkaew S, Talubmook C, Sakuljaitrong S. Hypoglycemic effect of a leaf extract of Pseuderanthemum palatiferum (Nees) Radlk. in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol 2010; 132: 491-6.
[6] Nualkaew S, Padee P, Talubmook C. Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O-β-D-glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. J Med Plants Res 2015; 9(20): 629-35.
[7] Pulbutr P, Jaruchotikamol A, Cushnie B, Rattanakiat S, Nualkaew S. Anti-lipolytic,α-amylase inhibitory and antioxidant activities of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract. J Med Plants Res 2014; 8(28): 967-74.
[8] Ali H, Houghton PJ, Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006; 107: 449-55.
[9] Elya B, Basah K, Mun'im A, Yuliastuti W, Bangun A, Septiana EK. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J Biomed Biotechnol 2012; 2012: 281078.
[10] Al Kazaz M, Desseaux V, Marchis-Mouren G, Prodanov E, Santimone M. The mechanism of porcine pancreatic α-amylase. Inhibition of maltopentaose hydrolysis by acarbose, maltose and maltotriose. Eur J Biochem 1998; 252: 100-7.
[11] Desseaux V, Koukiekolo R, Moreau Y, Santimone M, Marchismouren G. Mechanism of porcine pancreatic α-amylase: inhibition of amylose and maltopentaose hydrolysis by various inhibitors. Biologia 2002; 57(Suppl 11): 163-70.
[12] Kumar S, Kumar V, Prakash O. Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. Biomed Res Int 2013; 2013: 382063.
[13] Nkobole N, Houghton PJ, Hussein A, Lall N. Antidiabetic activity of Terminalia sericea constituents. Nat Prod Commun 2011; 6: 1585-8.
[14] Rungprom W, Siripornvisai S, Keawsawal S, Songtrai M.α-Glucosidase inhibitors from medicinal plants for diabetes therapy. Agric Sci J 2010; 41(3/1): 301-4.
[15] Giang PM, Bao HV, Son PT. Phytochemical study on Pseuderanthemum palatiferum (Nees) Radlk., Acanthaceae. J Chem 2003; 41: 115-8.
[16] Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006; 52: 149-53.
[17] Dubois M, Vacher P, Roger B, Huyghe D, Vandewalle B, Kerr-Conte J, et al. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology 2007; 148: 1605-14.
[18] Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol 2012; 364: 1-27.
*Corresponding author:Pawitra Pulbutr, Pharmaceutical Chemistry and Natural Product Research Unit, Faculty of Pharmacy, Mahasarakham University, Maha Sarakham 44150, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Prevalence of refractive errors among primary school children in a tropical area, Southeastern Iran
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
- Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
