比熱和直流磁化率證明N+H…O?氫鍵的電子自旋翻轉(zhuǎn)在D-和L-丙氨酸單晶中的不對(duì)稱相變
王文清 沈新春 吳季蘭 龔 ? 申國華 趙洪凱
(1北京大學(xué)化學(xué)與分子工程學(xué)院應(yīng)用化學(xué)系,北京分子科學(xué)國家實(shí)驗(yàn)室,北京100871; 2山東大學(xué)化學(xué)與化工學(xué)院,濟(jì)南250061)
比熱和直流磁化率證明N+H…O?氫鍵的電子自旋翻轉(zhuǎn)在D-和L-丙氨酸單晶中的不對(duì)稱相變
王文清1,*沈新春1,2吳季蘭1龔 ?1申國華1趙洪凱1
(1北京大學(xué)化學(xué)與分子工程學(xué)院應(yīng)用化學(xué)系,北京分子科學(xué)國家實(shí)驗(yàn)室,北京100871;2山東大學(xué)化學(xué)與化工學(xué)院,濟(jì)南250061)
為了解決D-和L-丙氨酸在約270 K相變的分岐和機(jī)理,對(duì)其單晶、多晶粉末及原料利用微分掃描量熱儀測定比熱.用三線法以藍(lán)寶石作校正,并與手冊(cè)的D-和L-丙氨酸標(biāo)準(zhǔn)比熱值比較.在單晶中,實(shí)驗(yàn)觀察到吸熱相變峰最高處時(shí)的溫度及熱焓為:D-丙氨酸,Tc=272.02 K,ΔH=1.87 J·mol-1;L-丙氨酸,Tc=271.85 K,ΔH= 1.46 J·mol-1;熱焓差為0.41 J·mol-1.參比晶體D-纈氨酸,Tc=273.59 K,ΔH=1.75 J·mol-1;L-纈氨酸,Tc= 273.76 K,ΔH=1.57 J·mol-1;熱焓差為0.18 J·mol-1.實(shí)驗(yàn)發(fā)現(xiàn)已測量過的單晶磨成多晶粉末后再測,相變峰消失.說明相變與晶格有關(guān).變溫中子衍射排除了D→L的構(gòu)型相變,但發(fā)現(xiàn)N+H…O-氫鍵沿D-和L-丙氨酸單晶的c軸反向變化.變溫偏振拉曼散射反映相變機(jī)制與N+H…O-中電子的軌道磁偶極矩相關(guān),觀察到偏振光的不對(duì)稱散射.在外加磁場強(qiáng)度H為+1 T和-1 T下,變溫測定D-和L-丙氨酸晶體的直流磁化率,證明在270 K有電子自旋翻轉(zhuǎn)的相變.電子自旋的向上或向下,取決于晶格中NH+3的扭曲振動(dòng)及N+H…O-氫鍵沿晶體c軸的方向.由于自旋的定軸性,可以解釋單晶和多晶粉末比熱結(jié)果的分岐.
比熱;直流磁化率;N+H…O-氫鍵;電子自旋翻轉(zhuǎn);不對(duì)稱相變;D-和L-丙氨酸單晶
1 Introduction
Why is life based on L-amino acids and D-saccharides rather than D-amino acids and L-sacharides?A theory on the origin of life suggests that the preferential emergence of the L-amino acids in proteins could be due to intrinsic differences in energy between enantiomers as a result of the weak forces. The weak-neutral current between electrons and nucleons introduces a small energy difference between enantiomers of a chiral molecule owing to parity violation.Calculation of the parity violating energetic differences(PVED)between L-and D-amino acids suggested that L-enantiomers are more stable than D-enantiomers by 10-17kTR,where k is Boltzman constant and TRis room temperature for amino acids.1The tunneling time from left-handed state to the right-handed state,then back to the left,is extremely long of order millions of years for typical amino acids.In 2004,MacDermott and Hegstrom2proposed a novel method to measure the PVED between enantiomers using chiral ammonia-like molecules(NHDT)with tunneling time between 10-7and 10-2s.The direct measurement of energy difference displays a small but possibly detectable electroweak optical rotation for D-and L-amino acids.
The concept of chirality comes from α-C atom with four different substituents based on Cahn-Ingold-Prelog rules with respect to geometry which is a pseudo-scalar without connection to the physical world.Amino acid molecules not only have a spatial arrangement of atoms but also possess a spatial arrangement of electric dipoles.D-and L-alanine crystallizes as a linear chain of molecules with the carboxylate anion(COO-)and the nitryl cation(NH+3)at opposite ends.All three protons of the NH+3group form N-H…O bonds.The intermolecular N+H…O-bond links up along the c(z)-direction.There are two inequivalent hydrogen centers in D-and L-alanine:one chiral center is Hα-C(hydrogen bonded to α-carbon),however,αCH…O interaction is too weak and appear to be non-directional; the second chiral center is polar H+N(hydrogenbonded to nitrogen).Neutron diffraction and polarized Raman vibration spectra showed the up and down directions of the strongest bond N+H…O-in the crystals of D-and L-alanine.This article is in focus on the temperature-dependence measurement of heat capacity and dc-magnetic susceptibility to search for the transition mechanism.
