Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
Faizal Rajeeb Mangudadatu Hussin, Rodel Jonathan Santos Vitor II*, Julie Ann Oraa Joaquin,Melody Mendoza Clerigo, Anamy Ma. Caterial PaanoBiology Department, College of Science, De La Salle University, 40 Taft Avenue, 09 Manila, PhilippinesChemistry Department, College of Science, De La Salle University, 40 Taft Avenue, 09 Manila, Philippines
?
Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus)
Faizal Rajeeb Mangudadatu Hussin1, Rodel Jonathan Santos Vitor II1*, Julie Ann Oraa Joaquin2,
Melody Mendoza Clerigo2, Anamy Ma. Caterial Paano21Biology Department, College of Science, De La Salle University, 2401 Taft Avenue, 0922 Manila, Philippines
2Chemistry Department, College of Science, De La Salle University, 2401 Taft Avenue, 0922 Manila, Philippines
Original article http://dx.doi.org/10.1016/j.apjtb.2015.04.013
Tel: +63 2 524 4611x460
E-mail: rodel.vitor@dlsu.edu.ph
All experimental procedures involving animals were conducted in accordance to PALAS Code of Practice for the Care and Use of Laboratory Animals in the Philippines and approved by the Institutional Animal Care and Use Committee of De La Salle University.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
Foundation Project: Supported by the University Research Coordination Office of De La Salle University with Challenge Grant No. 500-033.
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 9 Jan 2015
Received in revised form 22 Jan, 2nd revised form 30 Mar 2015
Accepted 29 Apr 2015
Available online 10 Dec 2015
Keywords:
Anti-hyperglycemic
Glucose
Lenzites betulina
ICR mice
Oral glucose tolerance test
ABSTRACT
Objective: To examine the anti-hyperglycemic effects of aqueous Lenzites betulina (L. betulina) extracts on normoglycemic glucose-loaded mice.
Methods: Different doses of aqueous extract from L. betulina were administered to 45 ICR mice (Mus musculus) to determine whether there was an effect of L. betulina extracts on the blood glucose level of the ICR mice. Aqueous extracts of L. betulina were orally gavaged to mice using oral glucose tolerance test. A total of five groups were used to determine the effect of the fungi on blood glucose of the mice. Group A (positive control) was given 16.7 μg/kg glimepiride; Group B (negative control) was given distilled water; Group C (low dosage) was given 200 mg/kg aqueous extract; Group D (mid dosage) was given 400 mg/kg aqueous extract and Group E (high dosage) was given 800 mg/kg aqueous extract. Baseline blood glucose value was firstly acquired before induction of hyperglycemia through D-glucose, after which another check on blood glucose was made after 0.5 h. Immediately, after the acquisition of hyperglycemic blood glucose level, the individual administration of treatments were done. After that, three blood collections were done spanning 3 h with 1 h interval.
Results: The low dose (200 mg/kg) and the mid dose (400 mg/kg) of L. betulina extracts were significantly different (P<0.05) from their respective baseline values throughout the whole experiment with the latter surpassing its baseline value during the 3rd hour. On the other hand, the high dose (800 mg/kg) during the 1st hour after administration was not significantly different (P>0.05) from its corresponding baseline value, acting faster than the positive control (glimepiride), which only became significantly different (P<0.05) at the 2nd hour.
Conclusions: Aqueous L. betulina extract is able to produce hypoglycemic effects on the mice with all doses, which are able to normalize blood glucose levels at varying times.
1. Introduction
Various species of Lenzites sp. are not only widespread around Asia, but also well distributed around the world. The species Lenzites betulina var.flaccida can be found in almost all parts of the world, while some species, such as Lenzites vespacea, are only found in Asia, Africa and Oceania. Other species like the Lenzites heteromorpha is native to Europe [1]. In Philippines, multiple species of Lenzites are identified to be present such as Lenzites repanda which can be found in the islands of Bataan, and Lenzites acuta which can be found in Cagayan Province [2].
