Ordered water monolayer on ionic model substrates studied by molecular dynamics simulations?
SHAO Shi-Jing(邵士靖),GUO Pan(郭盼),ZHAO Liang(趙亮),and WANG Chun-Lei(王春雷)
1Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2University of Chinese Academy of Sciences,Beijing 100049,China
Ordered water monolayer on ionic model substrates studied by molecular dynamics simulations?
SHAO Shi-Jing(邵士靖),1,2GUO Pan(郭盼),1,2ZHAO Liang(趙亮),1,2and WANG Chun-Lei(王春雷)1,?
1Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2University of Chinese Academy of Sciences,Beijing 100049,China
The molecular behaviors of interfacial water molecules at the solid/liquid interface are of a fundamental signif i cance in a diverse set of technical and scientif i c contexts,thus have drawn extensive attentions.On certain surfaces,the water monolayer may exhibit an ordered feature,which may result in the novel wetting phenomenon.In this article,based on the molecular dynamics simulations,we make a detailed structure analysis of the ordered water monolayer on ionic model surface with graphene-like hexagonal lattices under various charges and unit cell sizes.We carefully analyze the water density prof i les and potential of mean force,which are the origin of the special hexagonal ordered water structures near the solid surface.The number of hydrogen bonds of the ordered water monolayer near the solid surface is carefully investigated.
Ordered water monolayer,Hydrogen bond,Molecular dynamics simulations
I.INTRODUCTION
The complex behaviors of interfacial water[1–9],which are of great importance in research f i elds of protein stability and folding[10],molecular self-assembly[11],manipulating biomolecules[12],rearrangement of immunodef i ciency virus[13]etc.,have drawn extensive attentions[5–7,14], since the molecular structure and dynamics of the interfacial water molecules are usually different from the bulk properties[15].Interfacial water molecules play an important role in biophysical process.For example,water effectively catalyzes chiral interconversion of thalidomide[16],and dewetting transition promotes the amyloid f i brils formation[17]. Owing to the interaction between the interfacial water and the hydrophilic solid substrate,the diffusion of interfacial water[18]is slower,and the lifetime of hydrogen bonds[19]is longer,than that of the bulk water,as having been conf i rmed by experiments[20–22].Recently,ordered structure of the interfacial water conf i ned[23]at one or two dimensions has been studied extensively by both experimental and theoretical methods.In 2009,we reported a liquid water droplet on a water monolayer,termed as“ordered water monolayer does not completely wet water”on a model surface at room temperature[24].Later,similar phenomena were observed by several experiments on sapphire c-plane electrolyte surface[25]and on self-assemble monolayer(SAM)surfaces with the?COOH terminal[26,27].In addition,theoretical simulations found similar phenomenon on hydroxylated metal oxide surfaces of Al2O3and SiO2[4],Talc[28]and Pt(100)metal surfaces[29].We also explored the effect of morphology[30]and the critical length of the charge dipolesof the solid surface[31]on the structures of interfacial water and the surface wetting behaviors.
In this article,based on molecular dynamics simulations, we investigate the structure and hydrogen bonds to show detail information of the ordered water monolayer on ionic model surface having graphene-like hexagonal lattices with various charges and unit cell sizes.The article is organized as follows.The ordered structure of water monolayer near the surface is described in Sec.III.A.In Sec.III.B,the water density and the potential of mean force(PMF)[28]are studied.InSec.III.C,thenumberofhydrogenbondsiscalculated to show the stable formation of hydrogen bonds network in the ordered water monolayer.Finally,a short conclusion is presented in the last section.
II.SIMULATION DETAILS
We conf i gured a hexagonal solid lattice with 1664 solid atoms and the neighbor bond lengthlwas described in Fig.1, the same as our previous studies[24,32].The initial systems for the molecular dynamics simulations contained a water layer of about 4.0nm thick on the ionic model surface, where positive and negative charges were located diagonally in neighboring hexagon,and it was found that the charge had great inf l uence on the f l ux of water molecules in nanotube[12,32].All the simulations were performed atT= 300K(NVT ensemble),with Gromacs 4.5.4[34]by using a time step of 1.0fs.The Lennard-Jones parameters of the solid atoms wereεss=0.105kcal/mol andσss=33.343?A,and SPC/E water model[35]was used.The particle-mesh Ewald method[13]with a real space cutoff of 1nm was adopted for the long-range electrostatic interactions and a 10?A cutoff was used for the van der Waals interactions.The periodic boundary conditions were applied in three directions.The simulation time for every system was 4ns and the last 2ns data was collected for analysis.