2 Experimental
2.1 Crystallization of D-and L-alanine
The polycrystalline powders of D-and L-alanine(Sigma Chemical Co.)were twice recrystallized from sterilization aqueous solution at 277 K by slow evaporation for 2 to 3 weeks,producing well formed crystal elongated along the c axis and with principal faces{110}3,4washed with dropping absolute alcohol,evacuated and kept in a desiccator.Crowell et al.5pointed out that evaporation time of less than one week increased the inhomogeneous contribution to the phonon line shapes and concluded no further improvement in crystal quality passed through thrice recrystallization.According to the Kosic et al.6report,the most possible residual impurities in L-alanine are glycine and D-alanine.The crystal purity was identified by the following procedure.Firstly,the positive-ion electrospray mass spectra(EI-MS)were recorded using a Bruker APEX IV 7.0T Fourier transform ion cyclotron resonance (FT-ICR)mass spectrometer(Bruker Daltonics,Billerica,MA USA)equipped with an external ESI source(Analytica,Branford Inc.USA),which produced detection limits of 2 fmol for organic impurities.L-alanine crystal was dissolved in a solvent system composed of methanol/water/formic acid(49.4:49.4: 0.2,volume ratio),to the concentration of 0.3μg·mL-1.Sample solution was electrosprayed by 1μL·min-1flow rate from a capillary(ID:0.2μm)biased at-4 kV.The infusion rate was 60μL·h-1and the scan range was from m/z 50 to 1000.EI-MS could detect the impurity less than 2×10-13g·mL-1.7Secondly, the purity of the single crystals of D-,L-,DL-alanine and D-, L-,DL-valine were identified by gas chromatography mass spectrometry(GC-MS)of their N-trifluoroacetyl(TFA)isopropyl esters on Chirasil-Val capillary columns.8Atomic force microscopy(AFM)images clearly showed an ordered molecular morphology of orthorhombic alanine and monoclinic valine unit cells,which are in fair agreement with the X-ray diffraction data.9Thirdly,FT-IR spectrometer(Nicolet iN 10 MX, USA)was with detection limits of 10μg.
2.2 Heat capacity measurements
The heat capacity measurements of D-and L-alanine were performed from 240 to 320 K with a differential scanning calorimeter(pyris diamond DSC,Perkin Elmer Corp,USA),which is of the heat-flux type.All of the crystals were dried for several days at 10-4mm,in a system trapped with liquid nitrogen. For the pyris diamond system,dry N2gas was purged through the DSC cells with a flow rate of 10 mL·min-1for 60 min before the measurement.The empty reference pan had a mass of 29.08 mg for all runs.No mass losses which could be considered significant occurred.The scan rate was 10 K·min-1from 240 to 320 K.Final heat capacity calibrations were made with 31.33 mg of sapphire,and the sample measurements were carried out with sample and reference pan.Triple curve method was used for precise temperature measurements of the specific heat of the sample.10
The heat capacities of D-and L-alanine crystals and polycrystalline powders of Sigma products were measured.The rigorous drawing of the baseline is based on Mraw?s model11and the transition enthalpy can be calculated from the area enclosed by the baseline and the trace recorded in the experiments.12,13In order to get high precision of heat capacity data by standard DSC,the following procedure had to be carried out before the heat capacity measurement.
