Nectin-like Molecule 1 Inhibits the Migration and Invasion of U251 Glioma Cells by Regulating the Expression of An Extracellular Matrix Protein Osteopontin△
Bin Yin,Ke-han Li,Tai An,Tao Chen,and Xiao-zhong Peng*
National Laboratory of Medical Molecular Biology,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
NECTIN-like molecule 1 (NECL1) is a member of the immunoglobulin superfamily,specifically expressed in nervous system.1,2Members of this superfamily have the same conservative structure∶a N-terminus consisting of three extracellular Ig-like domains,a single transmembrane domain,and a short C-terminus intracellular region.Extracellular domains of NECL1 could form homo-or heterodimer by itself or with other members of the family.1,3The short C-terminus contains two relatively conservative motifs– FERM and PDZ.FERM motif is the binding motif of protein 4.1,while the PDZ binding motif can interact with other proteins containing PDZ domain.2,4Expression of NECL1 focuses in neuronal cell bodies in a variety of brain regions,including the cerebellum,cerebral cortex,and hippocampus.2It is reported that NECL1 is lost in 12 different glioma cell lines,and that the lose of NECL1 in human glioma cell lines and tumor tissues could be epigenetically caused by histone deacetylation.5,6NECL1 could inhibit the proliferation of T98G cells by inducing cell apoptosis,7and inhibit migration and invasion of U251 glioma cells.8Those effects of NECL1 on tumor cells could explain its limiting the tumor growth in xenograft model.6However,the molecular mechanism of this inhibiting effect on tumorigenesis remains unclear.
In this research,we studied the migration of U251 glioma cells,analyzed their differential gene expression profile after NECL1 restoration,and probed into the relation between NECL1 and extracellular matrix proteins in the migration and invasion of U251 cells.
MATERIALS AND METHODS
Cell line and culture conditions
Human glioma cell line U251 was purchased from Cell Center of Peking Union Medical College.The cells were cultured in Minimum Essential Medium Eagle’s with Earle’s Balanced Salts (MEM/EBSS) supplemented with 10% fetal bovine serum at 37°C in 5% CO2.
Recombinant adenovirus package and infection
Empty adenovirus (Ad) and adeno-NECL1 (Ad-NECL1)(obtained from National Human Genome Center,Beijing,China) at a concentration of 108pfu/mL were used to infect U251 cells incubated in serum-free MEM/EBSS.Cells were cultured after adenovirus infection and shaken gently every 15 minutes.The cells were then maintained in complete medium after 2-hour incubation.Fluorescence was observable 24 hours after that.
Wound healing assay
The cell supernatant was collected after infection with Ad-NECL1 or Ad,8in which U251 cells were cultured.At the same time,a scratch was drawn in the center of the well.The distance between the cells bordering the scratchwas measured every 12 hours within 36 hours.
Transwell assay
Transwell,with or without 300 μg/mL matrigel pre-coating at 37°C for 3 hours,was incubated in serum-free MEM/EBSS for 30 minutes.U251 cells were harvested by centrifugation(2 000 ×g)for 5 minutes,then resuspended in serum-free medium at a density of 106cells/mL.Altogether 100 μL of the cell suspension was put onto the upper layer of transwell,while the same kind of cell supernatant as used in wound healing assay was collected and put onto the lower layer of transwell.After 24 hours,the cells were fixed by formalin and stained by 4’,6-diamidino-2-phenylindole (DAPI).
Microarray screening
Total cellular RNA was extracted from U251 cells infected with Ad-NECL1 or Ad,using the Trizol Reagent (Invitrogen,Carlsbad,CA,USA) according to the manufacturer’s instructions.Microarray analysis was performed by Phalanx Biotech Group,Taiwan,China.A fluorescence value higher than 100 and a coefficient of variation (CV) lower than 0.2(CV=SD/mean) were considered positive hybridization signal.While an R value over 2 or under 0.5 (|log2R|≥1)was considered to indicate differential expression.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA was the same as that used in the microarray analysis.Oligo (dT) primers (Beijing TransGen Biotech,China) were used for reverse transcription.Detailed methods have been described in the manufacturer's instructions.RT-PCR exponential phase was determined on 25-35 cycles to allow semi-quantitative comparisons among cDNAs developed from identical reactions,performed in triplicate independently.
