Chinese Guidelines for the Diagnosis and Management of Atrial Fibrillation
Chang-Sheng MA,Shu-Lin WU,Shao-Wen LIU,Ya-Ling HAN;Chinese Society of Cardiology,Chinese Medical Association ;Heart Rhythm Committee of Chinese Society of Biomedical Engineering
ABSTRACT Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia,significantly impacting patients’ quality of life and increasing the risk of death,stroke,heart failure,and dementia.Over the past two decades,there have been significant breakthroughs in AF risk prediction and screening,stroke prevention,rhythm control,catheter ablation,and integrated management.During this period,the scale,quality,and experience of AF management in China have greatly improved,providing a solid foundation for the development of guidelines for the diagnosis and management of AF.To further promote standardized AF management,and apply new technologies and concepts to clinical practice in a timely and comprehensive manner,the Chinese Society of Cardiology of the Chinese Medical Association and the Heart Rhythm Committee of the Chinese Society of Biomedical Engineering have jointly developed the Chinese Guidelines for the Diagnosis and Management of Atrial Fibrillation.The guidelines have comprehensively elaborated on various aspects of AF management and proposed the CHA2DS2-VASc-60 stroke risk score based on the characteristics of AF in the Asian population.The guidelines have also reevaluated the clinical application of AF screening,emphasized the significance of early rhythm control,and highlighted the central role of catheter ablation in rhythm control.
1.INTRODUCTION
Atrial fibrillation (AF) is the most commonly sustained cardiac arrhythmia that significantly increases the risk of death,stroke,heart failure,cognitive impairment,and dementia[1-3]and severely impacts patients’ quality of life.The prevalence of AF increases with age,[4]and as population aging advances,AF will continue to impose a heavy burden on society and healthcare systems.Over the past two decades,there have been breakthroughs in the fields of AF risk prediction and screening,stroke prevention,rhythm control,catheter ablation,and integrated management.The use of non-vitamin-K-antagonist oral anticoagulants (NOACs) has completely changed the landscape of anticoagulant therapy,significantly increasing the rate of anticoagulation in the AF population and continuously reducing the risk of stroke.[5]The new-generation oral anticoagulants,Factor Ⅺ inhibitors,promise to prevent the risk of thromboembolism with a lower risk of bleeding,which may result in a new revolution in AF anticoagulation.[6]Catheter ablation has gradually become the first-line treatment for rhythm control in AF,which can reduce AF episodes,improve quality of life,delay the progression from paroxysmal to persistent AF,[7,8]and improve the prognosis of AF patients with concomitant heart failure.[9,10]For patients diagnosed with AF within one year,rhythm control is superior to rate control in improving prognosis.[11]Advances in devices and techniques have greatly reduced the difficulty and complication rates of left atrial appendage closure.[12]New evidence from evidence-based medicine continues to emerge,and new technologies and concepts such as wearable devices,telemedicine,and artificial intelligence are bringing about significant changes in AF management.These advancements provide a solid basis for the development of guidelines for the diagnosis and management of AF.
The scale,quality,and experience of atrial fibrillation management in China have greatly improved in recent years.To promote the timely and comprehensive application of new technologies and concepts of AF management in clinical practice and improve the quality of life and prognosis of AF patients,the Chinese Society of Cardiology of Chinese Medical Association and the Heart Rhythm Committee of Chinese Society of Biomedical Engineering jointly developed theChinese Guidelines for Diagnosis and Management of Atrial Fibrillation(hereinafter referred to as the “Guidelines”).
The Guidelines adopt the internationally accepted wording for the classification of recommendations [Table 1] and levels of evidence [Table 2].[13]
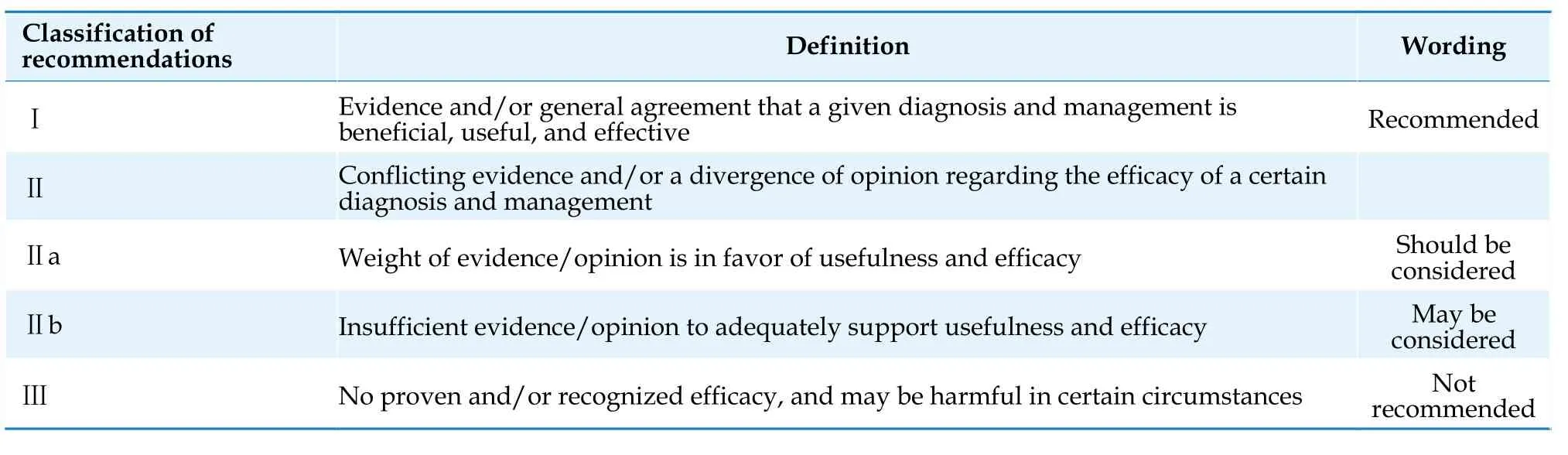
Table 1 Classification of recommendations.

Table 2 Level of evidence.