Two series of simulations were performed to investigate the ordered water monolayer formation on a hexagonal polarity solid surface.In the f i rst series of simulations, the chargeqof the solid atoms increased from 0.6e to 1.0e with 0.1e interval and there were 5252 water molecules in the simulation boxes with the volume of 6.395nm×6.816nm×20.110nm.The value of the neighboring bond length of solid atoms was kept as the constant ofl=0.142nm.In the second series of simulations,the bond lengthlwas set at 0.120nm,0.130nm,0.142nm, 0.150nm and 0.160nm,withq=0.8e,and the water layer thickness was kept at about 4.0nm,with the water molecules of 3525,4314,5252,5721 and 6564,in the simulation boxes of 5.404nm×5.760nm×20.110nm,5.854 nm×6.240 nm×20.110 nm,6.395nm×6.816nm×20.110nm, 6.755nm×7.20nm×20.110nmand7.205nm×7.680 nm×20.110 nm,respectively.
III.RESULTS AND DISCUSSIONS
A.Structure analysis of the water monolayer
To study the structure of water molecules in the water monolayer on the solid surface,two angle parametersθand?are introduced as illustrated in Fig.2(c)and 2(d),whereθis def i ned as the angle between a water molecule dipole and z axis,and?is the angle formed between the projection onto x-y plane of a water dipole and a crystallographic direction[30].Here,the def i nition of the water monolayer is the water molecules in the f i rst layer next to the solid surface with an average thickness of 0.4nm,the same as our previous work[24],which is also consistent with the existence of an experimentally observable monolayer[36].The second layer is def i ned as the water molecules with an average thickness of 0.4nm above the water monolayer.
As shown in Fig.2(e),two peaks of angleθconf i rm the two states,namely,state 1 and state 2 as depicted in Figs.2(a) and 2(b).The left peaks atθ≈60°represent state 1 with oxygen atoms attracted by the positive charged atoms,while the right peaks atθ≈120°represent state 2 with?OH bonds pointing towards the negative charged atoms.Fig.2(f)is the normalized probability distributions of angle?with three peaks at?≈0°,120°and 240°,which demonstrate that the water molecules in the monolayer can form a 2D hexagonal conf i guration(Fig.2(d)),the same as our previous work[24]. Asqincreases from 0.6e to 1.0e,all the peaks in Figs.2(e) and 2(f)become higher and the water molecules in the monolayer become ordered due to the larger binding of the surface charged atoms.However,the peaks are quite different as the bond lengthlincreases.Atl=0.142nm andq=0.8e,the peaks are the highest(Figs.2(g)and 2(h)),hence the most ordered water molecules in the water monolayer.As theldeparts from 0.142nm,the ordered hexagonal water monolayer gradually disappears.These results show that the ordered water structure greatly depends on the surface charge and suitable cell size.
B.Water density distribution prof i les and PMF curves
Figures.3(a)and 3(b)show the water density as a function ofzat differentqandl.The referencez=0 corresponds to the solid surface.Two peaks can be seen for all curves locating atz=0.3nm and 0.6nm.Due to the strong binding of charges on the surface,we can observe a quite high density peak near the solid surface forming the monolayer.The density increases with the charge,reaching the largest atq= 0.9eand1.0e.Withincreasingcellsize,thedensityincreases fi rst untill=0.142nm,where it begins to decrease,indicating the formation and break-down of the ordered structure, respectively.
Thedensityrelatestopotentialofmeanforce(PMF),F(z), by the expression[28],
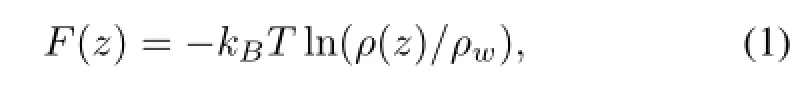
Fig.2.(Color online)(a)State 1 with water molecule adsorbed by positive binding charge and three negative neighbor charges marked with A,A’,B.(b)State 2 with water molecule adsorbed by negative binding charge and three positive neighbor charges marked with C,D,D’.(c) Schematic of angleθdef i ned as the angle between a water molecule dipole and z axis.(d)Schematic of angle?def i ned as the angle formed between the projection onto x-y plane of a water dipole and a crystallographic.(e)Probability distribution ofθin the monolayervs.q.(f) Probability prof i le for angle?in the monolayervs.q.(g)Probability prof i les ofθin the monolayervs.l.(h)Probability distribution of?in the monolayervs.l.