Protein extraction and Western blot
U251 cells infected with Ad-NECL1 or Ad were extracted with cold lysis buffer (50 mmol/L Tris-HCl pH 7.4,150 mmol/L NaCl,1 mmol/L ethylene diamine tetraacetic acid pH 8.0,1% NP-40,10% glycerol,25 mmol/L NaF,1 mol/L NaVO3,100 μg/mL dithiothreitol,100 μg/mL phenylmethanesulfonyl fluoride,1 μg/mL aprotinin,1 μg/mL leupeptin,and 1 μg/mL pepstatin).After being lyzed on ice for 30 minutes,the lysate and supernatant were centrifuged at 12 000 ×gfor 20 minutes,and the supernatant was collected for Western blot.Protein concentration was determined using the Bradford method.We separated the lysate with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred the gel onto nitrocellulose membrane.The protein was probed with rabbit anti-Human osteopontin(OPN) (1∶100,Beijing Aviva Systems Biology,China) and horseradish peroxides (HRP)-conjugated goat anti-rabbit antibody (1∶1000,Zhong Shan Gold Bridge Biotechnology,Beijing,China),followed by visualization with ECL.
Immunohistochemical analysis of primary glioma tissues
Human normal brain and glioma tissues were obtained from the Department of Neurosurgery of Beijing Tiantan Hospital,classified into normal,Grade II,Grade III,and Grade IV according to the third edition of the histological typing of tumors of the central nervous system (WHO,2000).Written informed consents were obtained from all patients involved before the surgery.We performed immunohistochemical assay as previously described6to examine the expression pattern of OPN in those tissues.Briefly,the 15 μm formalin-fixed,frozen tissue sections were incubated with anti-Human OPN antibody (1∶100,Aviva) at 4°C for 16 hours,followed by incubation in HRP-labeled secondary antibodies for 2 hours at room temperature.3,3’-diaminobenzidine (DAB) was used as the chromogen.
Statistical analysis
Numerical data obtained in each experiment were analyzed with Student’sttest.APvalue lower than 0.05 was considered statistically significant.
RESULTS
Wound healing and transwell assays
Since many secreted proteins such as the extracellular matrix proteins have been reported to be involved in migration and invasion of tumor,cultured supernatant from the U251/Ad-NECL1 and U251/Ad cells was used for wound healing and transwell assays to further verify that secreted proteins are involved in NECL1’s inhibiting the migration and invasion of tumor cells.The healing speed of the scratch in U251 cells cultured with supernatant from U251/Ad was higher than that in U251 cells cultured with supernatant from U251/Ad-NECL1 (P<0.05) (Fig.1A).Transwell assay also revealed that U251 cells cultured in supernatant from U251/Ad migrated faster than U251 cells cultured in supernatant from U251/Ad-NECL1 (P<0.0001)(Fig.1B).Those findings implied that some secreted proteins in the culture supernatant did participate in NECL1’s inhibiting effect on the migration and invasion of U251 cells.
DNA microarray results
To further explore how NECL1 exerts its function,microarray was used to screen the genes whose expression changed after the restoration of NECL1 in U251 glioma cells.A total of 195 differentially expressed genes were identified,in which 175 were down-regulated,and 20 up-regulated.These genes were cluster analyzed according to their different functions such as involved in signaling pathway and translation,or encoding extracellular matrix proteins (Fig.2).Among them,there are 9 extracellular matrix proteins down-regulated.
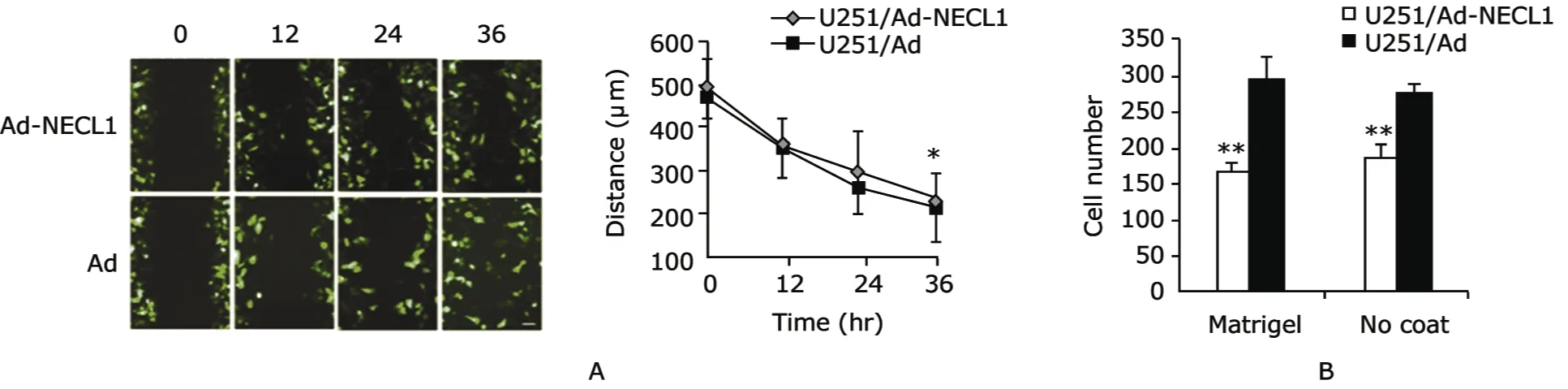
Figure 1.U251 cell migration in wound healing and transwell assays after incubated with the cell supernatant from U251 infected with adeno-nectin-like molecule 1 (Ad-NECL1) or empty adenovirus (Ad).