2.EPIDEMIOLOGY AND COMPLICATIONS OF AF
Several large-scale epidemiological surveys have shown the prevalence of AF in China.The prevalence of AF among individuals aged 35 to 85 years was 0.61% in 2003,[14]and 0.71% among individuals aged ≥ 35 years between 2012 and 2015.[15]Between 2014 and 2016,the prevalence of AF among individuals aged ≥ 45 years in China was 1.8% (1.9% for male and 1.7% for female patients).The prevalence of AF increased with age,and among individuals aged ≥75 years,the prevalence was 5.4% for male and 4.9%for female patients.[4]Based on the prevalence of AF in this study and the data from the seventh national census in 2020,it is estimated that there are approximately 12 million AF patients in China.However,given that approximately one-third of the patients are unaware of their condition and the underdiagnosis of paroxysmal AF,the actual number of AF patients in China is likely higher than this estimated figure.[4]
The risk of death for patients with AF is 1.5-1.9 times higher than that of patients without AF.[16]This may be related to increased risk of thromboembolism,heart failure,and the synergistic effect of comorbidities.The incidence of stroke,transient ischemic attack,and systemic embolism in AF patients who have not received anticoagulant therapy is approximately 34.2 per 1,000 person-years,[17]which is 3-5 times higher than that of individuals without AF.[2]AF-related stroke is often more severe,with higher rates of disability,mortality,and recurrence than non-AF-related stroke.[18]Approximately 20%-30%AF patients have concomitant heart failure,which may be related to AF with rapid ventricular rate,atrioventricular systolic dyssynchrony,ventricular strain dyssynchrony,and AF-related cardiomyopathy.[19,20]The incidence of dementia in AF patients is approximately 4.1% per year,which is 1.5 times higher than that of individuals without AF,[21]and this may be related to mechanisms such as stroke,intracranial hemorrhage,and cerebral hypoperfusion.[3]More than 60% AF patients present with symptoms of varying degrees,with 16.5% experiencing severe or disabling symptoms.[22-25]The hospitalization rate for AF patients is high,reaching 43.7 admissions per 100 person-years,with cardiovascular hospitalizations(26.3 admissions per 100 person-years) being more common than non-cardiovascular hospitalizations(15.7 admissions per 100 person-years).[26]
3.CLINICAL EVALUATION OF AF
3.1 Etiology of AF
The pathogenesis of AF is complex,and multiple factors can increase the susceptibility to AF and promote its occurrence and maintenance.These factors include aging;primary diseases (including cardiovascular diseases such as hypertension,valvular heart disease,coronary artery disease,congenital heart disease,and cardiomyopathy,as well as non-cardiovascular diseases such as endocrine disorders(e.g.,hyperthyroidism);respiratory diseases (sleep apnea syndrome,chronic obstructive pulmonary disease);autoimmune diseases;tumors;unhealthy lifestyle (e.g.,overweight/obesity,alcohol consumption,smoking,excessive/inadequate physical activity);and genetic factors.Additionally,severe illness such as severe infection and surgical procedures can increase the risk of AF.Identifying and correcting reversible factors that contribute to AF episodes and promoting a healthy lifestyle can prevent a significant number of AF cases caused by reversible factors.Therefore,AF is largely a preventable disease.[27]
3.2 Diagnosis and Classification of AF
AF can be diagnosed when a single-lead electrocardiogram (ECG) (≥ 30 s) or a 12-lead ECG (≥ 10 s)shows the disappearance of P waves,which are replaced with fibrillation waves (f waves) with irregular amplitude,morphology,and duration,as well as absolute irregularity in the RR intervals.
According to the duration of AF episodes,the difficulty in the conversion of AF and maintenance of sinus rhythm,and the choice of treatment strategies,AF can be classified into paroxysmal AF,persistent AF,long-standing persistent AF,and permanent AF.The specific definitions can be found in Table 3.[28]
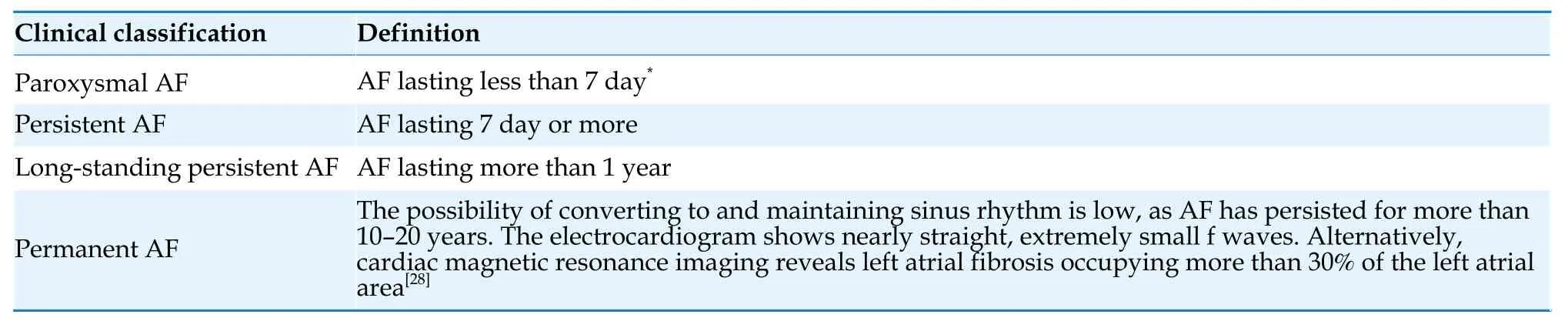
Table 3 Classification of AF.
3.3 Clinical Manifestations of AF
3.3.1 Symptoms and clinical history
The most common symptoms of AF are palpitations,decreased exercise tolerance,and chest discomfort.Some patients may also experience dizziness,anxiety,and increased urine output-due to increased secretion of atrial natriuretic peptide.The severity of AF symptoms varies greatly among individuals,and some patients may gradually tolerate the symptoms owing to their non-specific or mild symptoms.Approximately,25% patients self-report being asymptomatic.[25]Complications such as thromboembolism or heart failure can also be the initial manifestations of AF.Unstable hemodynamics caused by AF onset are often associated with structural heart disease and impaired cardiac function and can also present when AF transitions to atrial flutter or when it is accompanied by preexcitation syndrome,leading to extremely rapid ventricular rates.Syncope in AF patients is most commonly observed during the termination of a paroxysmal AF episode and is characterized by a long RR interval.Syncope can also occur in cases of severe thromboembolic events,hemodynamically unstable conditions caused by extremely rapid ventricular rates,and in patients with underlying heart diseases such as hypertrophic cardiomyopathy and aortic valve stenosis.Additionally,AF is the most common cause of tachycardia-induced cardiomyopathy in adults.[29]
3.3.2 Examination
3.3.2.1 Physical examination
The main signs of AF include an irregularly irregular pulse,variable intensity of the first heart sound,and pulse deficit.
3.3.2.2 Laboratory tests
Patients with newly diagnosed AF should undergo tests for complete blood count,serum electrolytes,liver and kidney function,coagulation profile,thyroid function,B-type natriuretic peptide or Nterminal pro-brain natriuretic peptide (NT-proBNP),and relevant laboratory tests for comorbidities.
3.3.2.3 Surface ECG
The typical electrocardiographic manifestations of AF include (1) Disappearance of P waves,replaced by irregular fibrillation waves (f waves) with a frequency of 350-600 beats/min;and (2) Absolute inequality of RR intervals.When interpreting the ECG of patients with AF,attention should also be paid to the presence of signs of myocardial ischemia,myocardial hypertrophy,pre-excitation syndrome,electrolyte disturbances,and pulmonary embolism,and indices such as heart rate,QRS duration,and QT interval should be evaluated.
3.3.2.4 Dynamic electrocardiography and other long-term electrocardiographic monitoring methods
Helpful for diagnosing asymptomatic AF,assessing AF burden,and evaluating ventricular rate during AF.
3.3.2.5 Household wearable devices such as ECG patches and ECG smartwatches
These can show a wide range of potential applications for AF diagnosis,burden evaluation,and screening.
3.3.2.6 Chest radiographic examination
Used to evaluate the morphology and size of the heart,lung diseases and can also be used to monitor the lung condition in patients taking amiodarone.
3.3.2.7 Transthoracic echocardiogram
A routine examination for patients with AF,which can provide information on the presence of structural heart disease,atrial size,and the structure and function of the ventricles and valves.
3.3.2.8 Transesophageal echocardiography
This is the gold standard for detecting left atrial thrombus.However,in a few cases,the pectinate muscles of the left atrial appendage may be misdiagnosed as a thrombus.Transesophageal echocardiography combined with three-dimensional image reconstruction can help with differentiation.
3.3.2.9 Left atrial and pulmonary vein CT imaging
This can be used to clarify the anatomical characteristics of the left atrium,left atrial appendage,and pulmonary veins,as well as screening for left atrial thrombus before AF ablation.[30,31]Due to the dead tract-like structure of the left atrial appendage,as well as the slow blood flow resulting from the significantly reduced or disappeared contraction function of the left atrial appendage during AF,a falsepositive diagnosis of filling defects in the left atrial appendage can easily occur during left atrial CT examination.Delayed-phase scanning can improve the accuracy of thrombus diagnosis.[31]Some falsepositive thrombi revealed by left atrial CT examinations can be further confirmed as pre-thrombus states rather than true thrombus formation through transesophageal echocardiography or intracardiac echocardiography.
