Pure Mucinous Carcinoma of the Breast:a Clinicopathologic Analysis with 56 Patients
Li Peng ,Qiang Sun *,Zhi-yong Liang ,Yi-dong Zhou ,Feng Mao ,and Jing-hong Guan
1Department of Breast Surgery,2Department of Pathology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
MUCINOUS carcinoma of the breast was first described in 1826,but it was and remained one of the uncommon pathological categories in breast cancer.1,2It accounts for 1-4% of all breast cancers and is associated with a better prognosis than infiltrative ductal carcinoma.3,4Mucinous carcinoma of the breast is further divided into pure and mixed subgroups according to the quantification of tumor cellularity.The pure type consists exclusively of tumor tissue with extracellular mucin production,while the mixed type also contains other components without mucin.5,6Pure mucinous carcinoma (PMC) of the breast is rare and considered to display indolent behavior.In the present study,we retrospectively reviewed the medical records of patients with pathologically proved PMC of the breast in our hospital,in order to assess the recent trends and prognostic features in the treatment of this disease.
PATIENTS AND METHODS
Patients
A retrospective review was performed with patients presenting with PMC of the breast between December 1982 and June 2008 in Peking Union Medical College Hospital.Only those with a pathological diagnosis of PMC of the breast were included.We reviewed the patients’ medical records and evaluated the clinical characteristics,surgical treatment,tumor size and pathological grade,presence of hormone receptor,lymph node status,and the use of adjuvant chemotherapy,endocrine therapy,or radiotherapy.
Statistical analysis
Fisher’s exact test was used to compare tumor characteristics.P<0.05 was considered statistically significant.All statistical analyses were performed using the SSPS15.0 statistical software.
RESULTS
We reviewed the medical records of 3 386 women with breast cancers diagnosed between December 1982 and June 2008 in our hospital.Among those patients,a total of 56 (1.65%) were recognized as having PMC.Patient information and tumor characteristics are listed in Table 1.
The mean age of the included patients was 53.7±13.3 years (range,26-82 years).The laterality of the lesions was right-sided in 30 (53.6%) patients and left-sided in 26(46.4%).Twenty-four patients underwent fine needle aspiration before operation and 10 (41.6%) of them had positive results.
The mean tumor size was 2.6 cm in diameter (range,0.3-6.5 cm).Forty-eight patients underwent modified radical mastectomy and 1 patient had mastectomy with immediate reconstruction (49/56,88%);only 3 patients received breast-conserving therapy with adjuvant radiotherapy (3/56,5%);4 patients underwent lumpectomy without radiotherapy (4/56,7%).Of the last group,1 underwent lumpectomy with axillary node dissection and the other 3 just received lumpectomy.Fifty-three patients(95%) received axillary node dissection,of which 7 (13%)had positive results.The remaining 3 patients did not have axillary node dissection because of advanced age or significant comorbidities.
Forty-four patients had complete record of pathological stage.Histological section of carcinoma breast shows clusters of malignant cells floating in mucus pool (Fig.1A).The majority of those 44 patients (40,90.9%) presented with early stage disease,of which 15 (34.1%) were at stage I and 25 (56.8%) were at stage II.Only 4 (9.1%)patients were diagnosed at stage III and none at stage IV.
The mean diameter was taken as the cutoff point.The tumor size was over the average level in 21 patients,among whom 4 showed lymph node metastasis;the tumor size was below the average level in 23 patients,in which 2 had lymph node metastasis.However,no significant association was found between tumor size and lymph node metastasis (P>0.05) (Table 2).

Table 1.Patient information and tumor characteristics (n=56)
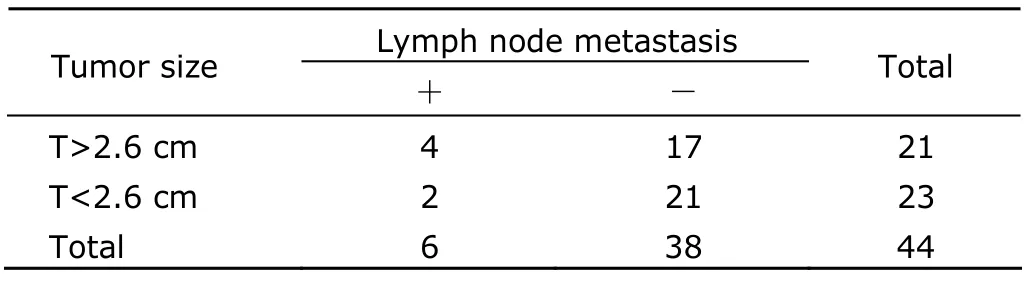
Table 2.Tumor size and lymph node metastasis
Thirty-seven patients had immunohistochemistry test for estrogen and progesterone receptors.The results were positive in 64.9% (24/37) and 62.2% (23/37) of tumors,respectively.Twenty-nine patients had immunohistochemistry test for estrogen and HER-2 receptors,in which only 2 (6.9%) had positive results in HER-2 receptor detection (Fig.1).
