Changes of Serum Trace Elements, AFP, CEA, SF, T3, T4 and IGF-Ⅱ in Different Periods of Rat Liver Cancer
Hong-xu Zhang, Dan-dan Liu, Bai-jie Jin, Ya-wei Wang, Qi Liu, Ru-bing Duan, Peng Zhao, Ming-xia Ma
College of Life Science, Henan Normal University, Xinxiang 453007, China
Changes of Serum Trace Elements, AFP, CEA, SF, T3, T4 and IGF-Ⅱ in Different Periods of Rat Liver Cancer
Hong-xu Zhang*, Dan-dan Liu, Bai-jie Jin, Ya-wei Wang, Qi Liu, Ru-bing Duan, Peng Zhao, Ming-xia Ma
College of Life Science, Henan Normal University, Xinxiang 453007, China
Objective: Based on liver cancer model built in SD rats, the contents of trace elements (Cu, Fe, Zn, Ca and Mg), AFP, CEA, SF, TH and IGF-II in serum were measured at different stages to explore the molecular changes during the rat liver cancer development.
Methods: The SD rat liver cancer model was built by using diethylnitrosamine (DENA) as the mutagen. During 16 weeks of DENA gavage, blood samples were taken in the 14th, 28th, 56th, 77th, 105th and 112th days respectively after the first day of gavage with DENA, then the contents of five trace elements (Cu, Fe, Zn, Ca and Mg), T3, T4, IGF-II, AFP, CEA and SF in serum were determined.
Results: During the development of the rat liver cancer, in the test group, the Cu content significantly increased in serum, while the contents of Fe, Zn and Ca significantly decreased. The content of Mg showed no significant change. AFP and CEA of the test group showed same expression level with the control group; while the content of SF was lower than that of the control group when cancerization appeared. T3 and T4 increased at the first stage and then went down, and the content of IGF-II was always high.
Conclusion: Cu, Fe, Zn, Ca, T3, T4, SF and IGF-II are closely related to the development of liver cancer. The changes of their contents in the development of cancer could enlighten the researches on cancer pathogenesis and prevention.
DENA; SD rats; Liver cancer; Trace elements; Biochemical components
INTRODUCTION
The interrelations among the changes of trace elements, hormones and proteins in serum during the development of tumor are the focus of studies of most researchers devoted in the investigation of liver cancer[1]. The malignant tumor's development process is usually accompanied by the abnormality of trace element Cu, Fe, Zn, Ca, and Mg[2-5]. But these researches mostly come from the clinical patients, so the samples from patients are usually lack of the continuity and complete data of the different stages, especially in early stage of carcinogenesis are difficult to obtain. At the same time, it also difficult to compare between the different results is from various studies because of the big difference. The changes of thyroxine [thyroid hormone (TH)] T3, T4 and insulin-like growth factor II (IGF-II) in liver cancer have iiiiiiiiiiibeen paid more attention in recent years. Alphafetoprotein (AFP) is the first tumor maker to detect liver cancer, and carcinoembryonic antigen (CEA) and serum ferritin (SF) are gradually used as clinical diagnosis signs of cancer.
In this study, the SD rat liver cancer model was built by using diethylnitrosamine (DENA) as the mutagen, and the contents of trace elements (Cu, Fe, Zn, Ca and Mg), AFP, CEA, SF, TH and IGF-II in serum at different stages during the development of carcinogenesis were measured. The aim of this study is to explore the relationship between the changes of trace elements and biochemical components during the development of carcinogenesis in rat liver.
MATERIALS AND METHODS
Experimental Animals
A total of 78 male SD rats (III Rank), 112±25 g, were divided randomly into two groups. The test group was 58 rats and the control contained 20.
The animal experiments were approved by the Institutional Animal Care and Use Committee of theCollege of Life Sciences, Henan Normal University.
Carcinogenesis Model
DENA was used as the mutagen to build the rat liver cancer model. The experimental rats were administered by gavage with 1% DENA at the dose of 20 mg/ml once a week, after 16 weeks, fed normally. The control was administered by gavage with physiological saline of 0.9% at the same time. Rats were fed with a standard diet and waterad libitum.
