Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
Suzhou, China
Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
Feng Chen, Lin Tang, Zhi-Qi Zhang, Bing-Wei Jin, Wei-Feng Dong, Jian Wang and Shun-Gen Huang
Suzhou, China
BACKGROUND:Pancreaticobiliary maljunction is a high risk factor of pancreatitis and biliary tract cancer. How this maljunction affects the liver remains obscure. This study aimed to examine the effects of pancreaticobiliary maljunction on the liver, pancreas and gallbladder in a cat model.
METHODS:A model of choledocho-pancreatic side-to-side ductal anastomosis was created in ten cats. Before the procedure, a small piece of tissue from the liver, pancreas and gallbladder was collected as a control. The common channel formation was checked by cholecystography. The livers, pancreases and gallbladders of these cats were harvested for histological examination. The expression of proliferating cell nuclear antigen in the gallbladder was examined with immunohistochemistry.
RESULTS:Seven of the 10 cats survived for 6 months after surgery. The color of the liver was darker in the PBM model than the control specimen, with nodules on the surface. Histological examination showed ballooning changes and infammatory infltrations and the histopathological score increased signifcantly (P<0.05). Also, mitochondria swelling and lipid droplet in cytoplasm were observed under an electron microscope. The pancreas also appeared darker in the PBM model than the control specimen and dilated pancreatic ducts were found in three cats. Histopathological examination revealed vascular proliferation and infammatory infltration with numerous neutrophils. Gallbladder epithelial cells were featured by expanded endoplasmic reticulum, increased intercellular space and cellular nucleus deformation. The positive cells ofproliferating cell nuclear antigen were increased signifcantly (P<0.05).
CONCLUSION:The present study demonstrated that pancreaticobiliary maljunction can lead to the injuries of the liver, pancreas and gallbladder. (Hepatobiliary Pancreat Dis Int 2015;14:90-95)
pancreaticobiliary maljunction;animal model; ballooning change; mitochondria swelling; endoplasmic reticulum expanding
Introduction
Pancreaticobiliary maljunction (PBM) is a congenital malformation defned as the pancreatic and biliary ducts join anatomically outside the duodenal wall.[1]Oddi's sphincter could not regulate the outfow of bile and pancreatic juice, resulting in two-way regurgitation. Pancreatic juice frequently refuxes into the biliary tract and activates pancreatic enzymes and secondary bile acid, damages the biliary mucosa, and provokes the risk of biliary tract cancer.[1,2]Bile refuxes into the pancreatic duct and causes pancreatitis.
Advancement of imaging, magnetic resonance cholangiopancreatography (MRCP),[3,4]improved PBM diagnosis. This maljunction leads to various pathologic conditions including gallbladder carcinoma and acute pancreatitis.[5,6]However, the relationship between PBM and these diseases remains obscure and the effect of PBM on the liver is neglected.
Our previous study[7]showed that the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased signifcantly in patients with PBM. To further investigate the effects of PBM on the liver, pancreas and gallbladder, we created a cat model of PBM without bile duct dilatation[8]and examined the morphological features of these three organs.
Methods
Animals
Ten cats weighing 3.0 to 4.0 kg were purchased from the Animal Center of Soochow University. The animals were treated according to the institution's guidelines for animal use in experimentation. All animals were fasted for 12 hours before surgery. All experiments were performed in accordance with the Animal Experimental Guide of our hospital.
PBM model
A vertical incision of approximately 6 cm was made on the abdominal wall under general anesthesia. Small pieces of tissue from the liver, pancreas and gallbladder were collected before the surgery to serve as a control. The capsule of the pancreas was opened to expose the pancreatic duct near the common bile duct (Fig. 1A). A 4 to 6 mm incision was made in the pancreatic duct and the common bile duct (Fig. 1B). The two incised edges were anastomosed with continuous 6-0 nylon monoflament suture to make a wide and long communication (Fig. 1C). Cholangiography was used to confrm the formation of the common channel (Fig. 1D).
Biochemical assessments
Liver function and liver injury parameters were assessed in blood samples by routine clinical chemistry.