2.2.1 D-and L-alanine single crystals and Sigma
polycrystalline powders
The heat capacity of L-alanine single crystal(12.26 mg), L-alanine Sigma powder(A-5824 Lot 84H02461,99%,15.04 mg)and D-alanine single crystal(14.01 mg),D-alanine Sigma powder(A-7377 Lot 16H1584,98%,10.28 mg)were measured from 250 to 320 K at the scan rate of 10 K·min-1by triple curve method.
2.2.2 L-and D-valine single crystals and Sigma polycrystalline powder
The heat capacity of L-valine single crystal(14.01 mg), L-valine Sigma powder(V-500 Lot 80H0457,98%,12.78 mg) and D-valine single crystal(12.80 mg),D-valine Sigma powder(V-0250 Lot 16H1206,98%,10.99 mg)were measured from 250 to 320 K at the scan rate of 10 K·min-1by triple curve method.
2.2.3 Ground powders from pre-measured D-,L-alanine and D-,L-valine crystals
It is worthy to note that the transition peak around 270 K was not observed in the Sigma products of D-and L-alanine (-valine),the polycrystalline L-alanine(47.431 g),L-valine (44.380 g),and D-alanine by Hutchens14and Huffman15et al. For the aim to understand the transition mechanism,we ground the pre-measured single crystals of D-and L-alanine(-valine) then performed the heat capacity measurement.
2.3 DC-magnetic susceptibility
DC-magnetic susceptibility measurement of D-and L-alanine single crystals was performed in the temperature range of 2-320 K with SQUID-based magnetometers of Quantum Design model MPMS-XL-5.The crystals were selected(mass of 390.89 mg for D-alanine and 338.50 mg for L-alanine)and rectangularly mounted in the long plastic tube.The crystals were placed that the c axis was exactly parallel to the direction of the magnetic field(H//z axis).The external magnetic field strengths(H=±1 T)were applied.The magnetic moments were measured under three scannings.
2.4 Neutron diffraction
The neutron diffraction data of D-and L-alanine at 295 and 60 K were performed on the ISIS Facility of CLRC Rutherford Appleton Laboratory,16and the temperature-dependent data of D-alanine at 300,260,250,and 240 K were measured at the VIVALDI neutron beam line.17,18
2.5 Raman vibration spectroscopy
Temperature-dependent polarized Raman spectra of the?NH+3torsion mode in D-and L-alanine were performed with b(cc)b scattering geometry from 80 to 300 K.The wavenumber accuracy of the instrument is±1 cm-1.The repeatability is 1 pixel with a standard CCD(1024 pixel×256 pixel of 26 μm)under the condition of temperature stability(±1°C).In the alanine molecule,the?NH+3group could undergo simultaneously a dynamic distortion and a lifting of the degeneracy of its ground and excited states,producing the splitting of the-NH+3torsional mode(493 cm-1,80 K)into N-H…O(481 cm-1)and N+H…O-(464 cm-1)modes at 290 K,which was discovered by Forss.19The Stokes vibrational Raman line of N+H…O-mode was measured with the spectrometer T64000.
3 Results and discussion
3.1 Identification of samples
In Fig.1,the MS spectrum of alanine(M=89.09)showed the first peak at m/z 71.305 and the second peak at m/z 107.081 which were due to loss of H2O and plus NH3from protonated molecular ion[M+H]+,respectively.
Nicolet photoacoustic spectrometer with a detection limit of 10 ng was used to preclude the possible H2O content in crystal as shown in Fig.2.
3.2 Heat capacity measurements

