Expression and Distribution Characteristics of Human Ortholog of Mammalian Enabled (hMena) in Glioma
Xue-tao Dong, Xue-jun Yang, Hua-min Wang, Wei Wang, Li Yu, Bin Zhang, Sheng-ping Yu Hao-lang Ming
Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin Neurological Institute, Tianjin 300052, China
Expression and Distribution Characteristics of Human Ortholog of Mammalian Enabled (hMena) in Glioma
Xue-tao Dong, Xue-jun Yang*, Hua-min Wang, Wei Wang, Li Yu, Bin Zhang, Sheng-ping Yu Hao-lang Ming
Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin Neurological Institute, Tianjin 300052, China
Objective: To investigate the utility of hMena, a family of enabled/vasodilator-stimulated phosphoprotein(Ena/VASP), we sought to characterize the expression profile and distribution characteristics of hMena in a large panel of glioma samples and determine whether hMena expression levels might correlate with the pathological grade of glioma.
Methods: Sixty-five specimens of glioma with different pathological grades and five control brain tissues werecollected. In 6 of the 21 glioblastoma patients, multi-specimens were obtained respectively from the main tumor mass, the junction zone between the tumor and the normal tissue, and adjacent brain tissue 1.5 cm away from the tumor boundary under assistance of neuronavigation system during the operation. Immunohistochemistry was used to detect the expression and distribution characteristics of hMena. hMena expression was analyzed by Western blot in 20 specimens.
Results: The hMena expression was negative in control brain tissue but positive in different grades of glioma.The expression rate of hMena was positively correlated with the increasing grade of the World Health Orgnization (WHO) classification (rs=0.682,P=0.000). hMena was located in cytoplasm. Positive cells only distributed around the vessels within the tumor mass in low grade glioma, while in high grade glioma, these cells were able to be detected not only in the tumor but also in the boundary zone and adjacent brain parenchyma. In the tumor mass, hMena expressed highly and diffusedly. In the junction zone, hMena positive cells formed radiolitic pattern around the vessels. In adjacent brain parenchyma, single positive cell was scattered. hMena expression was markedly elevated in Grade III and IV glioma compared with Grade II and I.
Conclusion: Our data suggested that the expression of hMena is closely related to malignant grade of glioma.hMena can label the migrating cells, and indicate the migrating path of glioma cells from the tumor to adjacent tissue along with the vascular basement membranes and tracts of white matter.
Glioma; hMena; Migration; Immunohistochemistry
INTRODUCTION
Glioma is the most common type of primary brain tumor and accounts for 40%–50% of the adult brain tumor. Malignant glioma is characterized by rapid, highly invasive growth and extensive neovascularisation and high mortality. The median survival of glioblastoma patients is from 12.1 to 14.6 months[1]. The key reason for the lack of successful therapy is the infiltration of tumor cells into the adjacent brain parenchyma.
Glioma invasion is a multi-step process in which tumor cells cccccccccdetach from primary lesions, establish new contacts with extracellular matrix (ECM) and neighboring cells, degrade and/or remodel ECM barriers and migrate to the adjacent normal brain tissue along with myelinated nerve fibre tracts, vessel basement membranes and the subependymal layers as major routes to form a new lesion[2]. Several recent reviews have highlighted the important role of ECM, proteinases, integrins and angiogenesis in glioma invasion and identified potential molecular targets for therapeutic intervention. This study focuses on the locomotion of the tumor cells. The movement of the tumor cells is a key process during migration.
Enabled/vasodilator-stimulated phosphoproteins (Ena/ VASP), a family of multi-functional actin-modulating proteins, are implicated in cell migration. They play an important role in linking signaling pathways to the remodeling of the actin cytoskeletal structure including the formation of lamellipodiaand filopodia which leads to cell motility[3,4]. hMena, an actin regulatory protein of Ena/VASP family, is overexpressed in many human tumors. It is found that the expression of hMena is correlated with the clinical stage and invasive nature and it plays a key role in the progress of the tumor[5,6]. In this study, we focused on the expression and distribution characteristics of hMena in glioma and explored its implication in tumor invasion.