Semi-quantitative RT-PCR and Western blot results
Based on the microarray data,secreted phosphoprotein 1 OPN was selected from the 9 down-regulated extracellular matrix proteins for further verification because of its marked reduction (the ratio of fluorescence value Ad-NECL1/Ad=0.191).Semi-quantitative RT-PCR results confirmed that the transcript of Opn gene was decreased in the U251 glioma cells infected with Ad-NECL1 (Fig.3A).The expression of OPN protein was also decreased in supernatant and cell lysate from Ad-NECL1-infected U251 cells (Fig.3B).
Immunohistochemical assay results
OPN expression was found increased along with the increasing malignant grade,demonstrated by the intensifying positive signal in the primary glioma tissues compared with the normal brain tissues (Fig.4).
DISCUSSION
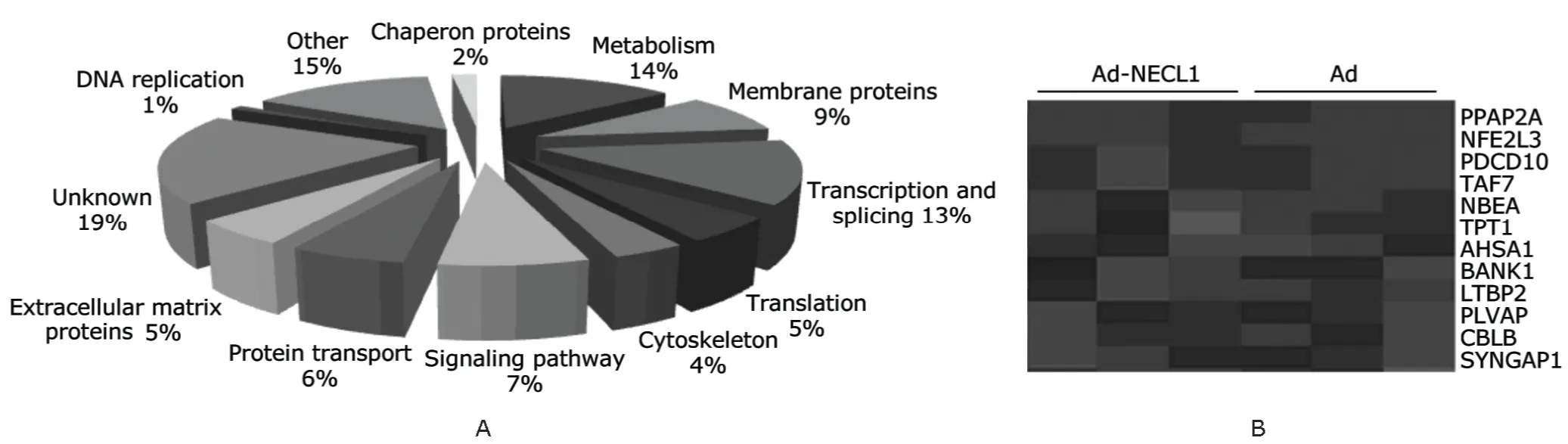
Figure 2.Gene expression profile after the restoration of NECL1 in U251 glioma cell lines detected by microarray.
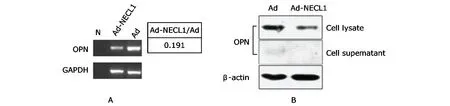
Figure 3.Osteopontin (OPN) expression detected by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot.
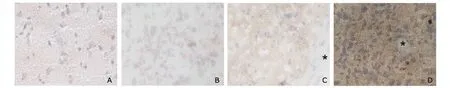
Figure 4.OPN expression in human normal brain and glioma tissues.×200
In conclusion,we confirmed that NECL1 could affect the migration and invasion characterization of glioma cell line.A link could be established between NECL1 and OPN based on the following evidence.There were obviously some secreted proteins in the surpernatants participating in the process of NECL1 inhibiting the migration and invasion of U251 cells.In the microarray analysis and following assays,we observed that OPN expression was up-regulated in glioma cell line and glioma tissues,and restoration of NECL1 was related to the decreased expression of this secreted protein.