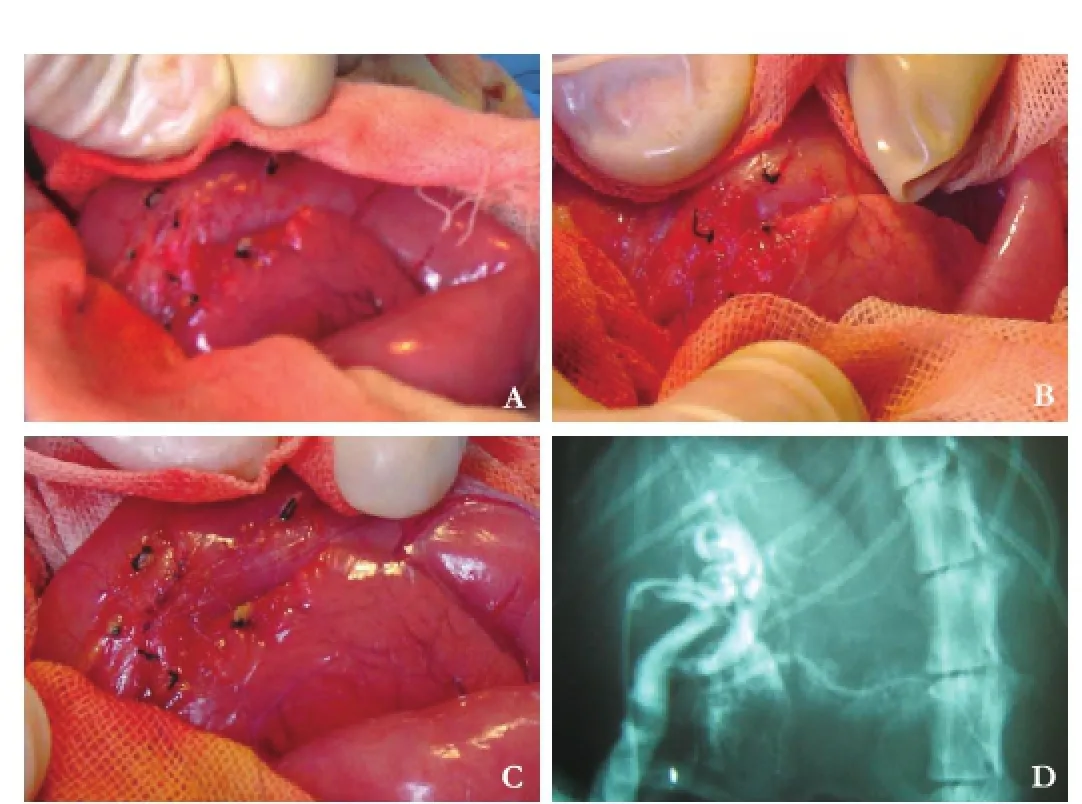
Fig. 1.Establishment of a cat PBM model: The capsule of the pancreas opened to expose the pancreatic duct near the common bile duct (A). Biopsies from the liver, pancreas and gallbladder were taken immediately before surgery to serve as the control. A 4 to 6 mm incision was made in the pancreatic duct and the common bile duct (B). The incised edges were anastomosed with continuous 6-0 nylon monofilament suture to make a wide and long communication between the pancreatic duct and the common bile duct (C). Cholangiography made 20 days later to confrm formation of the common channel (D).
These samples were obtained before and six months after PBM model establishment. The levels of ALT, AST, alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT) and total bilirubin (TB) were determined to assess liver damage. Albumin (ALB) was measured to evaluate liver synthesis function.
Histopathological examination
The liver, gallbladder and pancreas from the experimental cat that survived longer than 6 months postoperatively were examined and compared with the control specimen. All samples were preserved in 10% formalin, embedded in paraffn and sliced into 2 μm sections. The sections were stained with hematoxylin and eosin. The grading system was applied:[9]grade 0, normal hepatic cells; grade 1, ballooning change; grade 2, infammatory cell infltration; and grade 3, fbroplastic proliferation.
Immunohistochemistry
Immunohistochemistry was performed as described[10]with modifcations. The sections of gallbladders were incubated with proliferating cell nuclear antigen (PCNA) antibody (Sigma, St. Louis, USA) at a dilution of 1:100 at 4 ℃ overnight. After 3 washes, the sections were incubated with biotinylated rabbit anti-mouse antibody (Sigma, St. Louis, USA) for 60 minutes and washed for three times with PBS, and then covered with peroxidaseconjugated streptavidin. After further 3 washes, the peroxidase activity was detected by diaminobenzidine. PCNA labeling index was calculated as previously reported.[10]
Electron microscopy
The liver, gallbladder and pancreas samples collected from two cats at random were fxed in 3.5% glutaraldehyde and sliced with a microtome. The tissues were processed according to the standard procedure and examined under a transmission electron microscope.
Statistical analysis
Statistical analyses were performed on SAS 8.2 software (SAS Institute, Cary, NC, USA). Data were expressed as the mean±SEM (PCNA) and analyzed with Student'sttest; scores of histopathology were analyzed with the Mann-WhitneyUtest. APvalue <0.05 was considered statistically signifcant.