Mean follow-up time was 39 (range,1-240) months.Three patients had distant recurrence.No known deaths related to breast cancer were identified in the studied patients.Among the four groups receiving different systemic therapies,no significant difference was found with Fisher’s exact test in terms of age and tumor size (P>0.05) (Table 3).
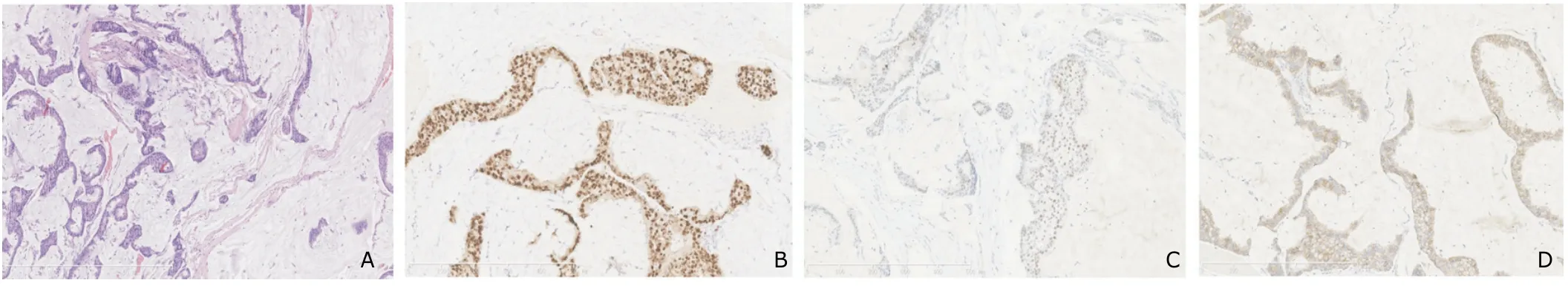
Figure 1.Pathological findings of pure mucinous carcinoma.
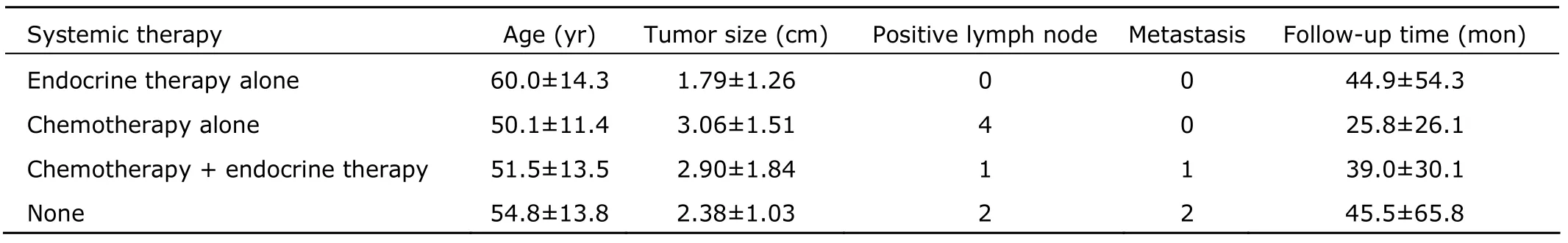
Table 3.Systemic therapy performed in the patients§
DISCUSSION
PMC of the breast is rare.Altogether 3 386 women were diagnosed as breast cancers in our hospital between December 1982 and June 2008,and 56 of them had PMC(1.65%),which falls just within the range of 1% to 4%commonly reported in literature.6,7Previous studies showed that PMC of the breast was often found in elderly women and only 1% of PMC patients were less than 35 years.8,9But in the present study,4 patients (7.1%) were under 35 years and 8 (14.2%) were under 40 years.The youngest patient was 26 years old at diagnosis.Although the sample size was small in this study,it might suggest an increase in incidence of PMC among young population.