Expressed in a variety of tissues,9osteopontin is a secreted phosphoprotein that has been reported playing an important role in tumor metastasis,10-12thus can be used as a biomarker for advanced disease and potential therapeutic target in the regulation of cancer metastasis.13Exploration into the molecular mechanism of OPN in tumorigenesis implied that its N-terminal can interact with integrin,and C-terminal can interact with CD44 to promote the migration of tumor cells.14OPN has also been found expressed at a high level in glioma.15The transcriptional regulation of OPN in the U-251MG and U-87MG human malignant astrocytoma cell lines reveals that a proximal promoter element (-24 to -94 relative to the transcription initiation site) is essential for maintaining a high level of OPN expression in the tumor cells,and that transcription factors Sp1,the glucocorticoid receptor,and the E-box-binding factors,Myc and OCT-1,participate in forming DNA-protein complexes.14
It could be interesting to find out how NECL1-related signaling pathway is involved in OPN expression in glioma,also to use OPN as a biomarker and a potential therapeutic target in glioma.
1.Kakunaga S,Ikeda W,Itoh S,et al.Nectin-like molecule-1/TSLL1/SynCAM3∶a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals,axons and glia cell processes.J Cell Sci 2005;118∶1267-77.
2.Zhou Y,Du G,Hu X,et al.Nectin-like molecule 1 is a protein 4.1N associated protein and recruits protein 4.1N from cytoplasm to the plasma membrane.Biochim Biophys Acta 2005;1669∶142-54.
3.Dong X,Xu F,Gong Y,et al.Crystal structure of the V domain of human Nectin-like molecule-1/Syncam3/Tsll1/Igsf4b,a neural tissue-specific immunoglobulin-like cellcell adhesion molecule.J Biol Chem 2006;281∶10610-7.
4.Ogita H,Takai Y.Nectins and nectin-like molecules∶roles in cell adhesion,polarization,movement,and proliferation.IUBMB Life 2006;58∶334-43.
5.Fukuhara H,Kuramochi M,Nobukuni T,et al.Isolation of the TSLL1 and TSLL2 genes,members of the tumor suppressor TSLC1 gene family encoding transmembrane proteins.Oncogene 2001;20∶5401-7.
6.Gao J,Chen T,Liu J,et al.Loss of NECL1,a novel tumor suppressor,can be restored in glioma by HDAC inhibitor-Trichostatin A through Sp1 binding site.Glia 2009;57∶989-99.
7.Gao J,Chen T,Yin B,et al.Effect of NECL1 on the proliferation of T98G glioma cell line.Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2008;30∶280-3.
8.Yin B,Chen T,Gao J,et al.Neural adhesion molecule NECL1 inhibits migration,invasion,and potentially induces differentiation of glioma cell.Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2009;31∶669-73.
9.Prince CW,Oosawa T,Butler WT,et al.Isolation,characterization,and biosynthesis of a phosphorylated glycoprotein from rat bone.J Biol Chem 1987;262∶2900-7.
10.Wai PY,Kuo PC.Osteopontin∶regulation in tumor metastasis.Cancer Metastasis Rev 2008;27∶103-18.
11.Shevde LA,Das S,Clark DW,et al.Osteopontin∶an effector and an effect of tumor metastasis.Curr Mol Med 2010;10∶71-81.
12.Jan HJ,Lee CC,Shih YL,et al.Osteopontin regulates human glioma cell invasiveness and tumor growth in mice.Neuro Oncol 2010;12∶58-70.
13.Agarwal D,Chen T,Irby R,et al.Osteopontin identified as lead marker of colon cancer progression,using pooled sample expression profiling.J Natl Cancer Inst2002;94∶513-21.
14.Wang D,Yamamoto S,Hijiya N,et al.Transcriptional regulation of the human osteopontin promoter∶functional analysis and DNA-protein interactions.Oncogene 2000;19∶5801-9.
15.Lamour V,Le Mercier M,Lefranc F,et al.Selective osteopontin knockdown exerts anti-tumoral activity in a human glioblastoma model.Int J Cancer 2010;126∶1797-805.
 Chinese Medical Sciences Journal2010年2期
Chinese Medical Sciences Journal2010年2期
- Chinese Medical Sciences Journal的其它文章
- Pure Mucinous Carcinoma of the Breast:a Clinicopathologic Analysis with 56 Patients
- Liquid Chromatography-tandem Mass Spectrometry for Analysis of Acylcarnitines in Dried Blood Specimens Collected at Autopsy from Neonatal Intensive Care Unit
- D-Tyr-tRNATyr Deacylase,a New Role in Alzheimer’sassociated Disease in SAMP8 Mice△
- Antagomir Dependent MicroRNA-205 Reduction Enhances Adhesion Ability of Human Corneal Epithelial Keratinocytes△
- A Second Protein Marker of Caveolae:Caveolin-2△
- Role of Acetylated p53 in Regulating the Expression of map2 in Retinoic Acid-induced P19 Cells△