Results
Seven of the 10 cats survived for 6 months after surgery. Three cats died of the postoperative complications inthree weeks.
Serological examination
There was a signifcant elevation of plasma ALT and AST levels in animals with PBM. GGT, ALP and TB levels were also increased six months after operation (P<0.05), the serum ALB level was not statistically different between the PBM model and the control specimen (Table 1).
Histological fndings of the liver
The morphological changes between the PBM model and control specimen are shown in Fig. 2. The liver in baseline was salmon pink (Fig. 2A) with normal morphology of hepatic cells (Fig. 2C). In the PBM model, the color of the liver was dark with nodus on the surface (Fig. 2B). Histopathological examination showed ballooning changes of liver cells and infammatory cell infltration (Fig. 2D), and the histopathological score was signifcantly higher than the control (P<0.05) (Table 2). Mitochondrial swelling and lipid droplet (Fig. 2F) were also found in cytoplasm under an electron microscope.
Pathological changes of the pancreas
The morphological changes of the pancreas between the PBM model and control specimen are illustrated in Fig. 3. The color of the pancreas in the control specimen was salmon pink (Fig. 3A) with normal pancreatic cells (Fig. 3C). In the PBM model, the pancreas was dark in color. Histological examination showed dilated pancreatic duct, infammatory cell (including neutrophil) infltration and vascular proliferation in 3 animals (Fig. 3B). Under an electron microscope, we found expanded rough endoplasmic reticulum, swollen mitochondria, and increased Golgi complex (Fig. 3F).