where,kBis the Boltzmann constant andρw=33nm?3is the number density of bulk water.F(z)is the potential of mean force for bringing a water molecule from the bulk to a distancezfrom the solid surface.Figs.3(c)and 3(d)show the PMF curves and for every curve there are two valleys atz= 0.3nm and 0.6nm.The two valleys account for the adsorption of the solid surface.The minimum PMF atz=0.3nm is about?0.9kcal/mol atq≥0.8e andl=0.142nm.The PMF reveals the adsorption interaction of the solid surfaces at the valleys.The adsorption increases with the charge,displaying a wide range of binding strength to attract the water molecules and form the ordered monolayer.This is different from the bulk water.Suitable cell size is quite important for adsorption interaction of the solid surface and formation ofthe monolayer.The PMF results indicate that the distribution of water molecules and formation of the ordered water monolayer are affected by the charge and cell size.
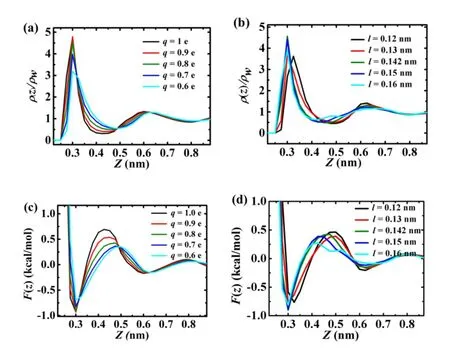
Fig.3.(Color online)(a)Density prof i le of water molecules away from the surfacevs.q,divided by the number density of bulk water,ρw= 33nm?3.(b)The density prof i levs.l,ρz/ρw.(c)Potential of mean forceF(z)vs.q.(d)F(z)vs.l.
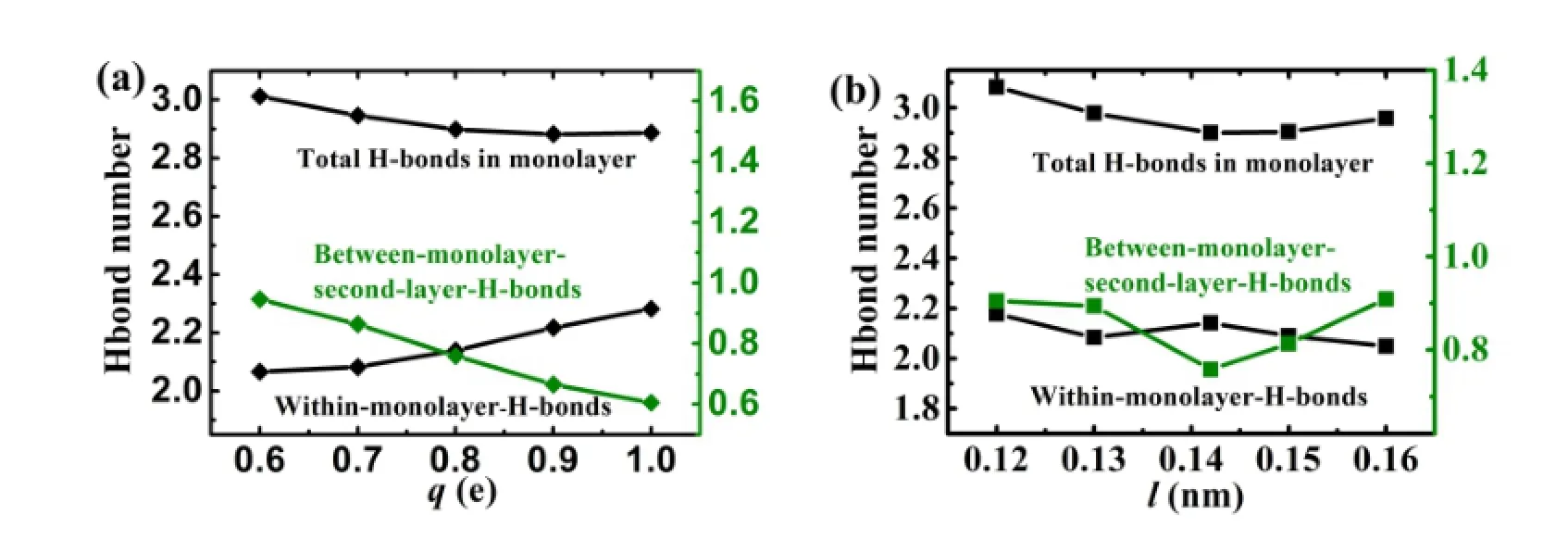
Fig.4.(Color online)Average number of hydrogen bonds of a water molecule to other water molecules in the same layer(◆),to water molecules in the second layer(?),and their sum(■)as function ofq(a)andl(b).