Lenzites spp. have no value as food since it has an extremely toughexterior[3].Therehavebeendifferentstudiesonthevarious medicinal uses of Lenzites spp. as scavengers of free radicals, antimicrobials, anti-oxidant, anti-viral and immunosuppressant[4–8]. Phytochemical analysis of compounds present in Lenzites spp. using three different solvents (ethanol, water and petroleum ether) was performed. The results showed that phenolic and steroids were present in the ethanol extract;flavonoids, tannins and steroids were present in the petroleum ether and only saponins were present in the aqueous extract[5]. However, there has been no study yet on the hypoglycemic effects of crude aqueous extract of Lenzites betulina (L. betulina).
The medicinal herbs have been used increasingly with greater advocacy for complementary and alternative medicine. Since then, there is a need for scientific-based research to find out the exact effects of different herbs and plants within the body. In the Philippines alone, 57.3% of the population is making use of herbal medicine to either control or prevent diseases [9,10]. Although herbal medicine has clinical substantiation for several years, mechanisms of how they provide medicinal effects are not well described. Since majority of the Philippine population is classified under the middle class, not all people can afford prescription medicine for different diseases and the use of L. betulina as a control of diabetes can serve as a cheap and accessible alternative prescription medication. Also, the value of L. betulina would increase especially in the medical sector and could possibly pave the way for the discovery and development of alternative medicine for the treatment of diabetes. Hence, the present study is aimed to investigate the hypoglycemic effects of crude aqueous extract of L. betulina.
2. Materials and methods
2.1. Fungi collection and processing
L. betulina was obtained from tree barks in Quezon, Palawan mostly in sites where kaingin was practiced. Bolos and knives were utilized for the removal of the mushroom from its attachment to the tree. It was authenticated by Edwin R. Tadiosa, Museum Researcher II, Botany Division at the National Museum of the Philippines.
Air-drying was done to prevent deterioration of the sample, after which the samples were then soaked in methanol for 24 h twice for the removal of low-molecular-weight compounds. The residues were extracted with distilled water at 100°C for 6 h, evaporated to a small volume and subjected to lyophilization in a vacuum [11]. The mushroom sample was dissolved in distilled water to obtain a final concentration of 100 mg/mL.
2.2. Animal procurement and housing
A total of 45 10-week-old female mice of the ICR strain were obtained from the Research Institute for Tropical Medicine in Alabang, Muntinlupa, Philippines. They were housed individually in standard-sized cages in the animal house of De La Salle University. All cages were cleaned twice a week and bedded with autoclaved soft wood shavings. Feeders and water bottles were cleaned and dried twice a week. Prior to the experiment, all mice were acclimatized for a period of one week to adjust to a 12 h-light: 12 h-dark cycle at (24±2)°C and relative humidity of 55%±10%. During the entire acclimatization and experimental period, food pellets and distilled water were supplied ad libitum. Proper handling and maintenance of the mice were observed and the Institutional Animal Care and Use Committee of De La Salle University approved the experimental protocol used.
2.3. Experimental procedures
Baseline blood glucose values were obtained through tail-tip nicking using an EasyTouch?GCU and immediately after, D-glucose solution (5 mg/kg body weight) was administered to all the mice to induce hyperglycemia. About 30 min after the induction of glucose, blood testing was performed to confirm hyperglycemia.
The mice were divided into 5 groups with 6 mice each. All treatments (glimepiride and L. betulina extracts) were diluted in distilled water. Then, Group A (positive control) was given glimepiride (16.7 μg/kg body weight); Group B (negative control) was given 0.2 mL of distilled water; Group C–E was given L. betulina at different concentrations (low dose: 200 mg/kg body weight; mid dose: 400 mg/kg body weight; high dose: 800 mg/kg body weight). Three more blood collections at 1st, 2nd and 3rd hour post-glucose administration were performed.
2.4. Statistical analysis
The differences in blood glucose levels (mean±SD) were analyzed using repeated measures ANOVA and multivariate ANOVA and means were compared using Tukey's test and SPSS version 22 to determine significant differences among the treatment groups at P<0.05.
3. Results
A total of 45 ICR mice were administered with three different doses of L. betulina extract (200 mg/kg, 400 mg/kg, 800 mg/kg), and glimepiride was given in the positive control (16.7 μg/kg) and distilled water was given in the negative control. A total of 5 blood collections were done to quantify blood glucose levels (mg/dL) during 3 h (Table 1).