3.3.2.10 Cardiac magnetic resonance imaging
Cardiac magnetic resonance can accurately assess the structure and function of cardiac chambers and can also be used to diagnose left atrial thrombus.Reports have shown that the degree of atrial fibrosis evaluated by delayed-enhanced magnetic resonance imaging was significantly correlated with the recurrence risk after catheter ablation.[28]However,ablation targeting the fibrotic areas guided by MRI does not improve the success rate of ablation in patients with persistent AF.[32]
3.3.3 Assessment of symptoms and quality of life
Symptoms and quality of life in patients with AF can be assessed and quantified using various tools,including the EuroQol Five Dimensions Questionnaire and the 36-item Short-Form,which are commonly used for assessing the quality of life in various diseases,as well as the Atrial Fibrillation Effect on Quality-of-Life Questionnaire,which is specifically designed to evaluate the quality of life in AF patients,and the European Heart Rhythm Association scale,which is used to assess AF symptoms.Common mental health disorders such as anxiety and depression in AF patients can be preliminarily assessed using the Patient Health Questionnaire and the Generalized Anxiety Disorder scale.
The prevalence of cognitive impairment is high in patients with AF.The commonly used cognitive function screening scales include the Mini Mental State Examination and Montreal Cognitive Assessment.[33]
3.3.4 AF Screening
3.3.4.1 AF screening in the general population
The screening strategies for AF include opportunistic screening (i.e.,AF screening through pulse palpation or ECG during routine visits for various reasons by general practitioners) and systematic screening (i.e.,systematic and detailed AF screening through regular or continuous electrocardiographic monitoring for high-risk individuals).Opportunistic screening using the single-lead ECG or combined with pulse palpation and blood pressure measurement in individuals aged ≥ 65 years did not significantly increase the detection rate of AF.[34-36]Systematic screening in individuals aged ≥ 70 years without AF can significantly increase the detection rate of AF,but the benefits of anticoagulation therapy based on screening results remain controversial.[37-39]Based on current evidence,the Guidelines recommend considering opportunistic screening for AF through pulse palpation or ECG during medical visits for individuals aged ≥ 65 years and considering systematic screening for AF through regular or continuous electrocardiographic monitoring for individuals aged ≥ 70 years.
The screening methods for AF include both ECG and non-ECG methods.The former includes standard ECG,ambulatory ECG monitoring,handheld or wearable ECG recorders,and cardiac implantable electronic devices.The latter includes pulse palpation,photoplethysmography pulse wave recording,and electronic blood pressure monitors with AF detection function.[40]Pulse palpation,blood pressure measurement,non-12-lead ECG,and mobile devices have similar sensitivity for detecting AF.Among them,pulse palpation has a lower specificity but is still a practical means of AF detection given its simplicity and ease of use.When non-ECG methods detect suspected AF,additional ECG monitoring is required for confirmation.There have been reports of the use of machine learning and artificial intelligence to identify AF based on sinus rhythm ECGs.[41]Artificial intelligence technology has the potential to reform AF screening strategies in the future.
3.3.4.2 AF screening in patients with cardiac implantable electronic devices
Cardiac implantable electronic devices with atrial sensing function can continuously monitor and detect atrial tachyarrhythmias,also known as atrial high-frequency events (AHREs),including atrial tachycardia,atrial flutter,and AF.[2]The duration and frequency of the definition of AHREs vary slightly among studies,and current guidelines and consensus recommend defining the lower limits of duration and frequency as 5 min and 175 beats/min,respectively.[42]A meta-analysis has shown that patients without a history of clinical AF who experience AHREs were 5.7 times more likely to have documented clinical AF and have a 2.4-fold increased risk of stroke during the follow-up period than those without AHREs.[43]Moreover,there is a significant correlation between the AF burden detected by cardiac implantable electronic devices and the risk of ischemic stroke,with patients who have an AF burden exceeding 1 h having a 2.11-fold higher risk of is chemic stroke than those with an AF burden of < 1 h.[44]Therefore,evaluation of AHREs and confirmation of AF diagnosis should be performed during routine programming to make timely adjustments to anticoagulation treatment decisions.Further clinical assessment is necessary for patients with recorded AHREs to confirm the diagnosis of AF.
3.3.4.3 Screening for AF in stroke patients
AF is an important cause of cryptogenic stroke.A meta-analysis showed that AF can be detected in 7.7% of patients with acute ischemic stroke or transient ischemic attack through initial emergency ECG examination,and combining various methods of ECG monitoring can detect newly diagnosed AF in 23.7% of patients.[45]Prolonging monitoring time and increasing monitoring frequency can improve the detection rate of AF,but the optimal monitoring method and duration are still unclear.[46-48]Studies have shown that serial long-term (7-14 day) intermittent monitors accumulating at least 28 d of annual monitoring provide estimates of AF burden comparable with implantable cardiac monitors.[49]Therefore,for patients with acute ischemic stroke or transient ischemic attack without known AF,it is recommended to consider using the above-mentioned methods to detect AF as much as possible and initiate timely treatment.Recommendations for AF screening in various populations are presented in Table 4.[37-39]
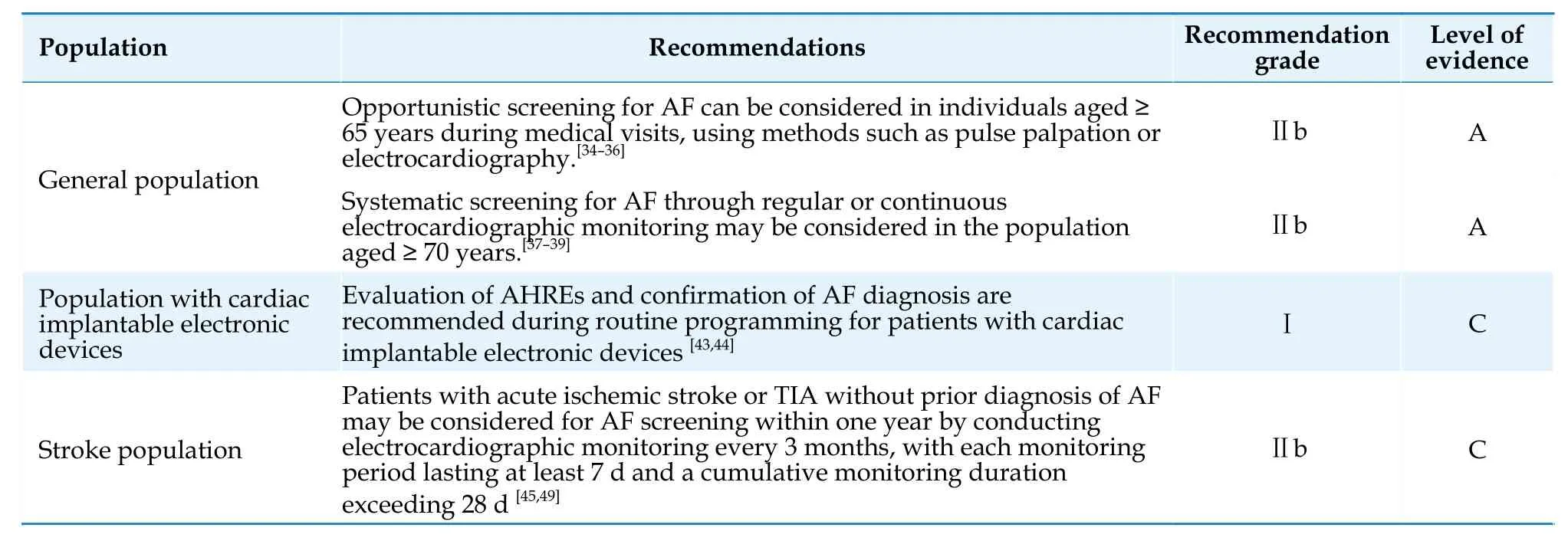
Table 4 Screening for AF.