Fig.1 MS spectra of L-alanine with electrospray ionizationfourier transform ion cyclotron resonance mass spectrometer

Fig.2 IR spectra of D-and L-alanine crystals compared with the standard spectrum of L-alanine(a)and L-valine and L-alanine crystals compared with the standard spectrum of H2O(b)
An endothermic transition occurs at 271.85 K for L-alanine and 272.02 K for D-alanine crystal,respectively.There is no peak present around 270 K in the Sigma powders of L-alanine and D-alanine.The heat capacity data of L-and D-alanine were compared with Sullivan et al.20and the standard values of Hutchens14and Huffman15et al.(Fig.3 and Fig.4,the inset figures show the sharp phase transition peak in this work).
An endothermic transition occurs at 273.76 K for L-valine crystal and 273.59 K for D-valine crystal,respectively.The heat capacity data of L-and D-valine were compared with the standard values of Hutchens(Fig.5 and Fig.6).There are no peaks present around 270 K in the Sigma polycrystalline powders of L-valine and D-valine(Fig.7).

Fig.3 Temperature-dependent heat capacity of L-alanine(●)Sullivan data20of L-alanine(ΔH=(20.3±0.1)J·mol-1);(■)Hutchens data14of L-alanine(47.431 g);(▲)this work,Sigma polycrystalline powder of L-alanine(A-5824,Lot 84H02461,99%,15.04 mg);(○)this work,L-alanine single crystal(12.26 mg).Inset:T(onset)=271.01 K,T(peak)=271.85 K, T(end)=272.51 K,ΔH=1.46 J·mol-1.Ms(L-alanine)=89.09 g·mol-1

Fig.4 Temperature-dependent heat capacity of D-alanine (●)Sullivan data20of D-alanine ΔH=(5.8±0.1)J·mol-1;(■)Huffman data15of D-alanine;(▲)this work,Sigma polycrystalline powder of D-alanine(A-7377 Lot 16H1584,98%,10.28 mg);(○)this work,D-alanine single crystal(14.01 mg).Inset:T(onset)=271.09 K,T(peak)=272.02 K, T(end)=272.64 K,ΔH=1.87 J·mol-1.Ms(D-alanine)=89.09 g·mol-1
The transition peak was vanished in the ground powder of D-,L-alanine and D-,L-valine(Fig.8)which demonstrates that the transition is observed only in pre-aligned molecules mediated by lattice modes.The transition parameters are given in Tables 1-3 and help to understand the difference in the Cpdata between our study and earlier reports.14,15,20,21
From the above results,endothermic transitions in single crystals of D-,L-alanine and D-,L-valine were verified.The heats of transition in D-,L-alanine and D-,L-valine are 1.87,1.46, and 1.75,1.57 J·mol-1,respectively.The transition peak was absent in the polycrystalline powders of D-,L-alanine and D-, L-valine of Sigma products and the ground powders from the pre-measured crystals,which excludes the unidentified impuritiesimpartedobservabledifferencesattransitiontemperature.
3.3 Transition mechanism of D-and L-alanine

Fig.5 Temperature-dependent heat capacity of L-valine(●)Sullivan data20of Sigma L-valine powder;(■)Hutchens data21of L-valine; (○)this work,L-valine single crystal(14.01 mg).Inset:T(onset)=272.72 K, T(peak)=273.76 K,T(end)=274.34 K,ΔH=1.57 J·mol-1. Ms(L-valine)=117.15 g·mol-1

Fig.6 Temperature-dependent heat capacity of D-valine(●)Sullivan data20of D-valine,ΔH=(17.8±0.1)J·mol-1;(■)Hutchens data21of L-valine;(○)this work,D-valine single crystal(12.8 mg).Inset:T(onset)= 272.86 K,T(end)=274.35 K,T(peak)=273.59 K,ΔH=1.75 J·mol-1. Ms(D-valine)=117.15 g·mol-1
3.3.1 DC-magnetic susceptibility evidence for electron spin-flip transition of H+Natom
The spin-flip transition in D-alanine is clearly observed in the plots of χρversus T and dχρ/dT versus T under H=1 T(N→S)and vice versa L-alanine experiences a peak around 270 K under H=-1 T(S→N),respectively(Fig.9).Because the dipole moment of L-alanine was originally aligned antiparallel to the direction of magnetic field at H=+1 T,it cannot turn to align itself parallel to the field unless it can release the same amount of energy.Zeeman effect involves the splitting of energy levels of an atom,due to the orientational potential energy of its magnetic dipole moment in an external magnetic field applied to the H+Natom of N+H…O-bonding.22

Fig.7 Temperature-dependent heat capacity of D-and L-valine Sigma powders(■)Hutchens data of L-valine polycrystal(44.380 g);(●)this work, Sigma L-valine powder(V-500 Lot 80H0457,98%,12.78 mg); (○)this work,Sigma D-valine powder(V-0250 Lot16H1206,98%, 10.99 mg).Ms(valine)=117.15 g·mol-1
Alanine molecules exist as parallel chains of hydrogen bonded zwitterions that form electric dipoles with magnetic moments μB(μB=1 Bohr magneton),which run parallel and antiparallel to the c axis in D-and L-alanine crystals.The magnitude of such a magnetic dipole moment would be of the order of μN(yùn)= e?/mp,in which the proton mass(mp)is 1837 times as that of the electron.The potential energy of the field is required to turn the magnetic dipole antiparallel to the field.The energy is-μBwhen the dipole is parallel to the field,and it is+μBwhen the dipole is antiparallel to the field.So the energy required to turn the dipole is 2μBB=1.16×10-4eV≈1 T.Nuclear magnetic dipole moments are three orders of magnitude small-er than the electronic magnetic dipole moments,so the source must be the electron.The energy difference of electron spinflip transition of N+H…O-bonding between D-and L-alanine was around 10-5eV·molecule-1(1 J·mol-1=1.036×10-5eV· molecule-1).