Billary aberrations and PCNA immunohistochemical staining
The structural comparison of gallbladder between the PBM model and control specimen is shown in Fig. 4. In the PBM model, the gallbladder wall was thicker than normal. Villous structure was obvious under a microscope (Fig. 4B). In addition, endoplasmic reticulum was expanded, intercellular space broadened and cellular nucleus distorted (Fig. 4D). The PCNA positive cells were signifcantly increased in the gallbladder epithelium of the PBM model (Fig. 5B), whereas only few stained epithelium was observed in the control specimen (Fig. 5A). The PCNA labeling index was 7.29% ±2.70% in the control specimen and 54.71%±10.90% in the PBM model (Fig. 5C,P<0.05).
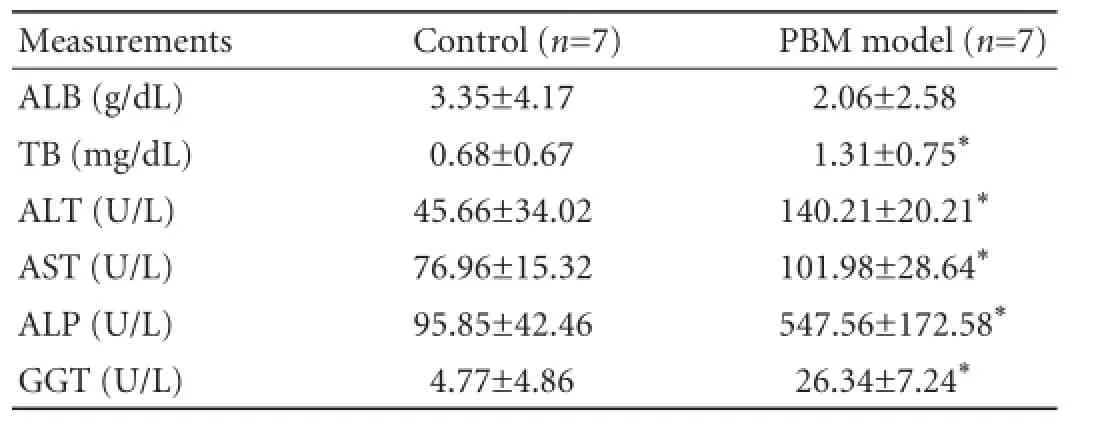
Table 1.The levels of ALB, TB, ALT, AST, ALP and GGT
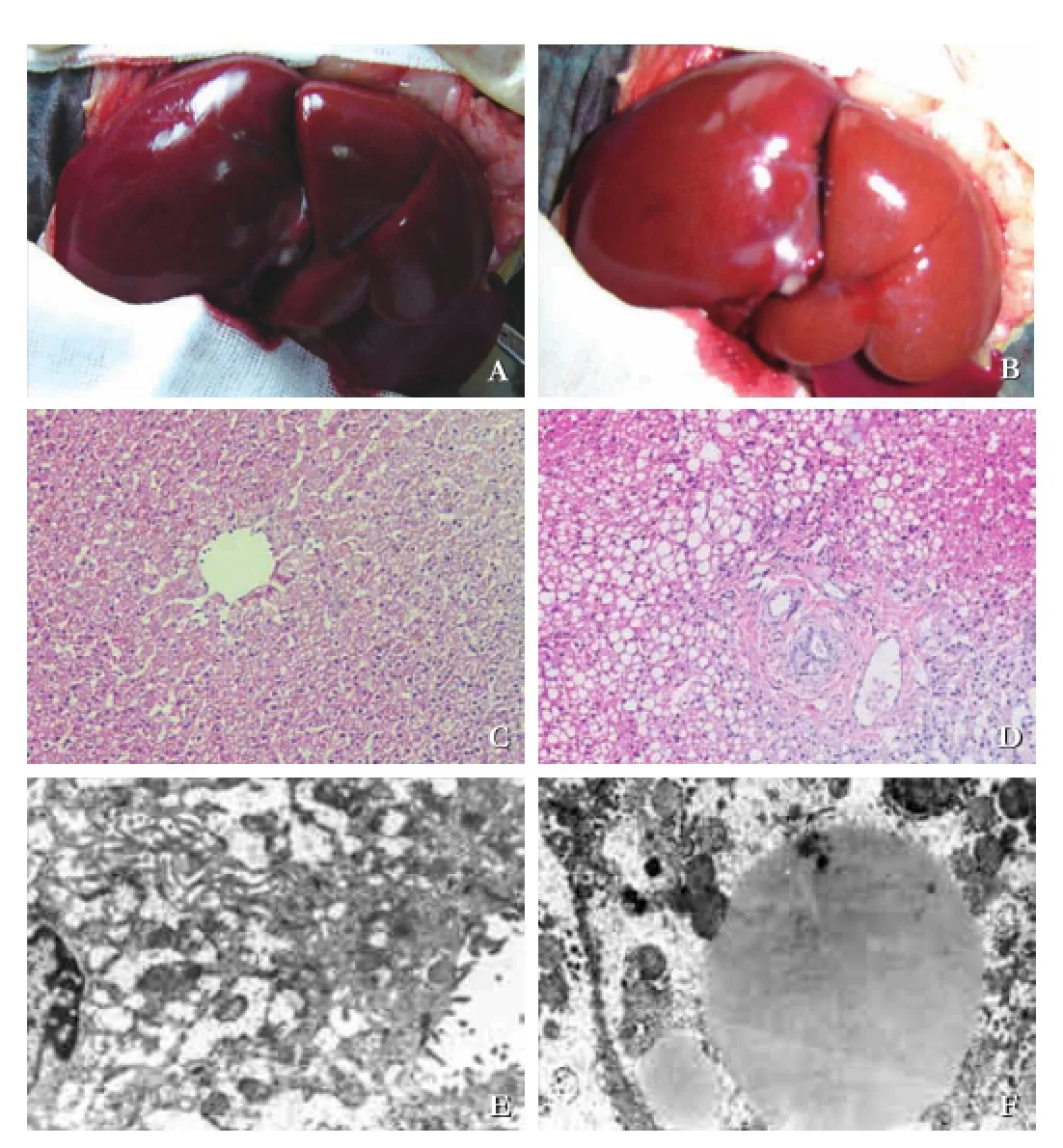
Fig. 2.Pathological findings of the liver. With unaided eye, we saw the normal liver of the control (A). The color became darker with nodus on the surface (B). Under a microscope, ballooning changes with inflammatory infiltrations (D, original magnification ×200), compared with the control (C, original magnifcation ×200). Under an electron microscope, mitochondria swelling and lipid droplets in cytoplasm (F, original magnifcation ×5000) in the PBM model, but not in the control (E, original magnifcation × 5000).