4.STROKE PREVENTION
4.1 Stroke Risk Assessment
AF is an independent risk factor for stroke.The CHA2DS2-VASc score is currently the most widely used tool for assessing stroke risk.[50]The scoring criteria include: congestive heart failure,1 point;hypertension,1 point;age ≥ 75 years,2 points;diabetes mellitus,1 point;stroke,2 points;vascular disease,1 point;age 65-74 years,1 point;female sex,1 point.[51]Observational studies have shown that female sex is not an independent risk factor for stroke,[52,53]rather a risk modifier: the stroke risk is equivalent between female patients with a CHA2DS2-VASc score of 1 and male patients with a CHA2DS2-VASc score of 0.However,when other risk factors(excluding sex category) have the same score,female patients with AF have a higher risk of stroke than male patients.[54]
Age is an important factor influencing the risk of stroke.It has been demonstrated that in Asian AF patients,an increment in stroke risk was observed in patients >50 years of age.[55]Asian patients with AF aged 55-59 years with no risk factors showed similar risk of stroke as patients with a nongenderrelated risk score of 1,and patients aged 65-74 years with no other risk factors had similar stroke risk as patients with nongender-related risk scores of 2.[56]Asian patients with AF aged >55 years can benefit significantly from oral anticoagulants therapy.[57]Considering the lower age threshold for increased stroke risk in Asian AF patients,the Guidelines adopt the CHA2DS2-VASc-60 score [Table 5] and assign 1 point for patients aged 60-64 years and 2 points for patients aged ≥65 years.In the future,whether to include the age group of 55-59 years as a lower age threshold for anticoagulation treatment will be determined based on new research evidence.It is recommended that male AF patients with a CHA2DS2-VASc-60 score of ≥ 2,or female AF patients with a score of ≥ 3,should use oral anticoagulants.[58-60]Male patients with a CHA2DS2-VASc-60 score of 1 and female patients with a score of 2 should also consider using oral anticoagulants after weighing the expected stroke risk,bleeding risk,and patient preferences.[60-63]Male patients with a CHA2DS2-VASc-60 score of 0 or female patients with a score of 1 should not use oral anticoagulants for stroke prevention.[58,63-65]Stroke risk factors are dynamic and studies have shown that about 16% patients with low stroke risk progress to be moderateto-high-risk patients within 1 year.Therefore,for male AF patients with a CHA2DS2-VASc-60 score of 0 or female patients with a score of 1,stroke risk should be reassessed at least annually to adjust anticoagulation strategies in a timely manner.[66]
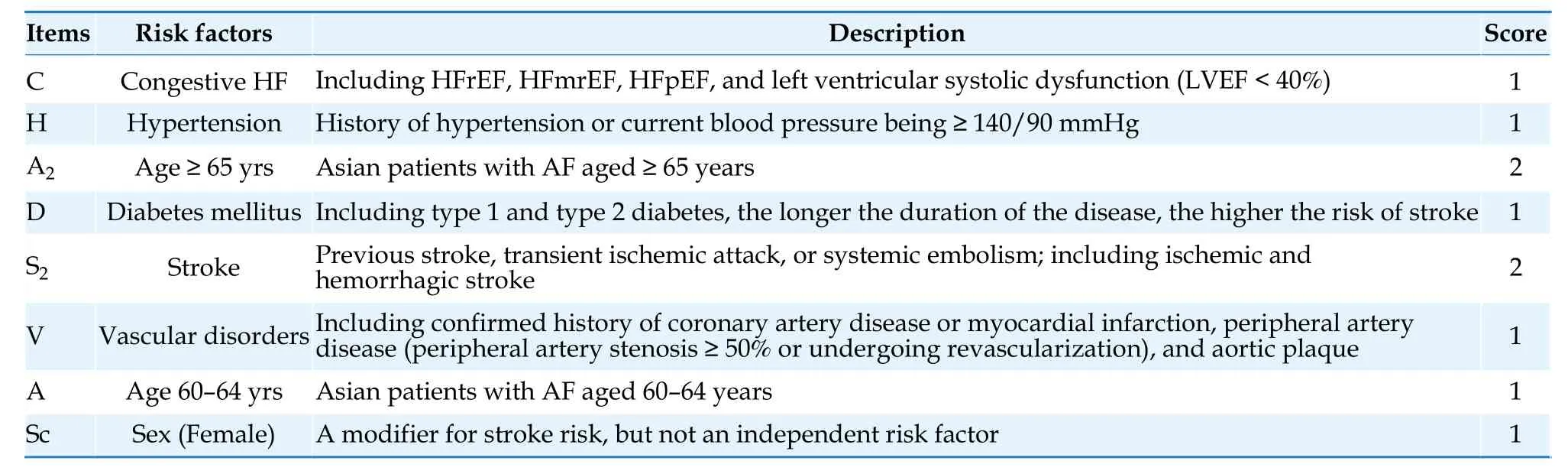
Table 5 CHA2DS2-VASc-60 score.
Atrial flutter also carries a significant risk of stroke,and the risk stratification and anticoagulation management for patients with atrial flutter are similar to those for patients with AF.[67]Recommendations on stroke risk assessment and anticoagulation therapy for AF are shown in Table 6.
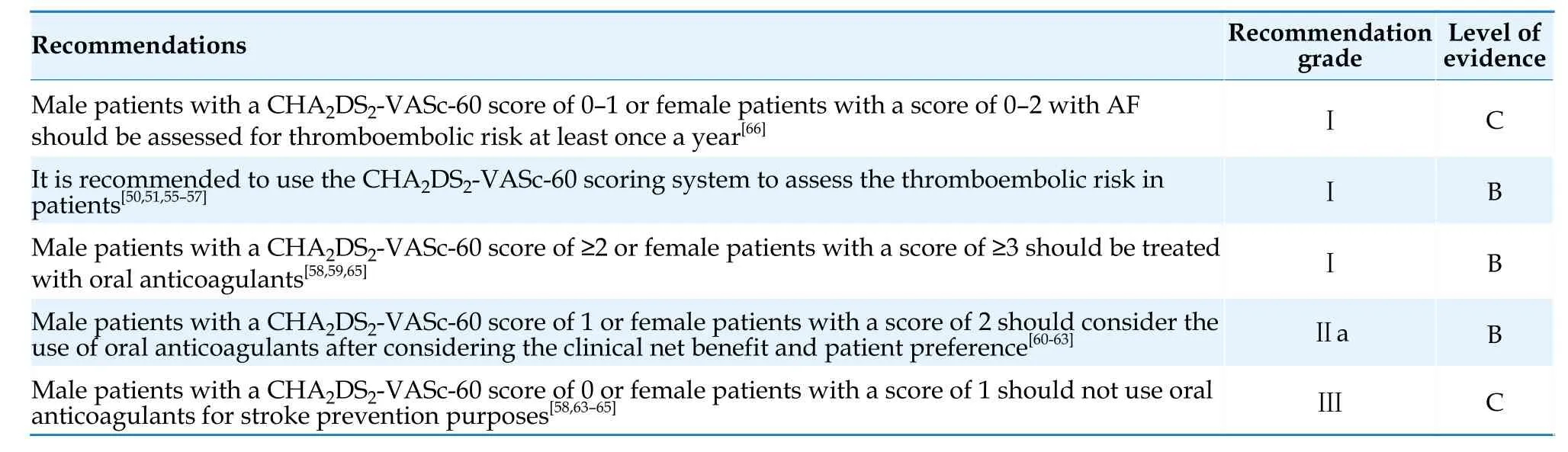
Table 6 Stroke risk assessment and anticoagulant therapy in AF.