Fig.8 Temperature-dependent heat capacity of ground powders from the pre-measured crystals(a)D-alanine,(b)L-alanine,(c)D-valine,(d)L-valine

Table 1 Transitional results of D-,L-alanine and D-,L-valine single crystals
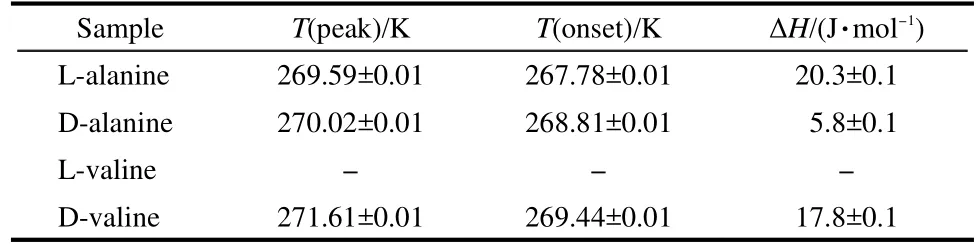
Table 2 Sullivan data20of D-,L-alanine and D-,L-valine crystals

Table 3 Comparison of Cpdata of D-,L-alanine and D-,L-valine between Sullivan,20this works and standard values in Handbook
3.3.2 Neutron diffraction recognized the direction of N+H(4)…O-bonding
Neutron diffraction can provide the hydrogen bond geometry of N-H…O and Cα-H…O weak contacts(Table 4).Two weak hydrogen bonds N-H(6)…O(2)and N-H(1)…O(1) formed layers parallel to the ab plane.The strongest bondN+H(4)…O-ran essentially along the c(z)axis which was shown up and down directions in D-and L-alanine(Fig.10).

Table 4 Hydrogen bond geometry from SXD experiment
3.3.3 Asymmetric Raman scattering of electron spin-flip of N+H(4)…O-bonding
Barron et al.23has shown that Raman EPR(electron paramagnetic resonance)could observe the spin-flip transition by an external magnetic field parallel to right and left circularly polarized incident lights along z axis.Two Raman lines shifted in wavenumber by the Zeeman splitting δ on either side of the Stokes vibrational Raman line.Ray et al.24have reported asymmetric scattering of polarized electrons by organized organic films of chiral molecules.
Gong25demonstrated an asymmetric scattering process between the incident and scattered photon in the Raman effect of chiral alanine crystal.The Raman wavenumber of D-alanine shifted from 465 cm-1to(474±1)cm-1(νD+σ+)and L-alanine shifted from 467 cm?1to(463±1)cm-1(νL-σ-)around 270 K (Fig.11).The predominant asymmetry in the photon scattering arises from the internal spin-orbit interaction of N+H(4)…O-bonding in chiral alanine which is recognized by polarized Raman vibrational spectroscopy with b(cc)b geometry.The -σ-)/(σ++σ-)=0.38,where σ+and σ-are the wavenumbers of scattered photons from D-and L-alanine molecules,respectively.The difference in scattering is found to be δ=|σ+-σ-|=(5.0±1.4)cm-1,which is characteristic of asymmetric spin-flip transitions in the scattering mode of N+H…O-within D-and L-alanine crystals. asymmetry can be expressed as A=(σ+

Fig.9 Temperature dependence of susceptibility χρof D-and L-alanine (a)H=+1 T(N→S),H parallel c(z)axis;(b)H=-1 T(S→N),H antiparallel c(z)axis;(c)dχρ/dT versus T of D-alanine(H=+1 T,H//c axis)

Fig.10 N-H…O bonding viewed down the axis of D-and L-alanine
4 Remark and prospects
Compton model26did not consider the zwitterions form in D-and L-alanine crystals.Barron27depicted the structures of L-and D-alanine crystals that are existed in two distinguishable mirror images with zwitterionic forms.