Prior to the induction of hyperglycemia to all mice, post hoc tests showed that there were no significant differences between the baseline levels of all groups. This was supported by the fact that all groups were subjected to the same conditions. The values obtained were also within the limit of the normal blood glucose level (160 mg/dL) [12]. This indicated that no hyperglycemic activity was present before the administration of any substance to the mice (Table 1).
After 30 min, the blood glucose values were again compared after the induction of hyperglycemia to all mice. While all groups exhibited the desired hyperglycemic condition and no significant differences were observed across all groups (Table 1).
During the 1st hour, the blood glucose level of the negative control was significantly higher from all the other groups surpassing the hypoglycemic effect of glimepiride. This indicated that the mushroom extract exhibited anti-hyperglycemic activity. Also, within the treated groups, the values from the high dose group were proved to be significantly lower than those from the low and mid dose groups, including the positive control (Table 1). This indicated that the mushroom extract at high levels provided hypoglycemic activity along with the natural ability of the mice to retain its normal blood glucose level.
After 2 h of baseline testing, continuous decrease in blood glucose levels for all groups was observed. This time, the high dose and the positive control groups were not significantly different from each other and glimepiride had the same effect with the high dose of the L. betulina extract. Both mid dose andlow dose groups still were not significantly different from each other, but they still were significantly different with the positive and high dose groups. This means that after 1.5 h of administration of the mushroom extracts, only the high dose group was able to reach the same hypoglycemic effect as the glimepiride (Table 1).
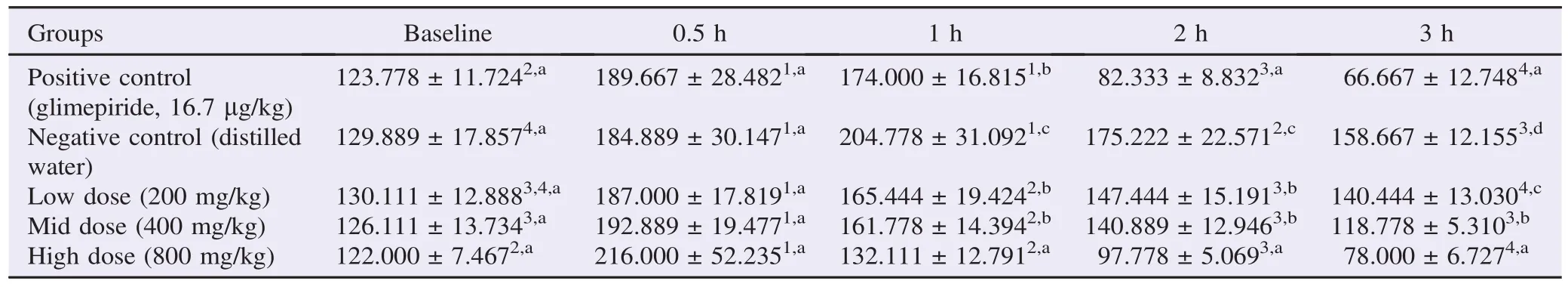
Table 1Blood glucose level of mice in different treatments.
At the 3rd hour of testing, blood glucose levels of all mice continued to decline. Still, only the high dose group was not significantly different from the positive control. The difference of this time showed that all Lenzites groups were significantly different from each other with the highest dosage exhibiting the highest hypoglycemic activity followed by the mid and low dose groups (Table 1). It was noted that the positive control group had the overall lowest blood glucose level at the end of the treatment.
Throughout the whole test, apart from baseline and when D-glucose was administered, the negative control group was constantly significantly different from all the other groups (Table 1). The negative control group was utilized to show the normalization of the blood glucose levels of the mice during the whole treatment to guarantee that the varying decrease of blood glucose for the mice was mainly because of the mushroom extract. Based from the results, the negative control group consistently held the highest blood glucose levels among all groups after the induction of hyperglycemia. Since the blood glucose levels in the negative control was significantly higher than the positive control and treatment groups, the decrease in blood glucose levels in all treatment groups was attributed to the hypoglycemic effects of the L. betulina extract.
Comparing the positive control, low dose, mid dose and high dose groups' blood glucose levels in the 3rd hour with their respective baseline values, different results were illustrated. Theoretically, among all groups, in 0.5 h, where the administration of the D-glucose was initiated, it was significantly different with its corresponding baseline blood glucose level as well as being the highest value across all time intervals since deliberate induction of hyperglycemia was done.