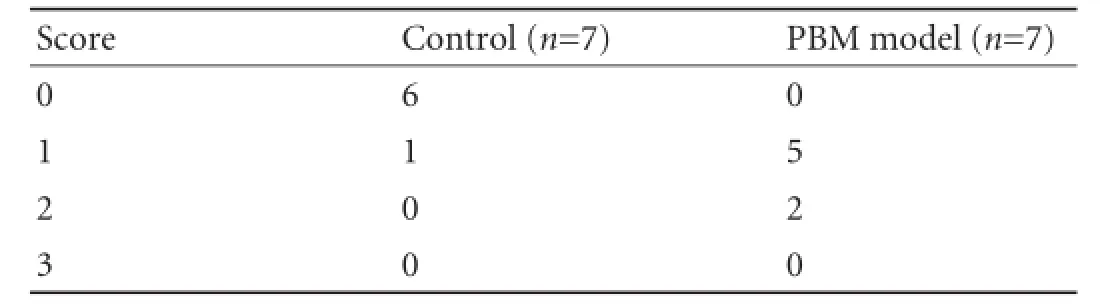
Table 2.Pathology scores of the liver
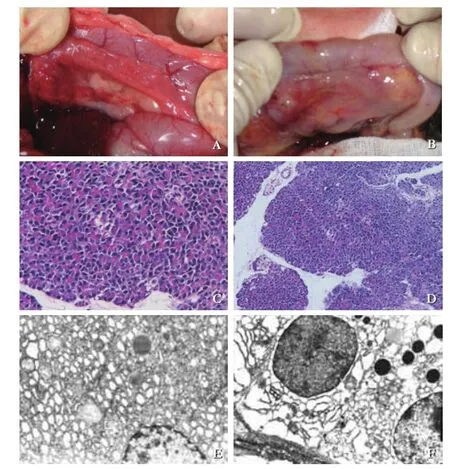
Fig. 3.Inflammatory changes of the pancreas. The pancreas became darker in color, with dilated pancreatic duct (B), compared with normal appearance (A). Neutrophil infltration in the PBM model (D, original magnifcation ×200), but not in the control (C, original magnifcation ×200). Rough endoplasmic reticulum expanding and Golgi complex becoming prosperous in the pancreas of the PBM model (F, original magnifcation ×5000), but not in the control (E, original magnifcation ×5000).
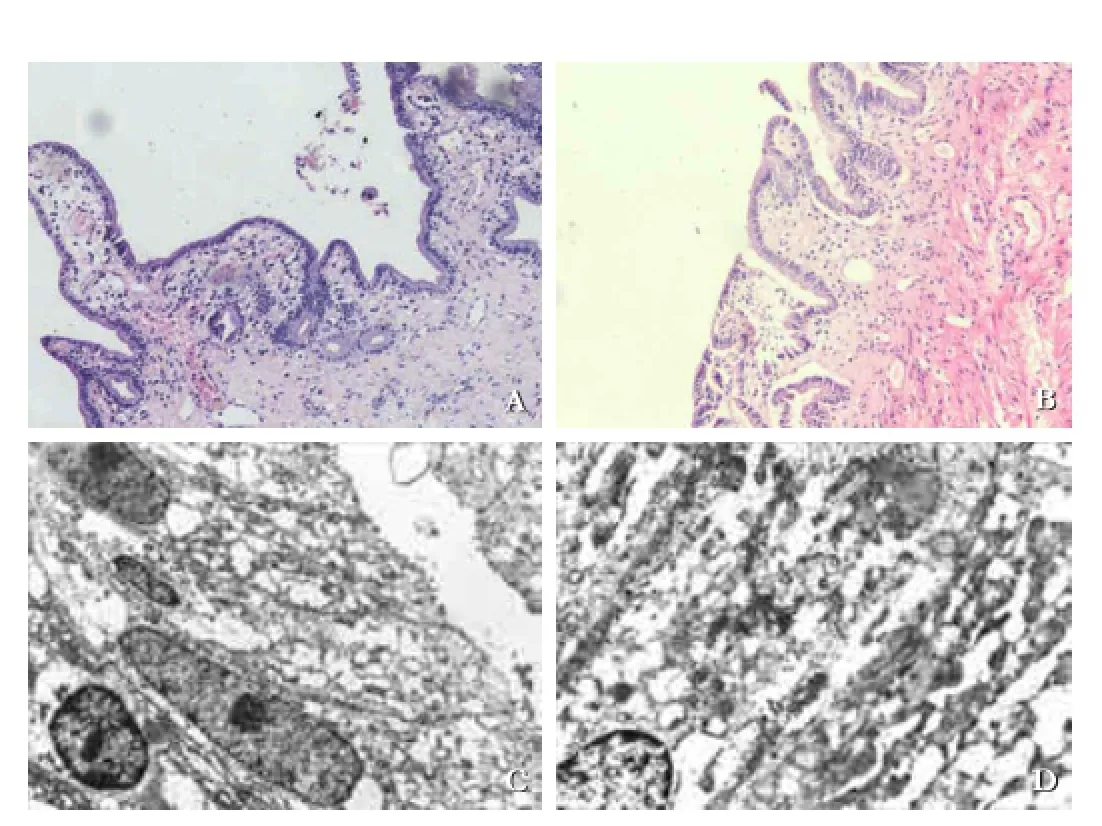
Fig. 4.Billary morphological aberrations. The epithelium of control was fat with few folds (A, original magnifcation ×200) and the gallbladder epithelium became villous in the PBM model (B, original magnifcation ×200). Intercellular space broadening and cellular nucleus deforming observed in epithelial cells of the gallbladder in the PBM model (D, original magnifcation ×5000), compared with normal nuclear and tightly arranged epithelium (C, original magnifcation ×5000) in the control.