C.Hydrogen bonds in the water monolayer
The ordered water monolayer affects the formation of hydrogen bonds of the water molecules in the interface.We calculated the average hydrogen bonds of a water molecule to its neighboring water molecules in the same monolayer(“in the monolayer”H bonds),and to water molecules in the second layer(“to the second layer”H bonds),as shown in Fig.4. The criteria characterizing existence of hydrogen bond between two water molecules is the geometric def i nition that their O?O distance is less than 3.5?A and simultaneously the angle H?O···O is less than 30°[37].
In Fig.4(a),the number of hydrogen bonds within the monolayer increases and the number of the hydrogen bonds between the monolayer and the second layer decreases as the increase of charge.Their sum remains at~2.9 whenq≥0.8e,which approaches 3,the maximum number of hydrogen bonds that any water molecule can form in the monolayer[24].The interaction energy between the monolayer and the charged surface is stronger when the charge increases as we calculate in Sec.III(B).The water molecules bound inthe monolayer make it easy to form hydrogen bond with the water molecule in the same layer.There is competition for formation of hydrogen bonds between the“in the monolayer”H bonds and“to the second layer”H bonds.The increase of former leads to the decrease of latter for weaker interaction between the water molecules in the monolayer and water molecules in the second layer.In Fig.4(b),whenl= 0.142nm andq=0.8e,the average number of hydrogen bonds among the water molecules in the monolayer is larger than the others,and the number of hydrogen bonds between the monolayer and the second layer is the smallest.The total number of hydrogen bonds per water molecule in the monolayer is also about 3.Thus,the large charge and the suitable unit cell size(l=0.142nm)make the water molecules in the monolayer prefer to form hydrogen bonds within the water monolayer,rather than form hydrogen bonds between the monolayer and water molecules in the second layer.Clearly, the unit cell size is also the key to the formation of hydrogen bonds of the water molecules near the solid surface.
IV.CONCLUSION
In summary,we study the structure,properties of free energy and hydrogen bonds of ordered water monolayer on ionic model surface with graphene-like hexagonal lattices with different charges and unit cell sizes by molecular dynamics simulations.The results indicate that both the charge and unit cell size have a great effect on the water molecular behaviors in the monolayer,such as water molecular conf i gurations and the hydrogen bond network.The charged surface displaying strong adhesive interaction is described by the water density prof i les and potential of mean force.We have also carefully investigated the number of hydrogen bonds of the ordered water monolayer near the solid surface.It is expected that the f i nding in this paper may help to deeply understand the ordered water monolayer on the surface.
ACKNOWLEDGEMENTS
We thank Prof.FANG Hai-Ping and Dr.XIU Peng for the helpful discussions and suggestions.
[1]Stirnemann G,Rossky P J,Hynes J T,et al.Faraday Discuss, 2010,146:263–281.
[2]Stirnemann G,Castrill′on S R V,Hynes J T,et al.Phys Chem Chem Phys,2011,13:19911–19917.
[3]Malani A and Ayappa K G.J Chem Phys,2012,136:194701.
[4]Phan A,Ho T A,Cole D R,et al.J Phys Chem C,2012,116: 15962–15973.
[5]Ostroverkhov V,Waychunas G A,Shen Y R.Phys Rev Lett, 2005,94:46102.
[6]Zheng J M,Chin W C,Khijniak E,et al.Adv Colloid Interfac, 2006,127:19–27.
[7]Sovago M,Campen R K,Wurpel G W H,et al.Phys Rev Lett, 2008,100:173901.
[8]Zanotti J M,Bellissent-Funel M C,Chen S H.Europhys Lett, 2005,71:91–97.