For the positive control group within the time intervals, it showed that during the 1st hour, the blood glucose level decreased, but it did not reach or surpass the baseline blood glucose value. It was only until the 2nd hour when a significant difference in blood glucose was noted which was observed up until the final hour of experimentation (Table 1).
With the low dose group, in 2nd hour from the administration of the mushroom extract, it showed a gradual decrease in blood glucose that blood values were not significantly different from the baseline only after the final hour of testing even though the values didn't go below the baseline. This signified that the low dose decreased the blood glucose level up to the normal baseline levels (Table 1). The same observations with the low dose group were seen for the mid dose except that during the final hour of testing, the blood glucose level was lower than the baseline.
The high dose group in 30 min after giving of the extract was already at level with the baseline values and this continued up until the final hour of administration. This was an indication that the higher dose had a faster hypoglycemic effect compared with the lower doses. Using the observations described above, we deduced that as the dose of mushroom extract increased, the time of onset of the hypoglycemic activity decreased (Table 1).
4. Discussion
This study showed that there was an anti-hyperglycemic effect on normoglycemic glucose-loaded mice after treatment with L. betulina. This effect can be attributed to saponins found in aqueous extracts of L. betulina [5].
Saponins are heterosides due to the presence of one or more sugars in their structures. Saponins are constitutive triterpenoid, steroid or steroidal glycoalkaloid molecules and are important in plant defense against microbial infection as well as possessing emulsifying properties [13]. It was studied that saponins can exhibit antidiabetic properties through modulation of calcium channels and beta cell invigoration. The role of calcium in the regulation of insulin was proven by the fact that nifedipine, a calcium channel blocker, significantly decreased the insulin levels when activated. Along with that, the secretion of insulin was halted once there were no extracellular Ca2+presence [14].
A study was done by Singh et al. who used triterpenoid saponin isolated from the leaves of Primula denticulate Sm. through chromatography and it was then identified with the use of nuclear magnetic resonance, UV and infrared ray spectroscopic methods [15]. It was administered to streptozotocin induced diabetic rats along with aqueous and ethanol extracts from the same plant. Results showed that it was able to lower the blood glucose level as well as restore the insulin level of the mice due to triterpenoid can stimulate insulin secretion by the pancreas.
Saponins from the ethanol extract of Momordica cymbalaria were given to streptozotocin-nicotinamide induced type 2 diabetic mice. Blood glucose, cholesterol, triglycerides and insulin levels as well as the histopathology of the pancreas were assessed. The results demonstrated that the saponin extract was able to lower the blood glucose, cholesterol and triglycerides, and increase the serum insulin values. This is attributed to the result of the assessment of the pancreas, in which there was an increase in beta cells and pancreatic islets [16].
Anothermechanismofsaponinasanantidiabeticcompoundis through stimulation of 5-adenosine monophosphate activated protein kinase and the insulin receptor/insulin receptor substrate 1/phosphatidylinositol 3'-kinase/Akt signaling pathways as well as increasing the activities of hexokinase and pyruvate kinase, key enzymes in glucose metabolism specifically glucose catabolism. Adenosine monophosphate activated protein kinase signals to stimulate glucose uptake in skeletal muscles and reduce hepatic glucose production and fatty acid oxidation in adipose tissues[17].Triterpenoidsaponinswereextractedfrom Stauntonia chinensis and were tested on human HepG2 cells. The results showed that the extract was able to boost glucose uptake and insulin sensitivity through the aforementioned mechanisms[18]. This study demonstrated that L. betulina extract was able to lower the blood glucose levels of ICR mice. Significant difference was observed between the three concentrations as well as compared to the positive control. During the 1st hour, the group given the highest dose was the sole group to produce a significant drop in blood glucose. For the 2nd hour, the positive control was able to achieve the same concentration as the high dose group, though all groups demonstrated steady hypoglycemic effects. During the last hour, all mushroom extract groups were significantly different from each other. The high dose group (800 mg/kg) exhibited a much faster reaction in relation to hypoglycemic activity compared to the other two mushroom extract groups. The rate of activation of the hypoglycemic effect was indirectly proportional to the dose of the extract given and it was able to surpass the effect of the positive control during the 1st hour of administration and became equaled at the end of the experiment. With this, it was deemed as the most effective dosage of the mushroom extract. Compounds such as saponins could have contributed to the anti-hyperglycemic effect of the plant. Less powerful effects were noted from the low and mid dose, which indicated that the strength of the antidiabetic property of L. betulina extract was dose-dependent and the plant could be used to be an alternative to most first generation sulfonylureas.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was partially funded by the University Research Coordination Office of De La Salle University with Challenge Grant No. 500-033.