4.2 Bleeding Risk Assessment
When initiating anticoagulant therapy,a thorough assessment of potential bleeding risk should be conducted.The HAS-BLED bleeding score (Table 7) is the most widely used bleeding risk prediction model.[68]A HAS-BLED score of ≤ 2 indicates low bleeding risk,while a score of ≥ 3 suggests high bleeding risk.Patients with high bleeding scores can still benefit significantly from anticoagulant therapy,therefore a high bleeding risk score should not be considered a contraindication to the use of oral anticoagulants.[69-71]Its significance lies in reminding clinicians to pay attention to and correct modifiable risk factors and to monitor and followup patients at high bleeding risk.The evaluation of bleeding risk factors prior to initiating anticoagulant therapy is crucial.Bleeding risk is dynamic and should be regularly reassessed during the course of anticoagulant therapy.[72-75]
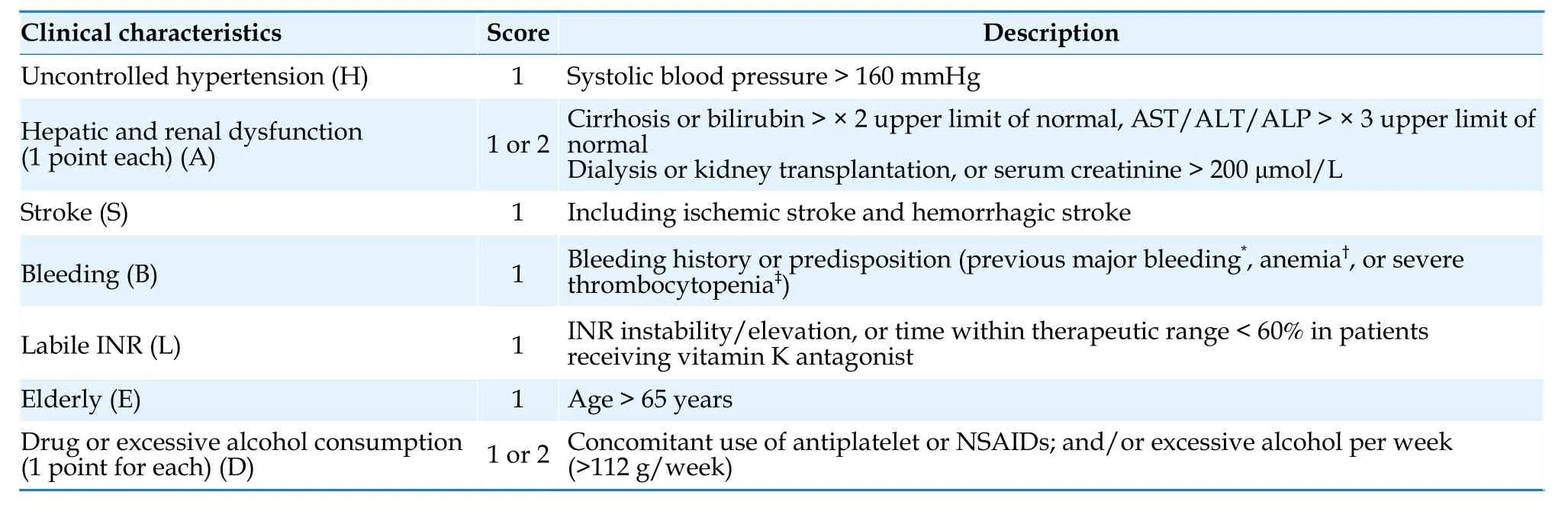
Table 7 HAS-BLED score.[68]
The risk factors for bleeding can be divided into modifiable factors,partially modifiable factors,and non-modifiable factors (Table 8).Identifying and modifying reversible bleeding risk factors is an important measure to reduce the risk of bleeding.For patients taking oral warfarin,efforts should be made to keep the international normalized ratio (INR) within the therapeutic range.For patients taking NOACs,appropriate drug dosages should be selected based on age,renal function,and concomitant medications.All patients should be educated on selfmonitoring for bleeding.For patients at high risk of gastrointestinal bleeding,especially those who need to concurrently receive aspirin or nonsteroidal antiinflammatory drugs (NSAIDs),the use of proton pump inhibitors in combination can reduce the occurrence of upper gastrointestinal bleeding.[76]Please refer to (Table 9) for recommendations on anticoagulant bleeding assessment.

Table 9 Anticoagulation-related bleeding risk assessment.
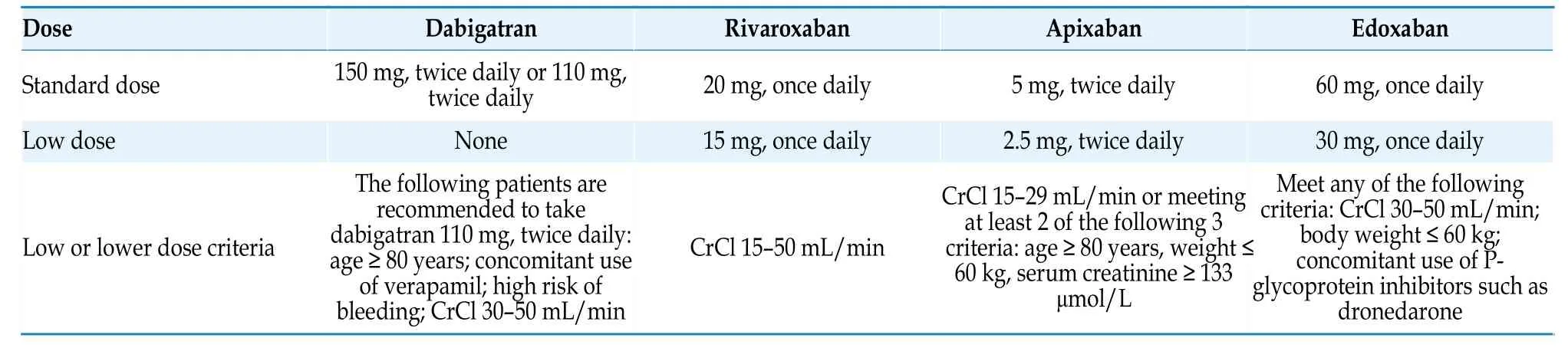
Table 11 NOACs dose recommendations.[82-85]
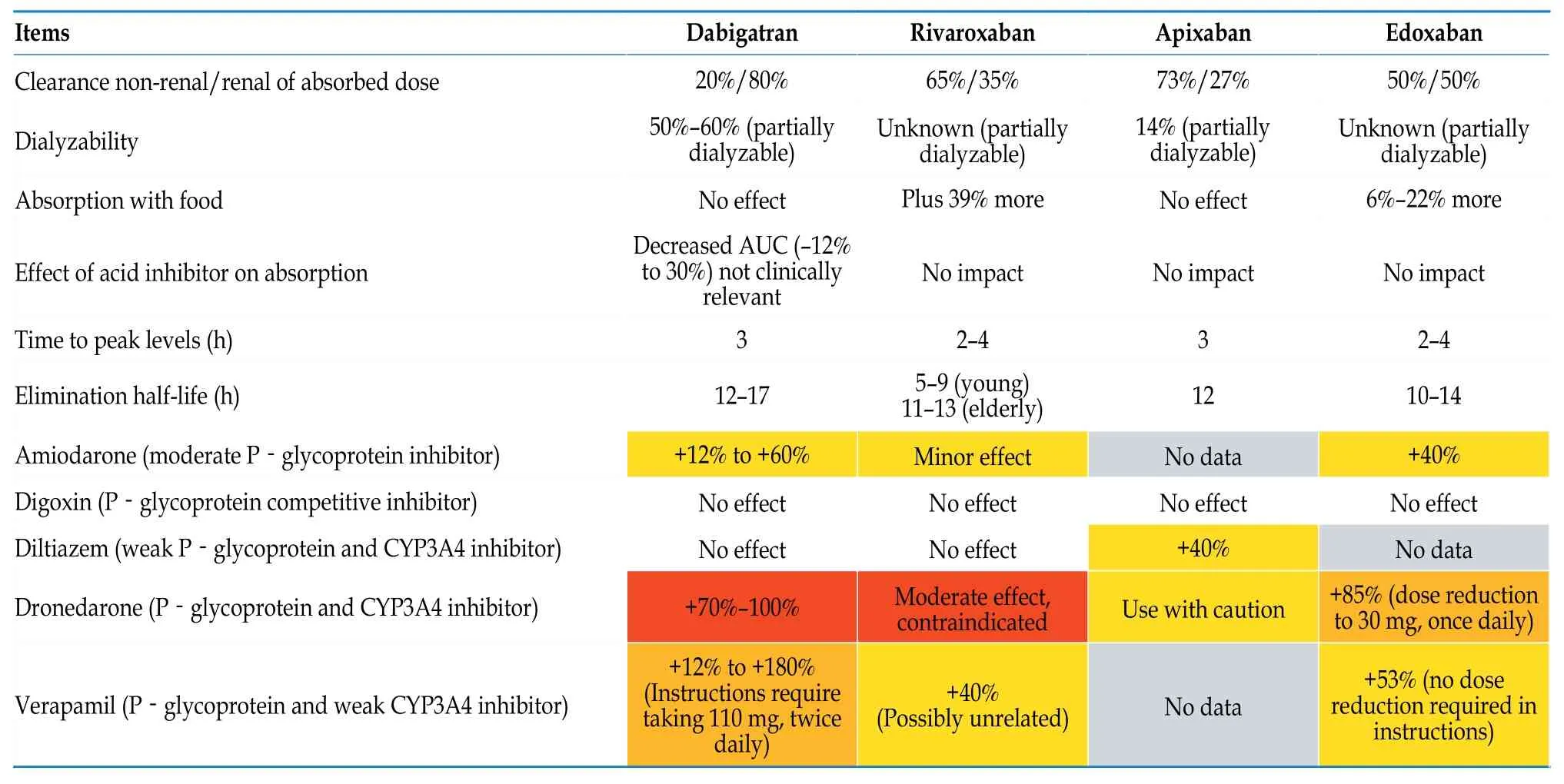
Table 12 Pharmacokinetics of NOACs and the influence of antiarrhythmic drugs on the anticoagulant effects of NOACs.[87]
Warfarin requires a relatively long time to take effect,has a long half-life,and a narrow therapeutic range.It is also easily influenced by various factors such as genetics,other medications,and diet.Patient education,follow-up,and monitoring of INR should be strengthened,especially when there are significant changes in diet or concurrent medication use.Monitoring frequency should be increased accordingly.Timely adjustment of warfarin dosage based on INR can improve TTR and enhance the effectiveness of warfarin therapy.
4.3 Oral Anticoagulants