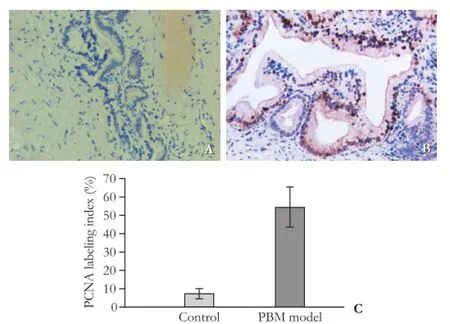
Fig. 5.PCNA immunostaining of gallbladders. The positive cells of PCNA were increased signifcantly in the gallbladder epithelium of the PBM model (B, original magnifcation ×200) compared with the control (A, original magnification ×200), with positive index listed inC.
Discussion
In this study, we successfully established a PBM model that was similar to patients with PBM. Using this model, we examined the effect of PBM on the liver, pancreas and gallbladder. We found that PBM caused obvious injuries in the liver, biliary tract and pancreas.
PBM, a congenital anomaly, has high impact on the hepato-biliary-pancreatic system.[11]It remains obscure how this malformation eventually leads to congenital choledochal dilation, cholangitis, gallbladder carcinoma, etc.[12-14]Using a cat model, this study examined the effects of PBM on the liver, biliary tract and pancreas. The anatomical relation between the pancreatic duct and the bile duct in cats is the same as in humans. Pathological changes observed in this model may provide useful information for patients with PBM.
Our previous study demonstrated that serum ALT, AST, ALP, GGT and TB levels were signifcantly increased in patients with PBM.[7]The present study also showed elevated levels of ALT, AST, ALP, GGT and TB in the PBM model, indicating liver injuries in PBM. However, no signifcant difference was found in ALB levels before surgery or at the time of PBM model matured, suggesting that liver synthetic function might not be affected. Morphological changes include on gross observation the color turned from light red into dark red, with superfcial nodes. Under a microscope, the hepatic cells showed ballooning changes, without bile thrombus in biliferous tubules. We also found lipid droplets accumulation in the cytoplasm, swelling mitochondria, expanding endoplasmic reticulum and newly formed collagens. Theliver, although much milder than the bile duct and pancreas, is still injured in PBM. We observed these injuries 6 months after model establishment and more severe damages might happen with disease progression. Morine et al[15]analyzed 514 adult patients with PBM without biliary dilatation, and found that the occurrence of liver cancer was 2.3%. Since the liver is far from the common channel of the pancreatic and biliary ducts, these injuries could not be explained by regurgitation of bile and pancreatic juice. The mechanisms of the injuries need further investigation. The absence of bile thrombus in biliferous tubules ruled out the possibility of obstruction. So free radicals might be involved in the damages, particularly reactive oxygen species (ROS) such as superoxide anion radical (O2-·), hydroxylion (OH-·) and hydrogen peroxide (H2O2). ROS attacks the double bonds in unsaturated fatty acids and oxide amino acid residue, leading to protein fragmentation and cell injury. Intriguingly, we found that anti-oxidant therapy using vitamin C and vitamin E signifcantly reversed liver injuries in the PBM model (data not shown), suggesting that ROS might play an essential role in liver damage in the PBM model.
In PBM patients, pancreatobiliary refux frequently leads to pancreatic disorders such as acute pancreatitis, chronic pancreatitis, pancreatic carcinoma and pancreas divisum. The common bile and pancreatic ducts are enlarged, whereas the gallstones usually incarcerate. Obstruction or spasm of the Vater ampulla and edema of duodenal papillary by gallstones result in refux of bile into the pancreatic duct, which may cause toxic injury to the pancreatic acini.[6,16-20]In our model, the color of the pancreas turned from pink to dark red, with dilated pancreatic ducts in 3 cases. Necrosis was found in the pancreatic parenchyma, with interstitial vascular hyperplasia, infammatory cell infltration and increased leukocytes adhering to endothelium. Infammatory indicators such as dilated endoplasmic reticulum, swelling and round mitochondria, and enhanced Golgi complex were found in the parenchyma of the pancreas, indicating the existence of pancreatitis.