References
[1] Oyetayo OV. Medicinal uses of mushrooms in Nigeria: towards full and sustainable exploitation. Afr J Tradit Complement Altern Med 2011; 8(3): 267-74.
[2] Graff PW. Philippine basidiomycetes-V. Bull Torrey Bot Club 1922; 49(8): 223-33.
[3] Christensen CM. Common edible mushrooms. Minneapolis: University of Minnesota Press; 1972.
[4] Oyetayo VO. Free radical scavenging and antimicrobial properties ofextractsofwildmushrooms.Braz JMicrobiol2009;40(2):380-6.
[5] Fakoya S, Oloketuyi SF. Antimicrobial efficacy and phytochemical screening of mushrooms, Lenzites betulinus, and Coriolopsis gallica extracts. TAF Prev Med Bull 2012; 11(6): 695-8.
[6] Liu K, Wang JL, Gong WZ, Xiao X, Wang Q. Antioxidant activities in vitro of ethanol extract and fractions from mushroom, Lenzites betulina. J Food Biochem 2013; 37(6): 687-93.
[7] Teplyakova TV, Psurtseva NV, Kosogova TA, Mazurkova NA, Khanin VA, Vlasenko VA. Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from Altai Mountains (Russia). Int J Med Mushrooms 2012; 14(1): 37-45.
[8] Fujimoto H, Nakayama M, Nakayama Y, Yamazaki M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem Pharm Bull (Tokyo) 1994; 42(3): 694-7.
[9] Haq I.Safetyofmedicinalplants.PakJMed Res2004;43(4):203-10.
[10] Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Pharmacol 2013; 2(2): 21-2.
[11] Mizuno T, Ohsawa K, Hagiwara N, Kuboyama R. Fractionation and characterization of antitumor polysaccharides from maitake, Grifola frondosa. Agric Biol Chem 1986; 50(7): 1679-88.
[12] Pineda MH, Dooley MP. McDonald's veterinary endocrinology and reproduction. Ames: Iowa State University Press; 2003, p. 597.
[13] Thakur M, Melzig MF, Fuchs H, Weng A. Chemistry and pharmacology of saponins: special focus on cytotoxic properties. Bot Targets Ther 2011; 1: 19-29.
[14] Koneri RB, Samaddar S, Ramaiah CT. Antidiabetic activity of a triterpenoid saponin isolated from Momordica cymbalaria Fenzl. Indian J Exp Biol 2014; 52(1): 46-52.
[15] Singh S, Farswan M, Ali S, Afzal M, Al-Abbasi FA, Kazmi I, et al. Antidiabetic potential of triterpenoid saponin isolated from Primula denticulate. Pharm Biol 2014; 52(6): 750-5.
[16] Firdous M, Koneri R, Sarvaraidu CH, Harish M, Shubhapriya KH. NIDDM antidiabetic activity of saponins of Momordica cymbalaria in streptozotocin-nicotinamide NIDDM mice. J Clin Diagn Res 2009; 3: 1460-5.
[17] Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 2014; 7: 241-53.
[18] Hu X, Wang S, Xu J, Wang DB, Chen Y, Yang GZ. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J Mol Sci 2014; 15(6): 10446-58.
*Corresponding author:Rodel Jonathan Santos Vitor II, Biology Department, College of Science, De La Salle University, 2041 Taft Avenue, 0922 Manila, Philippines.
 Asian Pacific Journal of Tropical Biomedicine2016年2期
Asian Pacific Journal of Tropical Biomedicine2016年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Evaluation of entomopathogenic Bacillus sphaericus isolated from Lombok beach area against mosquito larvae
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