Oral anticoagulants include warfarin and NOACs.The use of oral anticoagulants in patients with AF should be based on a careful consideration of the benefits and risks of bleeding.The decision to initiate anticoagulation therapy should be made through a shared decision-making process between the healthcare provider and the patient.Given the high disability and mortality rates associated with stroke,and the fact that most bleeding events do not result in long-term sequelae,even patients at high risk of bleeding can still obtain a clinical net benefit from anticoagulation therapy.[69-71]The decision to initiate or withhold anticoagulation therapy should not be based solely on high risk of bleeding.Absolute contraindications to oral anticoagulants therapy include severe active bleeding,bleeding-related comorbidities (such as severe thrombocytopenia with platelet count < 50 × 109/L[42]and hemophilia),or recent high-risk bleeding events such as intracranial hemorrhage.
4.3.1 Warfarin
Warfarin can reduce the risk of stroke in patients with AF by 64%.[77]Patients taking warfarin should have their INR regularly monitored and the warfarin dosage adjusted,to maintain the INR within the therapeutic target range of 2.0-3.0.[78]When the time within therapeutic range (TTR) of the INR is >70%,the overall risk of stroke and bleeding is relatively lower.[79]
4.3.2 NOACs
Currently,there are 4 types of NOACs available on the international market,including dabigatran,a direct thrombin inhibitor,rivaroxaban,apixaban,and edoxaban,all of which inhibit factor Ⅹa.FactorⅪ inhibitors that are still in the research stage can theoretically reduce the risk of bleeding associated with anticoagulant therapy and significantly improve the safety of anticoagulation therapy.[80]In the phase Ⅲ clinical trials comparing NOACs with warfarin,the efficacy of NOACs in preventing ischemic stroke and systemic embolism was either noninferior or superior to warfarin (Table 10),with a significant reduction in the risk of intracranial hemorrhage.[81-85]
The selection of NOACs should consider factors such as their bioavailability,metabolic pathways,potential drug interactions,elimination half-life,and presence of antagonists.Reducing or increasing the dosage without a clear indication will increase the risk of adverse events without increasing safety.[86]Different NOACs have different drug metabolic characteristics.When used in combination with antiarrhythmic drugs,attention should be paid to the impact of antiarrhythmic drugs on the blood concentration of NOACs,and a reasonable selection of drug types and dosage adjustments should be made (Tables 11 and 12).[87]
4.3.3 Antiplatelet drugs
Monotherapy with antiplatelet drugs does not reduce the risk of stroke in patients with AF.[88]Although dual antiplatelet therapy (DAPT) can reduce the risk of stroke in certain patients with AF,it significantly increases the risk of major bleeding.[89]Therefore,antiplatelet therapy is not recommended for the prevention of AF-related stroke.
Table 10 Comparison of the efficacy and safety of NOACs and warfarin.[82-85]
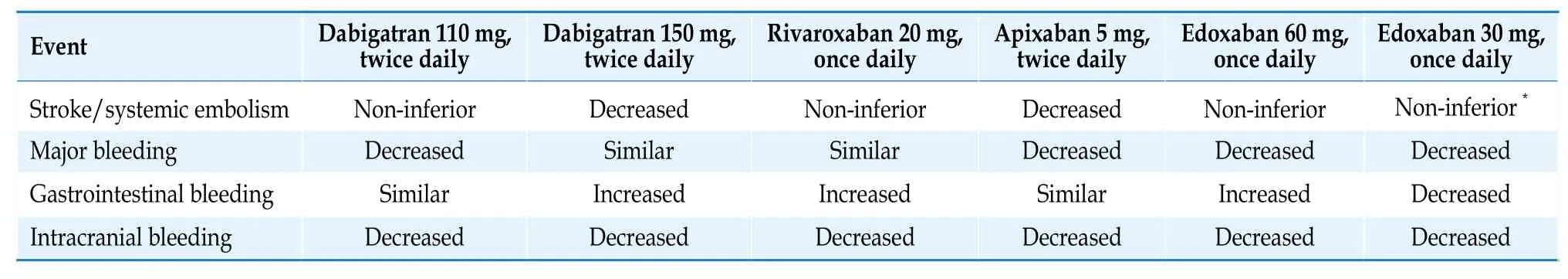
*Compared to warfarin,edoxaban 30 mg once daily increases the risk of ische mic stroke.[84] NOACs: non-vitamin K antagonist oral anticoagulant.
The principles of antithrombotic therapy for AF are summarized in Table 13.