The size of pure mucinous breast cancer at diagnosis varies widely,and the mean size in the present study was 2.6 cm.The relatively slow growth of pure mucinous breast cancer may allow diagnosis at a small size.6The prognostic significance of tumor size is an especially interesting point with PMC of the breast.The statistical analysis in this study,however,did not find significant association between size and axillary lymph node metastasis.This finding should not be surprising because mucin constituted a majority of the tumor volume.Therefore,the tumor could grow to a large size without impacting survival of the patient.10-12
Of the 44 patients with complete record of pathological stage,the majority (40/44,91%) presented at stage I or II,supporting the previous hypothesis that this disease displays indolent behavior.Modified radical mastectomy was the most frequently used surgical operation (49,87.5%) in this study.Just as tumor size,the extent of surgery also may not significantly influence survival.Adjuvant radiotherapy and endocrine therapy after breast-conserving therapy for mucinous cancer has become common.
Recent literature reported a high percentage of hormone receptor expression,a high rate of positive results for estrogen and progesterone receptors.10The present study is roughly in agreement with that statement.It suggests that most patients could take endocrine therapy after surgery.
The prognosis of PMC of the breast is favorable,with good overall and disease-specific survival.10A large retrospective comparative study showed that PMC of the breast had less aggressive behavior compared to infiltrative ductal carcinoma,and the favorable outcome still remained after 20 years.6In this study the mean follow-up was 39 months and no known deaths related to breast cancer were identified in the studied patients.Three patients had distant recurrence at a mean recurrence time of 48 months,again supporting the hypothesis that pure mucinous cancers are indolent,which take longer time to recur than other invasive cancers of the breast.It may explain the fact that we have not yet observed many late recurrences.
In conclusion,PMC of the breast has a favorable prognosis.Tumor size does not appear to significantly impact survival,perhaps because the large volume of mucin may lead to overestimation of tumor burden.
1.Geschickter CF.Gelatinous mammary cancer.Ann Surg 1938;108:321-46.
2.Li CI,Uribe DJ,Daling JR.Clinical characteristics of different histologic types of breast cancer.Br J Cancer 2005;93:1046-52.
3.Diab SG,Clark GM,Osborne CK,et al.Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas.J Clin Oncol 1999;17:1442-8.
4.Barkley CR,Ligibel JA,Wong JS,et al.Mucinous breast carcinoma:a large contemporary series.Am J Surg 2008;196:549-51.
5.World Health Organization (1982) Histological typing of breast tumors.Tumori 1982;68:181-98.
6.Di Saverio S,Gutierrez J,Avisar E.A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma.Breast Cancer Res Treat 2008;111:541-7.
7.Diab SG,Clark GM,Osborne CK,et al.Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas.J Clin Oncol 1999;17:1442-8.
8.Rosen PP,Lesser ML,Kinne DW.Breast carcinoma at extremes of age:comparison of patients younger than 35 years and older than 75 years.J Surg Oncol 1985;28:90-6.
9.Toikkanen S,Kujari H.Pure and mixed mucinous carcinomas of the breast:a clinicopathologic analysis of 61 cases with long-term follow-up.Hum Pathol 1985;20:758-64.
10.Komenaka IK,El-Tamer MB,Troxel A,et al.Pure mucinous carcinoma of the breast.Am J Surg 2004;187:528-32.
11.Park S,Koo J,Kim JH,et al.Clinicopathological characteristics of mucinous carcinoma of the breast in Korea:comparison with invasive ductal carcinoma-not otherwise specified.J Korean Med Sci 2010;25:361-8.
12.Monzawa SC,Yokokawa M,Sakuma T,et al.Mucinous carcinoma of the breast:MRI features of pure and mixed forms with histopathologic correlation.AJR Am J Roentgenol 2009;192:W125-31.
 Chinese Medical Sciences Journal2010年2期
Chinese Medical Sciences Journal2010年2期
- Chinese Medical Sciences Journal的其它文章
- Liquid Chromatography-tandem Mass Spectrometry for Analysis of Acylcarnitines in Dried Blood Specimens Collected at Autopsy from Neonatal Intensive Care Unit
- D-Tyr-tRNATyr Deacylase,a New Role in Alzheimer’sassociated Disease in SAMP8 Mice△
- Antagomir Dependent MicroRNA-205 Reduction Enhances Adhesion Ability of Human Corneal Epithelial Keratinocytes△
- Nectin-like Molecule 1 Inhibits the Migration and Invasion of U251 Glioma Cells by Regulating the Expression of An Extracellular Matrix Protein Osteopontin△
- A Second Protein Marker of Caveolae:Caveolin-2△
- Role of Acetylated p53 in Regulating the Expression of map2 in Retinoic Acid-induced P19 Cells△