MATERIALS AND METHODS
Tissue Samples
Sixty-five freshly resected glioma samples were collected in the Department of Neurosurgery at Tianjin Medical University General Hospital from August 2008 to December 2009. They were from 37 male and 28 female patients in the range of 16 to 77 years old. In 6 of 19 glioblastoma patients, multi-specimens were obtained from the main tumor mass, the junction zone between tumor and edema and the area of 1.5 cm away from the tumor mass under assistance of neuronavigation system during operation (Figure 1). As well, 5 nonneoplastic brain samples collected from brain injury patients were served as control. Parts of each sample were fixed in 4% paraformaldehyde and cut into serial sections (5 μm thick), and the left were snap-frozen in liquid nitrogen and kept at -80°C until use. The specimens were classified according to WHO classification of tumors of the central nervous system (2007). There were 9 cases of WHO grade I tumors, 16 cases of WHO grade II tumors, 19 cases of WHO grade III tumors, and 21 cases WHO IV grade tumors. No chemotherapy or radiotherapy was performed on the patients before the operation.
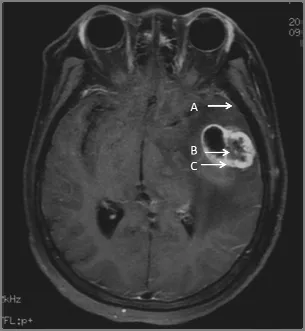
Figure 1. Specimens were collected from the glioblastoma under assistance of neuronavigation system during the operation. A: 1.5 cm away from the tumor boundary; B: the main tumor mass; C: the junction zone.
Immunohistochemistry
Paraffin-embedded tissue samples were rehydrated and then antigen retrieval was performed by incubation with citrate buffer (10 mmol/L, pH 6.0) at 96°C for 40 min. The sections were cooled to room temperature. After saturation with 5% bovine serum albumin (BSA), they were incubated with mouse anti-Mena monoclonal antibody (1:100, BD Biosciences, USA), at 4°C over night, and then the sections were washed and incubated with HRP-conjugated secondary antibodies. Immunoreaction was detected by Non-Biotin HRP Detection System (Zhongshan Corp., China). The sections were counterstained with Mayer's hematoxylin, dehydrated, and mounted for microscopic visualization.
Western Blot Analysis
Samples stored at ?80°C were lysed in the lysis buffer [62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol] and sonicated. Homogenates were clarified by centrifugation at 12,000 r/min for 15 min at 4°C, and protein concentrations were determined using the BCA protein assay kit (Pierce, USA). Samples were made equal in protein concentration and volume, and then transferred to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 8% SDS-acrylamide gel. Separate proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After being blocked in Tris-buffered saline with 5% nonfat dry milk, the membrane was probed with anti-Mena monoclonal antibody (1:1,000, BD Biosciences, USA) and then with an anti-mouse HRP-conjugated secondary antibody (1:2,000 Santa Cruz Biotechnology, USA). Specific signals were detected from quantitative gel and Western blotting imaging system (Becton Dickinson, Franklin Lakes, NJ, USA). The membrane was also reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:2,000, Santa Cruz Biotechnology, USA) using the same procedures described above.
Histological Assessment
The immunostaining results are independently evaluated by two pathologists. When an evaluation differed, the final decision was made by consensus. The immunohistochemical analysis of hMena expression was scored as follows: 0, no staining; +, staining of less than 10% of cells; ++, staining of 10%–60% of cells; +++, staining of greater than 60% of cells.
Statistical Analysis
All data were analyzed by SPSS 16.0. Kruskal-Wallis test was used to assess the positive rate in different grades of gllioma. Spearman correlations were used to verify the correlation between hMena staining intensity and the grades of glioma. The overall threshold of significance was 0.05 for both tests.
RESULTS
The hMena expression was negative in control brain tissue (0/5), and the positive rates of different grades of glioma were: WHO I 11.11% (1/9), WHO II 25.00% (4/16), WHO III 84.21% (16/19), andWHO IV 90.48% (19/21) (Table 1). The difference of hMena expression in glioma was significant (H=34.935,P=0.000). The expression rate of hMena was positively correlated with the increasing grade of WHO classification (rs=0.682,P=0.000). Western blot confirmed that hMena expression was markedly elevated in Grade III and IV glioma compared withGrade II and Grade I glioma (Figure 2). hMena was located in cytoplasm, especially at the leading edge of the cell (Figure 4).