Samples
During 16 weeks of DENA gavage, blood 6-8 ml was collected from each plucked eye rat on d14, d28, d56, d77, d105 and d112 after the first day of DENA gavage respectively, and serum was obtained by low temperature centrifugation. Rats were killed to collect sample liver tissues which were fixed in formalin for 20 h. The pathological sections from liver tissues were stained by hematoxylin-eosin (HE), and observed by two senior pathologists in different pathological periods.
Biochemical Component Measurements
The contents of five trace elements in serum were determined by atomic absorption spectrophotometry (Cu: λ=324.8 nm, Zn: λ=213.9 nm, Fe: λ=248.3 nm, Ca: λ=422.7 nm, Mg: λ=285.2 nm)[5], and the contents of T3, T4, IGF-II, AFP, CEA and SF were measured by radioimmunoassay[6].
Statistical Analysis
Normally distributed variables were expressed as (ˉx±s), and differences between control group and test group were identified using analysis of variance. Contents of biochemical factors were different between two groups. Furthermore, they changed constantly during the process of hepatocarcinogenesis. Two-tailed student’st-test was used in each stage of rat live cancer. Statistical significance was defined asP<0.05 while highly statistical significance was defined asP<0.01. Software SPSS13.0 was used to analyze all data.
RESULTS
Pathological Observation
In the control group, hepatic cells of the rats showed similar size, without obvious nuclear fission. Cytoplasm was abundant and acidophil. Moreover, the hepatic lobule appeared to be clear. In the test group, on the 14th day, the histological structure of hepatic tissue was normal. On the 28th day, there existed heterocysts, but obvious tubercle was not found. On the 56th day, the structure of hepatic lobules disappeared, and several tubercles at various sizes were formed. The ratio of adenoma-type hyperplasia was 100%, of which the ratio of no typical hyperplasia was 10%. On the 77th day, the ratio of untypical hyperplasia was 90%, and the tubercles became more and bigger, accompanied with the epidermic proliferation of biliary duct. On the 105th day, the ratio of cholangiocarcinoma was 40%, and the carcinoma was heteromorphic: the arrangement of cells was tubular and papillate and the carcinomatous cells intruded into the parenchyma of liver. On the 112th day, the ratio of cholangiocarcinoma was about 50%.
Changes of Trace Elements in Serum