The electron spin-flip transition only happened at the prealigned D-and L-alanine molecules with crystalline lattice. Structural chirality is time-reversal even and parity-reversal odd(P asymmetry),whereas spin chirality breaks the invariance of both time and parity(PT asymmetry).28Time does not have eigenvalues,so there is no conserved quantity analogous to parity(P).False chirality is exhibited by collinear electric and magnetic fields,but true chirality is exhibited by a magnetic field parallel or antiparallel to the propagation direction.In D-and L-alanine cases,temperature-dependent rotation of-NH+3group and N+H(4)…O-bond translating with“spin up”and“spin-down”electrons is parallel or antiparallel along the c(z) axis.The above results suggest that the electron spin-flip mechanism for generating chiral asymmetry in living systems may be viable if the molecules are pre-aligned,as in a crystal or by adsorption at an interface.The energy difference of electron spin-flip transitions between D-and L-alanine is around 10-5eV·molecule-1.

Fig.11 Peak wavenumber of the NH+3torsional modes from 80 to 300 K(a)and N+H(4)…O-mode from 240 to 300 K(b)
Acknowledgment: The authors thank Professor ZHANG Xiao-Hong(SINOPEC Beijing Research Institute of Chemical Industry)for supply of the instruments of Pyris Diamond DSC and Dr.CAI Chuan-Lun(SINOPEC Beijing Research Institute of Chemical Industry)for cooperation to perform heat capacity measurements.We are grateful to associate professor CHEN Qing-De(College of Chemistry and Molecular Engineering, Peking University)for theoretical discussion.
(1) MacDermott,A.J.Enantiomer 2000,5,153.
(2)MacDermott,A.J.;Hegstrom,R.A.Chemical Physics 2004, 305,55.
(3) Simpson,H.J.;Marsh,R.E.Acta Cryst.1966,20,550.
(4) Destro,R.;Marsh,R.E.J.Phys.Chem.1988,92,966.
(5) Crowell,R.A.;Chronister,E.L.Phys.Rev.B 1993,48,172.
(6) Kosic,T.J.;Raymond,E.C.,Jr.Chem.Phys.Lett.1983,103, 109.
(7) Gledhill,M.Analyst 2001,126,1359.
(8) Cronin,J.R.;Pizzarello,S.Science 1997,275,951.
(9)Wang,W.Q.;Gong,Y.;Liang,Z.;Sun,F.L.;Shi,D.X.;Gao, H.J.;Wang,Z.M.Surface Science 2002,512,L379.
(10) Perkin-Elmer Corporation,ThermalAnalysis Instrument Division,Shanghai,2008.
(11) Mraw,S.C.Rev.Sci.Instrum.1982,53,228.
(12) Pak,J.;Qiu,W.;Pyda,M.;Nowak-Pyda,E.;Wunderlic,B. J.Therm.Anal.Cal.2005,82,565.
(13)Morad,N.A.;Idrees,M.;Hasan,A.A.J.Therm.Anal.1995, 44,823.
(14) (a)Hutchens,J.O.;Cole,A.G.;Stout,J.W.J.Am.Chem.Soc. 1960,82,4813. (b)Handbook of Biochemistry and Molecular Biology; Physical and Chemical Data;CRC Press:Cleveland,1976; Vol.4.
(15)Huffman,H.M.;Borsook,H.J.Am.Chem.Soc.1932,54,4297.
(16)Wilson,C.C.;Myles,D.;Ghosh,M.;Johnson,L.N.;Wang,W. Q.New J.Chem.2005,29,1318.
(17) Wang,W.Q.;Liu,Y.N.;Gong,Y.Acta Phys.-Chim.Sin.2004, 20,1345.[王文清,劉軼男,龔 ?.物理化學(xué)學(xué)報(bào),2004, 20,1345.]
(18)Wang,W.Q.;Gong,Y.;Yao,N.Acta Phys.-Chim.Sin.2005, 21,774. [王文清,龔 ?,姚 楠.物理化學(xué)學(xué)報(bào),2005,21, 774.]
(19) Forss,S.A.J.Raman Spec.1982,12,266.
(20) Sullivan,R.;Pyda,M.;Pak,J.;Wunderlich,B.;Thompson,J. R.;Pagni,R.;Pan,H.J.;Barnes,C.;Schwerdtfeger,P.; Compton,R.J.Phys.Chem.2003,107,6674.
(21) Hutchens,J.O.;Cole,A.G.;Stout,J.W.J.Phys.Chem.1963, 67,1128.
(22) Eisberg,R.;Resnick,R.Quantum Physics of Atoms,Molecules, Solids,Nuclei,and Particlesm,2nd ed.;John Wiley&Sons: New York,1985;p 432.
(23) Barron,L.D.;Buckingham,A.D.Accounts Chem.Res.2001, 34,785.
(24)Ray,K.;Ananthavel,S.P.;Waldeck,D.H.;Naaman,R.Science 1999,283,814.