[9]Goertz M P,Houston J,Zhu X Y.Langmuir,2007,23:5491–5497.
[10]Hummer G,Garde S,Garc?a A E,et al.Chem Phys,2000,258: 349–370.
[11]Vauthey S,Santoso S,Gong H,et al.P Natl Acad Sci USA, 2002,99:5355–5360.
[12]Xiu P,Zhou B,Qi W P,et al.J Am Chem Soc,2009,131: 2840–2845.
[13]York D M,Darden T A,Pedersen L G,et al.Biochemistry-US, 1993,32:1443–1453.
[14]Gragson D E,McCarty B M,Richmond G L.J Am Chem Soc, 1997,119:6144–6152.
[15]Bandyopadhyay S,Tarek M,Klein M L.Curr Opin Colloid Int, 1998,3:242–246.
[16]Tian C,Xiu P,Meng Y,et al.Chem-Eur J,2012,18:14305–14313.
[17]Yang Z,Shi B,Lu H,et al.J Phys Chem B,2011,115:11137–11144.
[18]Chen S H,Gallo P,Bellissent-Funel M C.Can J Phys,1995,73:703–709.
[19]Li J,Liu T,Li X,et al.J Phys Chem B,2005,109:13639–13648.
[20]Riter R E,Willard D M,Levinger N E.J Phys Chem B,1998,102:2705–2714.
[21]Pal S K,Peon J,Bagchi B,et al.J Phys Chem B,2002,106: 12376–12395.
[22]Pal S K,Peon J,Zewail A H.P Natl Acad Sci USA,2002,99: 1763–1768.
[23]Pal S,Balasubramanian S,Bagchi B.J Phys Chem B,2003,107:5194–5202.
[24]Wang C,Lu H,Wang Z,et al.Phys Rev Lett,2009,103: 137801.
[25]L¨utzenkirchen J,Zimmermann R,Preoˇcanin T,et al.Adv Colloid Interfac,2010,157:61–74.
[26]James M,Darwish T A,Ciampi S,et al.Soft Matter,2011,7: 5309–5318.
[27]James M,Ciampi S,Darwish T A,et al.Langmuir,2011,27: 10753–10762.
[28]Rotenberg B,Patel A J,Chandler D.J Am Chem Soc,2011,133:20521–20527.
[29]Limmer D T,Willard A P,Madden P,et al.P Natl Acad Sci USA,2013,110:4200–4205.
[30]Wang C,Zhou B,Xiu P,et al.J Phys Chem C,2011,115: 3018–3024.
[31]Wang C,Zhou B,Tu Y,et al.Sci Rep,2012,2:358.
[32]Ren X P,Zhou B,Li L T,et al.Chin Phys B,2013,22:016801.
[33]Xu W,Tu Y,Wang C,et al.Nucl Sci Tech,2011,22:307–310.
[34]Hess B,Kutzner C,van der Spoel D,et al.J Chem Theory Comput,2008,4:435–447.
[35]Berendsen H J C,Grigera J R,Straatsma T P.J Phys Chem, 1987,91:6269–6271.
[36]Miranda P B,Xu L,Shen Y R,et al.Phys Rev Lett,1998,81: 5876–5879.
[37]Luzar A and Chandler D.J Chem Phys,1993,98:8160–8173.
10.13538/j.1001-8042/nst.25.020502
(Received January 8,2014;accepted in revised form February 24,2014;published online March 20,2014)
?Supported by the National Science Foundation of China(Nos.11290164 and 11204341),the Knowledge Innovation Program of SINAP,the Knowledge Innovation Program of the Chinese Academy of Sciences,Shanghai Supercomputer Center of China and Supercomputing Center of Chinese Academy of Science
?Corresponding author,wangchunlei@sinap.ac.cn
 Nuclear Science and Techniques2014年2期
Nuclear Science and Techniques2014年2期
- Nuclear Science and Techniques的其它文章
- A hybrid voxel sampling method for constructing Rad-HUMAN phantom?
- Measurement of keffwith an improved neutron source multiplication method based on numerical analysis?
- Simulation of neutron diffusion and transient analysis of MSR?
- A method for determination of the s orbital component of12Be ground state?
- Demonstration of Pm-147 GaN betavoltaic cells
- Upgrade to the front-end electronics of the BESIII muon identif i cation system