The changes of trace element contents in serum are indicated in Table 1. During the process of the rat liver carcinogenesis, in the test group, the Cu content significantly increased in serum after day 56 (P<0.05 orP<0.01), while the content of Fe significantly decreased in different stages (P<0.05 orP<0.01). The content of Zn significantly decreased from day 28 (P<0.05 orP<0.01). The content of Ca significantly went up after day 56 (P<0.05 orP<0.01). For the content of Mg, it showed no significant change (P>0.05)
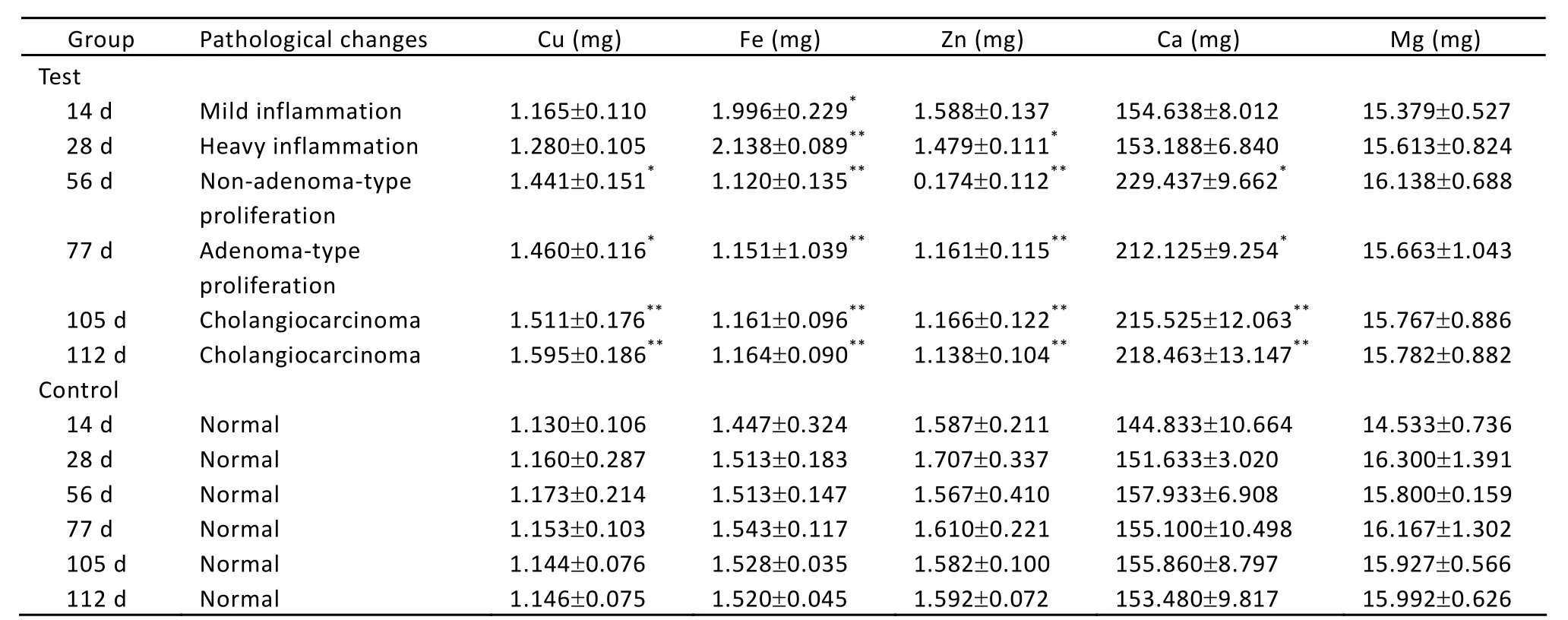
Table 1. Contents of Cu, Fe, Zn, Ca and Mg in serum of rats bearing liver cancer (ˉx±s)
Changes of Biochemical Components in Serum
Changes of biochemical components in serum are showed in Table 2. AFP and CEA of the test group indicated the same expression as the control group, while the content of SF is significantly lower than the control group only on day 112 (P<0.01). The content of T3 significantly went up in the first stage (P<0.05 orP<0.01), then reduced gradually to the normal level (day 112). T4 content was significantly higher than the control after day 28 (P<0.05 orP<0.01), the change trend of which was similar to that of T3, rising at the beginning of carcinogenesis (day 28) and then going down. The content of IGF-II was significantly higher than the control after day 28 (P<0.05 orP<0.01).
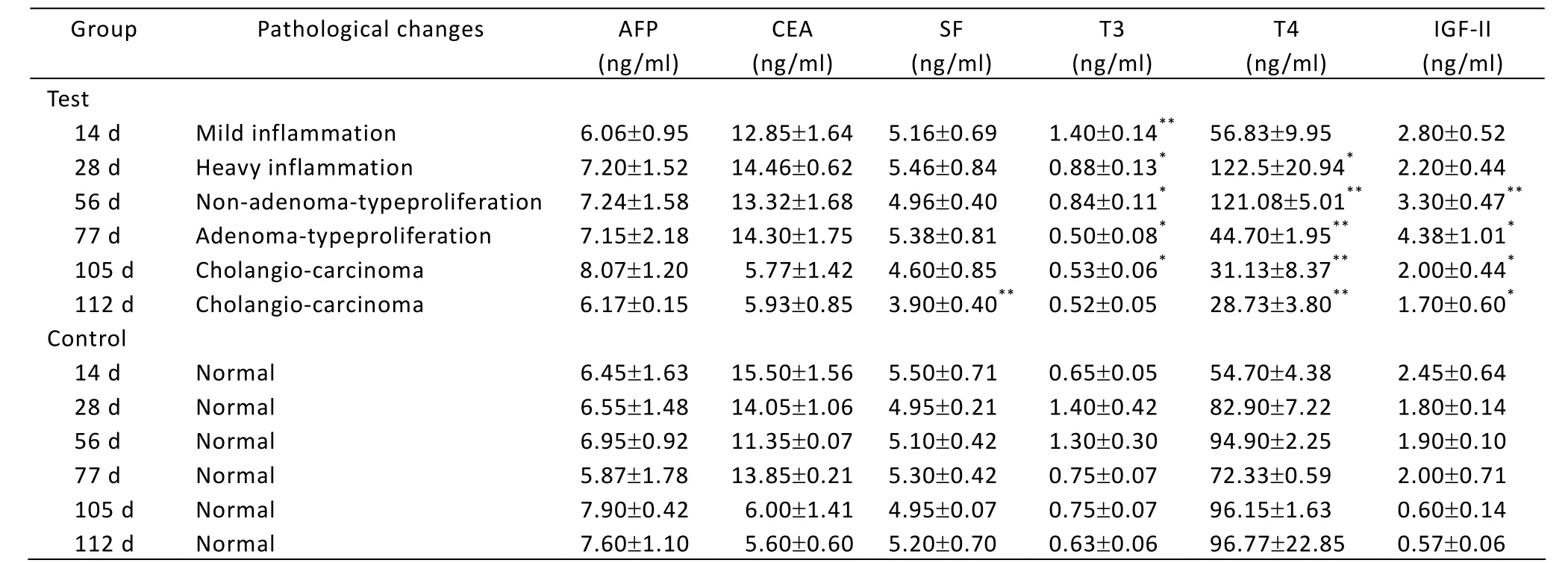
Table 2. Changes of AFP, CEA, SF, T3, T4 and IGF-II in serum of rats (ˉx±s)
DISCUSSION
According to the report from Kuo[2], Cu content in serum was elevated obviously in the malignant tumor patients. Han[3]also suggested that Cu plays an important role in the division and proliferation of tumor cells, and increases in serum in cancer patients. A. Al-Ebraheem[4]found that higher levels of Cu could decrease apoptosis and be an essential cofactor in angiogenesis during carcinogenesis and metastasis. In this study, when adenoma-type proliferation appeared in the experimental group, the content of Cu in serum was obviously higher than control (P<0.05) (Table 1). From the non-adenoma-type proliferation stage to the cholangiocarcinoma stage, the Cu content in serum was remarkably higher than the control (P<0.01). This result is coincident with that reported by Kuo[2]and A. Al-Ebraheem[4], and the elevation of Cu content in serum may be a potential marker which means the start of the malignant tumor pathological activity. On the other hand, the excessive Cu in the cell may propel carcinogenesis, resulted from the lipoperoxide and the biological damage caused by production of surplus free radical.