(25) Gong,Y.Origin of Homochirality in Biomolecule——Temperature-Induced Phase Transitions in D-/L-Alanine Single Crystals.Ph.D.Dissertation,Peking University,Beijing,2006. [龔 ?.生物分子手性起源——D-/L-丙氨酸晶體低溫相變研究[D].北京:北京大學(xué),2006.]
(26) Compton,R.N.;Pagni,R.M.Adv.At.Mol.Opt.Phys.2002,48, 219.
(27) Barron,L.D.Space Sci.Rev.2008,135,187.
(28) Lorenzo,J.E.;Boullier,C.;Regnault,L.P.;Ammerahl,U.; Revcolevschi,A.Phys.Rev.B 2007,75,054418.
October 31,2011;Revised:January 19,2012;Published on Web:February 13,2012.
Heat Capacity and DC-Magnetic Susceptibility Evidence for the Asymmetry of Electron Spin-Flip Phase Transition of N+H…O-Bond in Chiral Alanine Crystal
WANG Wen-Qing1,*SHEN Xin-Chun1,2WU Ji-Lan1GONG Yan1
SHEN Guo-Hua1ZHAO Hong-Kai1
(1Beijing National Laboratory for Molecular Sciences,Department of Applied Chemistry,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China;2School of Chemistry and Chemical Engineering, Shandong University,Jinan 250061,P.R.China)
With a view to understanding the argument of phase-transition mechanisms of D-and L-alanine at around 270 K,the temperature dependence of heat capacity measurements was investigated, for single crystals,ground powders,and polycrystalline products,using differential scanning calorimetry (DSC).The Cp(heat capacity under constant pressure)values of D-and L-alanine were calibrated with standard sapphire by the triple-curve method;these values coincide with the standard Cpvalues in the literature.Endothermic transition peaks were observed at Tc=272.02 K,ΔH=1.87 J·mol-1and Tc=271.85 K, ΔH=1.46 J·mol-1for D-and L-alanine,respectively,and Tc=273.59 K,ΔH=1.75 J·mol-1and Tc=273.76 K, ΔH=1.57 J·mol-1for the reference crystals D-and L-valine,respectively.The energy differences of 0.41 J· mol-1for D-and L-alanine and 0.18 J·mol-1for D-and L-valine,which were observed from pre-aligned molecules in the single crystals and vanished in the ground powders and polycrystalline products,show that the phase transition is related to the crystal lattice.Neutron diffraction results exclude the possibility of a D→L configuration change,and show that the hydrogen bonds run antiparallel to the c-axis in the D-and L-crystals.Polarized Raman vibrational spectroscopy shows that the transition mechanism may be related to the electronic orbital angular momentum and magnetic dipole moments of N+H···O-in the crystals.External magnetic fields,H=+1,-1 T,were applied parallel to the c(z)-axis of the D-and L-alanine crystalline lattices, respectively.The DC-magnetic susceptibilities show electron spin-flip transitions at around 270 K in D-and L-alanine.The spin is“up”or“down”relative to the direction of N+H···O-bond along the c(z)-axis.Based on spin rigidity and magnetic anisotropy,the results help to explain the discrepancies among heat capacity and magnetic susceptibilitydata forsingle crystals and polycrystalline powders ofD-and L-alanine.
Heat capacity;DC-magnetic susceptibility;N+H…O-bonding;Electron spin-flip; Asymmetry transition;D-and L-alanine crystals
10.3866/PKU.WHXB201202132
O642;O646
?Corresponding author.Email:wangwqchem@pku.edu.cn;Tel:+86-10-62752457.
The project was supported by the National Natural Science Foundation of China(21002006,20452002)and Special Program for Key Basic Research of the Ministry of Science and Technology,China(2004-973-36).
國家自然科學(xué)基金(21002006,20452002)和國家科技部基礎(chǔ)研究重大項(xiàng)目(2004-973-36)資助