Pancreatic juice might refux into the common bile duct because of the pressure gradient from the pancreatic duct to the common bile duct. The bile activates a variety of enzymes, such as phospholipase A2 and proteases which damage the epithelium of the common bile duct.[21]PBM is always concomitant with congenital choledochal dilation.[22]In the present study, the cholecystic wall was thicker and the choledochus was dilated. These changes might be due to repeated cholangitis by refux of pancreatic juice into the bile duct. Moreover, PBM is a risk factor of gallbladder carcinoma.[5,23-27]Our model as reported previously[8]demonstrated the thickening of the gallbladder wall with enhanced PCNA expression. In PBM, pancreatic juice and bile are constantly mixed. The repeatedly damaged and repaired biliary mucosa may result in multiple gene mutations, epithelial cell proliferation, and ultmately gallbladder carcinoma.[28]
In conclusion, PBM may lead to injuries of the liver, pancreas and biliary tract, and early treatment of PBM is necessary to prevent severe complications.
Contributors:WJ proposed the study. CF and TL performed the research and wrote the frst draft. ZZQ collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. CF and TL contributed equally to this paper. WJ is the guarantor.
Funding:This study was supported by grants from the Experimental Animal Special Purpose Foundation of Science and Technology Commission of Shanghai Municipality (13140902901) and the Technology Development Foundation of Pudong District (PKJ2013-Y67).
Ethical approval:This study was approved by the Institutional Animal Ethics Committee of Soochow University Affliated Children's Hospital.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kamisawa T, Ando H, Hamada Y, Fujii H, Koshinaga T, Urushihara N, et al. Diagnostic criteria for pancreaticobiliary maljunction 2013. J Hepatobiliary Pancreat Sci 2014;21:159-161.
2 Kamisawa T, Ando H, Shimada M, Hamada Y, Itoi T, Takayashiki T, et al. Recent advances and problems in the management of pancreaticobiliary maljunction: feedback from the guidelines committee. J Hepatobiliary Pancreat Sci 2014;21:87-92.
3 Fumino S, Ono S, Kimura O, Deguchi E, Iwai N. Diagnostic impact of computed tomography cholangiography and magnetic resonance cholangiopancreatography on pancreaticobiliary maljunction. J Pediatr Surg 2011;46:1373-1378.
4 Guo WL, Huang SG, Wang J, Sheng M, Fang L. Imaging fndings in 75 pediatric patients with pancreaticobiliary maljunction: a retrospective case study. Pediatr Surg Int 2012;28:983-988.
5 Deng YL, Cheng NS, Lin YX, Zhou RX, Yang C, Jin YW, et al. Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: meta-analysis. Hepatobiliary Pancreat Dis Int 2011;10:570-580.
6 Takuma K, Kamisawa T, Hara S, Tabata T, Kuruma S, Chiba K, et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreaticobiliary malformation. Adv Med Sci 2012;57:244-250.
7 Chen F, Wang J, Huang SG. Study on serum enzyme map in patients with congenital cholangiectases. J Clin Pediatr Surg 2005;4:286-288.
8 Kaneko K, Ando H, Umeda T, Murahashi O, Hiraiwa K, Niimi N, et al. A new model for pancreaticobiliary maljunction without bile duct dilatation: demonstration of cell proliferation in the gallbladder epithelium. J Surg Res 1996;60:115-121.
9 Loff S, Waag KL, Kr?nzlin B, Zovko D, Dzakovic A, Jester I, etal. Long-term total parenteral nutrition-induced hepatobiliary dysfunction in a rabbit model. J Pediatr Surg 1998;33:694-699.
10 Guo WL, Zhang Q, Wang J, Jin MF. Higher expression of phosphorylated myosin regulatory light chain in the common bile duct in pancreaticobiliary maljunction accompanied by bile duct dilatation in children: a post-mortem observational study. Pediatr Surg Int 2013;29:293-298.
11 Kamisawa T, Ando H, Suyama M, Shimada M, Morine Y, Shimada H, et al. Japanese clinical practice guidelines for pancreaticobiliary maljunction. J Gastroenterol 2012;47:731-759.
12 Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg 2009; 394:159-169.
13 Adham M, Valette PJ, Partensky Ch. Pancreaticobiliary maljunction without choledochal dilatation associated with gallbladder cancer: report of 2 European cases. Surgery 2005;138:961.
14 Kamisawa T, Honda G, Kurata M, Tokura M, Tsuruta K. Pancreatobiliary disorders associated with pancreaticobiliary maljunction. Dig Surg 2010;27:100-104.