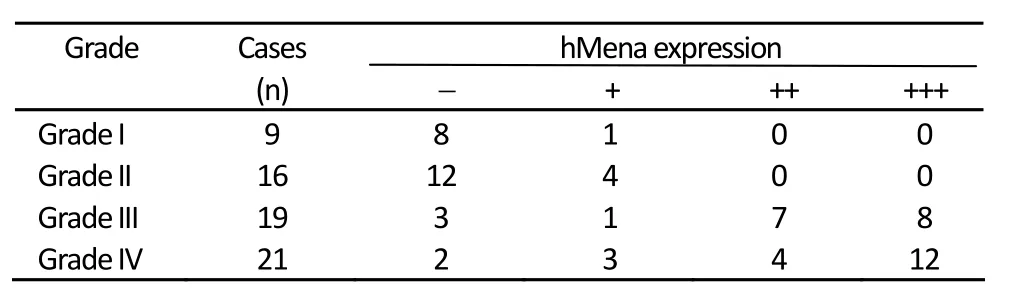
Table 1. The positive rates of different grades of glioma
In low grade glioma, hMena is expressed weakly, while widely and strongly in malignant glioma (Figure 3). We also found that the distribution characteristics of hMena are unique in malignant glioma: In the tumor mass, hMena is expressed in a high level and diffused. Around the vessel, hMena positive cells clustered and formed radiolitic pattern and the long axis of the cell are perpandicular to the vascular wall. In the area of 1.5 cm away from the tumor mass, hMena is expressed in some single cell (Figure 4).
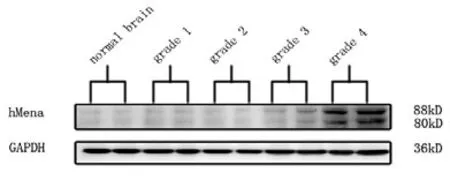
Figure 2. Western blot: the expression of hMena in control brain and glioma.
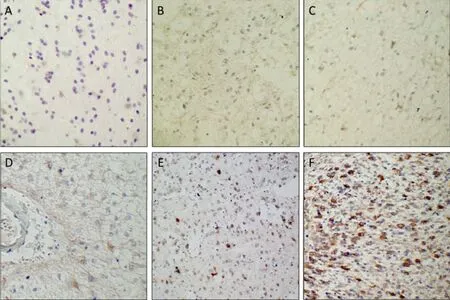
Figure 3. Immunohistochemistry for hMena expression in different grades of glioma. A: Control brain tissue (×100). B: Grade I (×100). C: Grade II (×100). D: Around vessel in Grade II. E: Grade III (×100). F: Grade IV (×100).
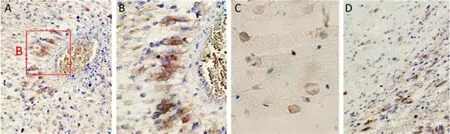
Figure 4. Immunohistochemistry for hMena expression in different areas of glioblastoma. A and B: Perivascular structures. C: 1.5cm away from tumor mass. D: In white matter.
DISCUSSION
Cell migration is a key process of tumor invasion, which requires dynamic remodeling of the actin cytoskeleton through assembly and disassembly, and forms lamellipodia and filopodia at the leading edge. Mena (mammalian enabled) is a member of Ena/VASP family, which plays a significant role in the development of the nervous system of vertebrate. The expression of Mena in high level is required in the formation and viability of several neural-derived structures, such as neural tube, craniofacial, spinal nerve and anterior commissure[7]. In the recent few years, it is found that hMena, regulators of actin cytoskeleton dynamics, is involved in epithelial-mesenchymal transitions in tumor progression and the invasive nature of the tumor[8-10]. It is found that hMena is overexpression in kinds of tumor including pancreatic cancer[11], colorectal carcinoma[5], lung cancer[12]and breast cancer[13]but not in corresponding normal tissues. In breast cancer, hMena over-expressed both in protein and mRNA levels. In addition, as the degree of malignancy increased, the positive rate of hMena significantly increased in the mammary lesion[8,14]. A recent research showing that there is a statistically significant increase of hMena transcripts in matched human colorectal carcinomas and adjacent non-neoplastic colorectal epithelium and an elevated hMena expression is correlated to the cases with advanced TNM stages of colorectal carcinomas. Furthermore, they observed intensified hMena staining in the invasive front of colorectal carcinomas, especially in tumor budding, a transition from glandular structure to single or small clusters of cells at the invasive front[5].
Our data indicates that the expression of hMena has a significant relationship with the grade of WHO classification and unique distribution characteristics in glioma. In low grade glioma, there are only few weakly staining cells locating around the microvessels, which exhibit a round cell morphology. While in high grade glioma, hMena positive cells spread widely. In the main tumor mass, hMena is diffused and the cells have no obvious directionality. In the junction zone, which is rich of capillary, the positive staining cells clustered and formed in a radiolitic pattern around the vessels. The cell morphology exhibits distinct polarity, and the major axis is perpendicular to the vascular wall. These cells form pseudopalisade structure. In the area of 1.5 cm away from the tumor mass, hMena positive cells could be found as well, but they are scattered and very few, which indicate that a few cells have migrated away from the tumor to adjacent brain parechyma, even to the further tissue. It is difficult to obtain the samples far away from the main tumor in patients, so whether hMena expressed in this field is unknown and need to be assessed in animal models in the future. The cells that strongly express hMena have an outstanding ability of migration and these cells usually lengthen their bodies indicating that they are in the moving process. Our data imply that most of the glioma cells are capable of invasion and migrate to the microvessels. We speculate one of the possible reasons of this phenomenon is that the microvesselsin situof tumor cannot provide enough oxygen and other nutrition while tumor cells proliferate widely. Under this circumstance, some tumor cells would migrate to seek new microenvironment around the microvessel driven by hypoxia or nutrition deficit. In addition, David Zagzag[15]reported that stromal cell-derived factor-1α (SDF-1α) is expressed in blood vessels, and white matter tracts while its receptor CXCR4 is expressed in invading glioma cells organized around blood vessels, and along white matter tracts. These cells migrate from tumor core to vessels according to the SDF-1α gradient formed around blood vessels[15]. Furthermore, some studies including our early research found that the distribution characteristics of CD133 are seriously similar to hMena, especially around the microvesse[16-18]. While CD133 positive cells, in another word, brain tumor stem cells (BTSCs), reside in perivascular niche and the surrounding necrotic tissue[19,20]. Evidence for the existence of vascular niche complex has been recently elucidated for malignant glioma, which is rich in a multitude of chemokines, growth factors and other signaling molecules. According to the similarity, we infer that some signaling molecules such as SDF-1α/CXCR4 induce the glioma cells or BTSCs to concentrate around blood vessels to obtain nutrition, and use nascent microvessels as an escalator for migration during development.
This study provides a new target and a clue for the invasive pattern of glioma. Rearrangement of actin cytoskeleton resulting into invasive behavior is involved in tumor progression and it is one of the reasons that glioma has an infiltrative nature[21-23]. As the actin regulatory protein, hMena involved in the control of cell motility, is over expressed in numerous tumors and plays a significant role in the tumor progression and at the beginning of invasion to adjacent tissue[5,8,13]. Further investigation on molecular mechanism of hMena in cell migrating and the relationship of hMena, BTSCs and chemokines should be carried to understand the invasive nature of glioma and may provide insights into the novel therapeutic strategies against malignant glioma.
Acknowledgment
We are grateful to Sun Jian, MD, Zeng Zheng, Wang Zeng-guang, MD for providing surgical tissue samples and sample collection; and Zhan An-ling PhD., Pan Qiang, Yue Xiao and Yang Yang for technical assistance.
REFERENCES
1. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005; 352:997-1003.
2. Nakada M, Nakada S, Demuth T, et al. Molecular targets of glioma invasion. Cell Mol Life Sci 2007; 64:458-78.
3. Scott JA, Shewan AM, den Elzen NR, et al. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin- adhesive contacts. Mol Biol Cell 2006; 17:1085-95.
4. Loureiro JJ, Rubinson DA, Bear JE, et al. Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phospho- protein (VASP) function during cell migration. Mol Biol Cell 2002; 13:2533-46.
5. Toyoda A, Kawana H, Azuhata K, et al. Aberrant expression of human ortholog of mammalian enabled (hMena) in human colorectal carcinomas: implications for its role in tumor progression. Int J Oncol 2009; 34:53-60.
6. Gurzu S, Jung I, Prantner I, et al. The expression of cytoskeleton regulatory protein Mena in colorectal lesions. Rom J Morphol Embryol 2008; 49:345-9.
7. Menzies AS, Aszodi A, Williams SE, et al. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J Neurosci 2004; 24:8029-38.
8. Di Modugno F, DeMonte L, Balsamo M, et al. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: epidermal growth factorincreases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res 2007; 67:2657-65.
9. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54.
10. Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2002; 2:91-100.
11. Pino MS, Balsamo M, Di Modugno F, et al. Human Mena+11a isoform serves as a marker of epithelial phenotype and sensitivity to epidermal growth factor receptor inhibition in human pancreatic cancer cell lines. Clin Cancer Res 2008; 14:4943-50.
12. Dertsiz L, Ozbilim G, Kayisli Y, et al. Differential expression of VASP in normal lung tissue and lung adenocarcinomas. Thorax 2005; 60:576-81.
13. Goswami S, Philippar U, Sun D, et al. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasionin vivo. Clin Exp Metastasis 2009; 26:153-9.
14. Di Modugno F, Bronzi G, Scanlan MJ, et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer 2004; 109:909-18.
15. Zagzag D, Esencay M, Mendez O, et al. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer's structures. Am J Pathol 2008; 173: 545-60.
16. Christensen K, Schroder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol 2008; 90:157-70.
17. Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007; 11:69-82.
18. Wang HM, Yang XJ, Dong XT, et al. Correlation between the distribution of CD133-positive cells and the proliferation of microvessels in glioblastoma multiforme. Zhonghua Yi Xue Za Zhi 2011; 91:781.
19. Seidel S, Garvalov BK, Wirta V, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain 2010; 133:983-95.
20. Li MW, Niu CS. Correlative study of distribution of brain tumor stem cell with micro-vascular system. Zhonghua Yi Xue Za Zhi 2010; 90:305-9.
21. Katsetos CD, Draberova E, Legido A, et al. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme. I. Class III beta-tubulin. J Cell Physiol 2009; 221:505-13.
22. Katsetos CD, Draberova E, Legido A, et al. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme. II. gamma-Tubulin. J Cell Physiol 2009; 221:514-20.
23. Salhia B, Rutten F, Nakada M, et al. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res 2005; 65:8792-800.
10.1007/s11670-011-0312-z
2011-01-12; Accepted 2011-06-23
This work was supported by National Natural Science Foundation of China (30772228).
*Corresponding author.
E-mail: ydenny@yahoo.com
? Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
 Chinese Journal of Cancer Research2011年4期
Chinese Journal of Cancer Research2011年4期
- Chinese Journal of Cancer Research的其它文章
- Prognostic Value of Promoter Hypermethylation of Retinoic Acid Receptor Beta (RARB) and CDKN2 (p16/MTS1) in Prostate Cancer
- Changes of Serum Trace Elements, AFP, CEA, SF, T3, T4 and IGF-Ⅱ in Different Periods of Rat Liver Cancer
- Mast Cells in Adjacent Normal Colon Mucosa rather than Those in Invasive Margin are Related to Progression of Colon Cancer
- Wild-Type KRAS and BRAF Could Predict Response to Cetuximab in Chinese Colorectal Cancer Patients
- Dosimetry Comparison between Volumetric Modulated Arc Therapy with RapidArc and Fixed Field Dynamic IMRT for Local-Regionally Advanced Nasopharyngeal Carcinoma
- Hepatocellular Tumors: Immunohistochemical Analyses for Classification and Prognostication