Compared with the control, the content of Zn decreased gradually along with the development of carcinogenesis, and significantly went down when the non-adenoma-type proliferation appeared (P<0.01) (Table 1). This is in accord with Nabarro[5]and A. Al-Ebraheem’s[6]reports about liver and kidney cancer. Zn is the essential element in the constructions of many proteins. Malignant tumor cells make use of more Zn due to the fast proliferation and metabolism, leading to its accumulation in liver or other organs and reduction in serum. The decreased Zn content in serum can influence thymus growth and the function of immune system, especially T cell function, reducing T cell number and causing imbalances of T cell subgroup, at last leading to damage of immunity. Therefore, the decrease of sensitivity of immunity cells to the pathological changes might become the potential hazard factor to propel carcinogenesis of liver cells.
Some previous reports showed that the content of Fe in serum was reduced compared with the control in throat cancer[7], but other researcher pointed out Fe content increased in thyroid cancer[8]. In this experiment, in mild inflammation, the temporary elevation of Fe content is possibly related to mutagen stress on rats. From heavy inflammation to cholangiocarcinoma, the content of Fe was significantly reduced compared with the control group (P<0.01), and this is consistent with the fact that there are more Fe metabolism acceptors in the surface of tumor cells than that of the normal cells[9]. More Fe was combined with the tumor cells. Therefore, the content of Fe decreased in serum of canceration rats. This result provides certain theory foundation for the Fe-eliminated therapy in the treatment of liver cancer.
Ca plays an important role in vital life activity, and is involved in many physiological processes such as cell contraction, glycogen metabolism, DNA synthesis, celldivision, chromosome movement and cell death. This research indicated that Ca content in serum tended to rise along with the extension of induced cancer and the aggravation of cell cancerization. This result is in accord with Wysolmerski’s suggestion[10]. In patients with breast cancer, the content of Ca is higher in serum before radiotherapy than after radiotherapy. In this study, at the beginning of inflammatory reaction, Ca content in serum did not show remarkable difference between the test group and the control group (P>0.05). From the adenoma-type-proliferation stage to the non-adenomatype-proliferation stage, the content of Ca was obviously higher in the test group (P<0.05). Once cholangiocarcinoma appeared, Ca content in serum remarkably increased compared with the control group (P<0.01). So these may be a close relationship between Ca content in serum and the development of liver cancer.
The content of Mg in serum was relative stable in the entire process of carcinogenesis and showed no remarkable difference between the two groups (P>0.05) (Table 1). This is consistent with Tan’s study[11]that the trace element Mg does not have relevance to the malignant tumor.
AFP, as a specific sign of tumor, could provide the important referential value for cancer diagnosis, histopathological classification and prognosis. Only when serum levels of AFP rise above 500 ng/ml, it is sufficiently specific for hepatocellular carcinoma (HCC)[12]. Marchesi, et al.[13]discovered the AFP content in tumor patient was remarkably higher than the normal tissue near carcinoma and the precancerous lesion tissue. Therefore, AFP has been used as a diagnostic sign of liver cancer in clinic. In this research, the content of AFP went up straightly from inflammatory reaction to cholangiocarcinoma (P>0.05), which is according with the results of Marchesi, et al[13].
CEA is an important tumor-associated antigen, and its overexpression has been used to identify or diagnose early colorectal, gastric, pancreatic, ovarian cancer and others[14]. Li, et al.[15]discovered that the overexpression of CEA in serum always existed in metastatic HCC, and not in primary HCC. In this research, when the rat liver tumor induced by DENA appeared, the CEA content in serum elevated slightly, but was not remarkably higher than the control (P>0.05). This is consistent with the research result of Li, et al[15]. This is because there appeared only primary not metastatic HCC in our experiment.
SF is associated with many statuses, including iron delivery, angiogenesis, inflammation, immunity, signaling and cancer[16]. Jacober, et al[17]discovered that many malignant tumor patients had extremely high content of SF. Currently, most researchers suggest that SF content can be used as a sensitive diagnostic marker of primary HCC. In this experiment, compared with the control, SF content did not show obvious change from the beginning of treatment with DENA to the non-adenoma-type-proliferation stage, only significantly decreased in cholangiocarcinoma stage (P<0.01).
Kotru[18]pointed out that, in normal condition, the SF content in serum was positively correlated with the Fe content in body. In this research, both Fe and SF contents were decreased from non-adenoma-type proliferation to cholangiocarcinoma. This conforms to the result reported by Kotru. It implies that the reduction of Fe and SF might be important factors in hepatocarcinogenesis. In the process of cancerization induced by DENA, the decrease of Fe content in serum induced the enhancement of SF catabolism in cells to release more Fe to meet the demand for Fe in the proliferation tissue. At last, it could promote the advance and formation of cancer.
With nodular proliferation in liver tissue, the contents of T3 and T4 in serum increased compared with the control (Table 2). It was suggested that T3 and T4 might take part in cell proliferation. Then the contents of T3 and T4 decreased from adenoma-type-proliferation to cholangiocarcinoma (P<0.05), this accorded with the report of Tjandra[19]. The reduction of T3 might be caused by metabolic disturbance of hepatocytes and the decrease of 5’-deiodinase activity under the liver lesion.
IGF-II is a kind of active mitogen, overexpressing in liver of embryo and newborn, and then going down rapidly after the birth. When the liver cancerization appears, IGF-II immediately recovers to express[20]. Nussbaum found that autocrine IGF-II overexpression promoted HCC motility, and activation of IGF-II/IGF-IR signaling was likely a progression switch selected by function that promoted liver tumor cell dissemination and aggressive tumor behavior[21]. This research indicated that the content of IGF-II increased in the process of the induced carcinoma, significantly went up at the adenoma-type-proliferation stage (P<0.05), and climbed up to top value at the cholangiocarcinoma stage in contrast with the control (P<0.01). It was demonstrated that the excessive expression of IGF-II was involved in the advance of HCC. Whether IGF-II can be used as a marker of hepatocyte differentiation and a standard of the auxiliary diagnosis of primary HCC needs to be further studied in the future.
REFERENCES
1. Gurusamy K. Trace element concentration in primary liver cancers-a systematic review. Biol Trace Elem Res 2007; 118:191-206.
2. Kuo HW, Chen SF, Wu CC, et al. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res 2002; 89:1-11.
3. Han CZ, Jing JX, Zhao XW, et al. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res 2003; 94:113-22.
4. A Al-Ebraheem, Mersov A, Gurusamy K, et al. Distribution of Ca, Fe, Cu and Zn in primary colorectal cancer and secondary colorectal liver metastases. Nuclear Instruments and Methods in Physics Research A 2010; 619:338-343.
5. Nabarro SS, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer 2007; 18:7-27.
6. Badiei K, Mostaghni K, Pourjafar M. Serum and tissue trace elements in Iranian camels. Comp Clin Pathol 2006; 15:58-61.
7. Serel TA, Turan T, Soyupek S, et al. Urine and serum free IGF-1 levels in patients with bladder cancer: a brief report. Urol Res2003; 31:58-61.
8. Al-Ebraheem A, Farquharson MJ, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl Radiat Isot 2009; 67: 470-4.
9. Taysi S, Akcay F, Uslu C, et al. Trace elements and some extracellular antioxidant protein levels in serum of patients with laryngeal cancer. Biol Trace Elem Res 2003; 91:11-8.
10. Wysolmerski J. Calcium handling by the lactating breast and relationship to calcium-related complications of breast cancer. J Mammary Gland Biol Neoplasia 2005; 10:101-3.
11. Tan M?, Y?lmaz C, Uygur MC, et al. Effects of combined androgen blockade on bone metabolism and density in men with locally advanced prostate cancer. Int Urol Nephrol 2002; 34:75-9.
12. Tan HT, Low J, Lim SG, et al. Serum autoantibodies as biomarkers for early cancer detection. FEBS J 2009; 276: 6880–904.
13. Marchesi MC, Conti MB, Pieramati C, et al. Assessment and behavior of Alphafetoprotein (AFP), Antigen Cancer15/3 (CA15/3), Carcinembryonal Antigen (CEA) in clinical oncology of the dog: preliminary study. Vet Res Commun 2007; 31:301-4.
14. Ladd J, Lu H, Taylor AD, et al. Direct detection of carcinoembryonic antigen autoantibodies in clinical human serum samples using a surface plasmon resonance sensor. Colloids SurfB: Biointerfaces 2009; 70:1-6.
15. Li N, Xiao B, Chen XB, et al. Relationship between expression of CEA, E-cadherin and liver metastasis in colorectal cancer. Chin J Clin Oncol 2008; 5:429-32.
16. Wang W, Knovich MA, Coffman LG, et al. Serum ferritin: Past, present and future. Biochim Biophys Acta 2010; 1800:760-76.
17. Jacober ML, Mamoni RL, Lima CS, et al. Anaemia in patients with cancer: role of inflammatory activity on iron metabolism and severity of anaemia. Med Oncol 2007; 24:323-9.
18. Kotru M, Rusia U, Sikka M. Evaluation of serum ferritin in screening for iron deficiency in tuberculosis. Ann Hematol 2004; 84:95-100.
19. Tjandra JJ, Reading DM, McLachlan SA, et al. Phase II clinical trial of preoperative combined chemoradiation for T3 and T4 resectable rectal cancer preliminary results. Dis Colon Rectum 2001; 44:1113-22.
20. Peters GP, Gonqoll SG, Langner C, et al. IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and predictive markers in colorectal-cancer. Virchows Arch 2003; 443:139-45.
21. Nussbaum T, Samarin J, Ehemann V, et al. Autocrine insulin-like growth factor-II stimulation of tumor cell migration is a progression step in human hepatocarcinogenesis. Hepatology 2008; 48:146-56.
10.1007/s11670-011-0301-2
2010-12-09; Accepted: 2011-05-26
This work was supported by the grant from the Zoology Key Subject of Henan Province.
*Corresponding author.
E-mail: hxzh5571@sina.com.cn
?Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
 Chinese Journal of Cancer Research2011年4期
Chinese Journal of Cancer Research2011年4期
- Chinese Journal of Cancer Research的其它文章
- Prognostic Value of Promoter Hypermethylation of Retinoic Acid Receptor Beta (RARB) and CDKN2 (p16/MTS1) in Prostate Cancer
- Expression and Distribution Characteristics of Human Ortholog of Mammalian Enabled (hMena) in Glioma
- Mast Cells in Adjacent Normal Colon Mucosa rather than Those in Invasive Margin are Related to Progression of Colon Cancer
- Wild-Type KRAS and BRAF Could Predict Response to Cetuximab in Chinese Colorectal Cancer Patients
- Dosimetry Comparison between Volumetric Modulated Arc Therapy with RapidArc and Fixed Field Dynamic IMRT for Local-Regionally Advanced Nasopharyngeal Carcinoma
- Hepatocellular Tumors: Immunohistochemical Analyses for Classification and Prognostication