15 Morine Y, Shimada M, Takamatsu H, Araida T, Endo I, Kubota M, et al. Clinical features of pancreaticobiliary maljunction: update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci 2013;20:472-480.
16 Sugiyama M, Atomi Y, Kuroda A. Pancreatic disorders associated with anomalous pancreaticobiliary junction. Surgery 1999;126:492-497.
17 Tahara K, Ishimaru Y, Fujino J, Suzuki M, Hatanaka M, Igarashi A, et al. Association of extrahepatic bile duct duplication with pancreaticobiliary maljunction and congenital biliary dilatation in children: a case report and literature review. Surg Today 2013;43:800-805.
18 Cheng L, Tian F, Zhao T, Pang Y, Luo Z, Ren J. Annular pancreas concurrent with pancreaticobiliary maljunction presented with symptoms until adult age: case report with comparative data on pediatric cases. BMC Gastroenterol 2013;13:153.
19 Zhang Y, Sun W, Zhang F, Huang J, Fan Z. Pancreaticobiliary maljuction combining with pancreas divisum: Report of four cases. Exp Ther Med 2014;7:8-10.
20 Nakamura T, Okada A, Higaki J, Tojo H, Okamoto M. Pancreaticobiliary maljunction-associated pancreatitis: an experimental study on the activation of pancreatic phospholipase A2. World J Surg 1996;20:543-550.
21 Lahmar A, Abid SB, Arfa MN, Bayar R, Khalfallah MT, Mzabi-Regaya S. Metachronous cancer of gallbladder and pancreas with pancreatobiliary maljunction. World J Gastrointest Surg 2010;2:143-146.
22 Watanabe M, Miura S, Terashita K, Kudou T, Mori Y, Uebayashi M. A case of diverticular form type of congenital choledochal dilatation with anomalous arrangement of pancreaticobiliary duct. Nihon Shokakibyo Gakkai Zasshi 2008;105:1384-1389.
23 Kaneko K, Ando H, Watanabe Y, Seo T, Harada T. Pathologic changes in the common bile duct of an experimental model with pancreaticobiliary maljunction without biliary dilatation. Pediatr Surg Int 2000;16:26-28.
24 Kasuya K, Nagakawa Y, Matsudo T, Ozawa T, Tsuchida A, Aoki T, et al. p53 gene mutation and p53 protein overexpression in a patient with simultaneous double cancer of the gallbladder and bile duct associated with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg 2009;16:376-381.
25 Ono S, Fumino S, Iwai N. Implications of intestinal metaplasia of the gallbladder in children with pancreaticobiliary maljunction. Pediatr Surg Int 2011;27:237-240.
26 Yoshida N, Esaki M, Kishi Y, Shimada K, Ojima H, Kanai Y, et al. Bile duct carcinoma involving the common channel associated with pancreaticobiliary maljunction shows an extension pattern similar to ductal carcinoma of the pancreas. Pathol Int 2013;63:415-418.
27 Tsuchida A, Nagakawa Y, Kasuya K, Matudo T, Kyo B, Suzuki Y, et al. Signifcance of CD44s and CD44v6 expression in pancreaticobiliary maljunction. Hepatogastroenterology 2011;58: 1877-1881.
28 Tsuchida A, Itoi T. Carcinogenesis and chemoprevention of biliary tract cancer in pancreaticobiliary maljunction. World J Gastrointest Oncol 2010;2:130-135.
Received December 17, 2013
Accepted after revision June 10, 2014
AuthorAffliations:Division of General Surgery, Shanghai Jiaotong University Affliated Sixth People's Hospital, Shanghai 200233, China (Chen F, Zhang ZQ, Jin BW and Dong WF); School of Basic Medical Sciences, Nanjing University of Chinese Medicine, Nanjing 210023, China (Tang L); Division of General Surgery, Soochow University Affliated Children's Hospital, Suzhou 215003, China (Wang J and Huang SG)
Jian Wang, MD, PhD, Division of General Surgery, Soochow University Affliated Children's Hospital, Suzhou 215003, China (Tel/Fax: +86-512-65224492; Email: wj196312@vip.163.com)
? 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60306-4
Published online November 14, 2014.
 Hepatobiliary & Pancreatic Diseases International2015年1期
Hepatobiliary & Pancreatic Diseases International2015年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Blunt abdominal injury with rupture of giant hepatic cavernous hemangioma and laceration of the spleen
- Enlarged pancreas: not always a cancer
- p38 MAPK inhibition alleviates experimental acute pancreatitis in mice
- Predictors of incidental gallbladder cancer in elderly patients
- Development of hybrid-type modifed chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells