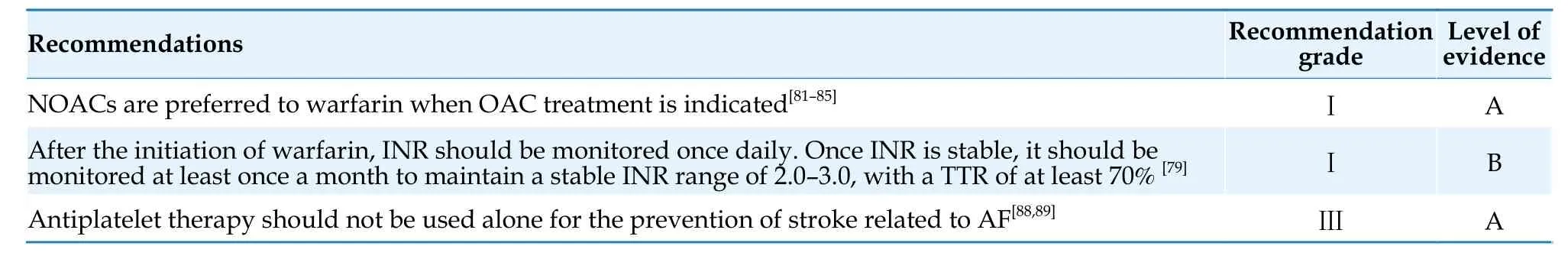
Table 13 Antithrombotic drugs for AF.
4.4 Management of Anticoagulation-related Bleeding Risk
If a bleeding event occurs in patients with AF treated with oral anticoagulants,the severity of bleeding,site of bleeding,and time of last oral anticoagulants dose,whether concomitant use of antiplatelet drugs should be assessed.Other risk factors that may affect bleeding,such as excessive alcohol consumption and liver or kidney dysfunction,should also be assessed.INRs should be monitored in patients receiving warfarin,while activated partial thromboplastin time,prothrombin time,diluted thrombin time,or ecarin clotting time should be measured in patients receiving dabigatran,and anti-Ⅹa activity or prothrombin time should be measured in patients receiving Factor Xa inhibitors.
According to the location and severity of bleeding,bleeding events can be generally classified as mild,moderate,severe,or life-threatening (Table 14).[90]Severe or life-threatening bleeding refers to bleeding that affects hemodynamic stability or occurs in important areas,such as intracranial,intraspinal,pericardial,retroperitoneal,intra-articular,or compartment syndrome.[91]Moderate bleeding refers to bleeding without hemodynamic compromise but requiring blood transfusion or medical intervention.Mild bleeding refers to bleeding that does not meet the above criteria (e.g.,limb bruising,hemorrhoids bleeding,subconjunctival hemorrhage,and self-limited epistaxis).Mild bleeding can be managed by discontinuation of oral anticoagulants and observation,as the half-life of NOACs is relatively short,and the anticoagulation effects significantly diminish after discontinuation for 12-24 h.Moderate-tosevere bleeding may require transfusion/fluid replacement therapy.In patients who have taken the last dose of NOACs within 2-4 h,activated charcoal or gastric lavage can be administered to reduce drug exposure.Upper gastrointestinal bleeding can be evaluated with endoscopy and appropriate endoscopic hemostasis measures can be taken.In cases of severe or life-threatening bleeding,immediate reversal of the anticoagulant effects of oral anticoagulants is necessary.Idarucizumab and andexanet alfa can be used to reverse the anticoagulant effects of dabigatran and factor Ⅹa inhibitors,respectively.[92]In patients who cannot be treated with NOACs reversal agents promptly or in those receiving warfarin,immediate administration of prothrombin complex concentrate (PCC) containing factors Ⅱ,Ⅶ,Ⅸ,and Ⅹ (or fresh frozen plasma if PCC is unavailable) is recommended.[93,94]In patients receiving warfarin,intravenous vitamin K takes 6-8 h to take effect.Once the cause of bleeding has been identified and corrected,anticoagulation therapy should be restarted as soon as possible in patients with high risk of stroke.[95]The management recommendations for management of bleeding associated with anticoagulation therapy in patients with AF are presented in Table 15.

Table 14 Definition of bleeding.[90,91]

Table 15 Management of bleeding associated with anticoagulation in AF.
4.5 Anticoagulation Therapy in Special Populations and Special Situations
4.5.1 Patients with AF and coronary artery disease
Approximately 20% to 30% of AF patients have coronary artery disease,including acute coronary syndromes (ACS) and chronic coronary syndromes(CCS).[96,97]The combined use of oral anticoagulants and antiplatelet drugs,especially triple antithrombotic therapy (oral anticoagulants plus aspirin and P2Y12receptor inhibitors),significantly increases the risk of bleeding.Therefore,for AF patients with coronary artery disease,careful assessment of the risk of thromboembolism and bleeding is necessary to select an appropriate antithrombotic strategy.[97]For the selection of oral anticoagulants,NOACs are preferred to warfarin.[98-101]When used in combination with antiplatelet drugs,lower doses of NOACs(e.g.,15 mg once daily of rivaroxaban or 110 mg twice daily of dabigatran) should be considered to reduce the risk of bleeding.[99,100]When a combination of antiplatelet and anticoagulant therapy is required,the duration of triple antithrombotic therapy,including oral anticoagulants plus DAPT,should be minimized.P2Y12receptor inhibitors are preferred as the single antiplatelet drug in combination with oral anticoagulants,and the use of potent P2Y12receptor inhibitors should be avoided (e.g.,Clopidogrel as the preferred drug of choice).[98-101]If vitamin K antagonist is used for anticoagulation in combination with antiplatelet drugs,the vitamin K antagonist dosage should be adjusted to maintain a target INR of 2.0-2.5[102]and a TTR > 70%.[42]
Thrombotic and bleeding risks are key factors determining antithrombotic strategies.Currently,there is no unified and prospectively validated risk assessment scheme in the guidelines and consensus.When the risks of bleeding and thromboembolism coexist,the antithrombotic strategy can be determined by considering the following thrombotic and bleeding risk factors.Thrombotic risk should be evaluated based on the likelihood of severe consequence (such as stenting of the left main stem,left main bifurcation disease,left main equivalent disease,or last remaining patent artery) caused by stent thrombosis,and thrombotic risk factors including (1) diabetes mellitus requiring treatment;(2) previous ACS or recurrent myocardial infarctions;(3)multi-vessel coronary artery disease;(4) concomitant peripheral arterial disease;(5) premature coronary artery disease (occurring at the age of < 45 years) or accelerated coronary artery disease (new lesion within 2 years);(6) chronic kidney disease (estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2);(7) non-low-risk ACS;(8) multi-vessel stenting;(9) complex revascularization (left main stenting,bifurcation lesion stenting,chronic total occlusion percutaneous coronary intervention (PCI),last patent vessel stenting);and (10) prior stent thrombosis despite adequate antiplatelet therapy;procedural factors (inadequate stent expansion,residual lesions,stent length > 60 mm.[42]Bleeding risk assessment should be carried out according to a dynamic evaluation based on the HAS-BLED score and ARCHBR criteria.[103]
4.5.1.1 ACS
For AF patients with concomitant ACS and/or undergoing PCI who require anticoagulation therapy,the What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) study demonstrated that dual antithrombotic therapy with warfarin and a P2Y12inhibitor significantly reduced bleeding risk compared to triple antithrombotic therapy,without increasing ischemic events.[104]Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With AF Who Undergo PCI (PIONEER AF-PCI),Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatranvs.Triple Therapy with Warfarin in Patients with Nonvalvular AF Undergoing PCI (REDUAL-PCI),Apixabanvs.vitamin K antagonist and Aspirinvs.Aspirin Placebo in Patients With AF and ACS or PCI (AUGUSTUS),and Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention(ENTRUST-AF-PCI) studies compared the bleeding risk of 4 NOACs (rivaroxaban,dabigatran,apixaban,and edoxaban) combined with P2Y12receptor inhibitors versus warfarin combined with DAPT in patients with concomitant ACS and/or undergoing PCI.The results showed that compared to triple antithrombotic therapy,NOACs combined with single antiplatelet therapy (mainly clopidogrel) reduced the risk of clinically relevant bleeding or major bleeding,as well as intracranial bleeding,by 17% to 47%,with no significant difference in cardiovascular death,stroke,or all-cause death.[98-101,105-107]However,there was a higher risk of cardiac ischemic events(mainly stent thrombosis).[106]In the PIONEER AFPCI,REDUAL-PCI,AUGUSTUS,and ENTRUSTAF-PCI studies,patients receiving NOACs combined with P2Y12receptor inhibitors were all treated with triple antithrombotic therapy,including aspirin,during the peri-PCI period (median time from PCI to randomization was 1 to 6 d).[98-101]However,a post-hoc analysis of the AUGUSTUS study showed that continuing aspirin for more than 30 d in patients undergoing PCI increased bleeding risk without a significant reduction in cardiovascular death,stent thrombosis,myocardial infarction,or stroke events.[108]
For patients undergoing PCI for ACS,if the risk of bleeding is higher than the risk of thrombosis,it is recommended to discontinue aspirin early (≤ 1 week) and use dual antithrombotic therapy with oral anticoagulants and P2Y12receptor inhibitors for 12 months.[98-101]If the risk of thrombosis is higher than the risk of bleeding,triple therapy should be used for 1 month after PCI[108,109]and continued with dual antithrombotic therapy with oral anticoagulants and P2Y12receptor inhibitors for 12 months.[110]
For patients with ACS who have not undergone PCI,it is recommended to use dual antithrombotic therapy with oral anticoagulants combined with P2Y12receptor inhibitors for up to 6 months,followed by long-term use of oral anticoagulants alone.[98-101]
4.5.1.2 CCS
For patients with CCS undergoing PCI,if the risk of thrombosis is higher than the risk of bleeding,dual antithrombotic therapy with oral anticoagulants and P2Y12receptor inhibitors should be considered for maintenance therapy for 6-12 months.If the risk of thrombosis is lower than the risk of bleeding,dual antithrombotic therapy should be considered for 6 months and then switched to oral anticoagulants monotherapy.[98-101,110]
For patients with AF and CCS who have not undergone PCI,the AF and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease (AFIRE) study demonstrated that the efficacy endpoint (composite endpoint of stroke,systemic embolism,myocardial infarction,unstable angina requiring revascularization,or all-cause death)of using rivaroxaban alone for treatment is non-inferior to using rivaroxaban in combination with antiplatelet therapy,with a significantly lower rate of major bleeding.[111]Therefore,it is recommended to use oral anticoagulants alone for AF patients with CCS who have not received PCI treatment.The antithrombotic treatment strategy for AF patients with concomitant coronary artery disease is summarized in Figure 1,and the relevant recommendations are presented in Table 16.
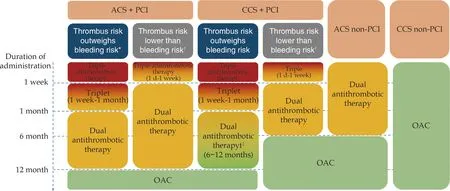
Figure 1 Antithrombotic therapy for atrial fibrillation complicated with coronary heart disease.*For patients with thrombotic risk factors but low risk of bleeding,or for patients with a high bleeding risk (HAS-BLED score ≥ 3) but with potential severe consequences of stent thrombosis;?For patients without complications after PCI and with a low risk of stent thrombosis,or for patients with thrombotic risk factors but with a high bleeding risk (HAS-BLED score ≥ 3);?Dual antithrombotic therapy until 12 months for patients with potential severe consequences of stent thrombosis.Triple antithrombotic therapy refers to the combination of OAC,aspirin,and a P2Y12 receptor antagonist (preferably clopidogrel).Dual antithrombotic therapy refers to the combination of OAC and a P2Y12 receptor antagonist (preferably clopidogrel).ACS: acute coronary syndrome;CCS: chronic coronary syndrome;OAC: oral anticoagulant;PCI:percutaneous coronary intervention.
Table 16 Antithrombotic therapy for patients with AF and CAD.
4.5.2 Patients with AF and Chronic Kidney Disease
Chronic kidney disease coexists in approximately 50% of patients with AF,[112]and increases the risk of stroke,bleeding,and mortality.[113,114]
Previous phase Ⅲ clinical trials on rivaroxaban,dabigatran,and edoxaban only included AF patients with a creatinine clearance (CrCl) > 30 mL/min.A meta-analysis of phase 3 clinical trials on multiple NOACs indicate a favorable efficacy and safety profile of all NOACs compared to warfarin in patients with renal function impairment (CrCl > 30 mL/min).[115]A meta-analysis including randomized controlled trials (RCTs) and observational studies showed that NOACs were superior to warfarin in preventing thromboembolism and reducing bleeding events in patients with CrCl values of 15-60 mL/min.[116]
Apixaban has the least dependence on renal metabolism among all NOACs,so its use in chronic kidney disease patients has always been highly regarded.The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation(ARISTOTLE) study included a part of AF patients with a CrCl of 25-30 mL/min,and the results showed that compared to warfarin,apixaban significantly reduced the risk of major bleeding,with a trend towards a decreased risk of stroke.[117]Observational studies have shown that factor Ⅹa inhibitors can be a safe and effective alternative to warfarin for AF patients with a CrCl of 15-29 mL/min.[118-120]Dabigatran has a renal metabolism proportion of up to 80% and is not recommended for patients with a CrCl < 30 mL/min.
Currently,there is insufficient evidence to support the benefit of oral anticoagulants in patients with stage 5 chronic kidney disease (CrCl < 15 mL/min)or those on dialysis.Meta-analysis demonstrated that the benefit of oral anticoagulants in non-dialysis patients with stage 4-5 chronic kidney disease is unclear,while in patients on dialysis,the use of oral anticoagulants significantly increases the risk of major bleeding without reducing the risk of stroke and death.The Renal Hemodialysis Patients Allocated Apixabanvs.Warfarin in Atrial Fibrillation(RENAL-AF) and Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease (AXADIA-AFNET4)studies-2 RCTs comparing apixaban with warfarin in patients on dialysis-were prematurely terminated and failed to answer whether apixaban was non-inferior to warfarin in reducing major bleeding events in patients with AF who are on dialysis.However,both studies showed that patients on dialysis who received oral anticoagulants had a significantly higher risk of major bleeding events than the risk of thromboembolic events,and these patients had difficulty maintaining an ideal TTR with vitamin K antagonist.[121,122]The benefit of anticoagulation therapy in high-risk stroke patients with stage 5 chronic kidney disease or those on dialysis is still unclear,and the decision to use warfarin or apixaban for anticoagulation therapy should be carefully considered after weighing the risks of stroke,bleeding,and patient preferences.When using NOACs in patients with chronic kidney disease,dose adjustment should be made based on renal function,as shown in Figure 2.The recommendations for anticoagulation therapy in patients with AF and chronic kidney disease are summarized in Table 17.[115-117,120-122]
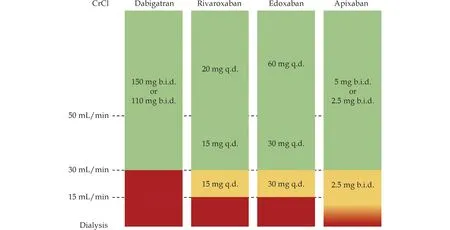
Figure 2 Oral anticoagulant (non-vitamin K antagonists) dosing adjusted for renal function.CrCl: creatinine clearance;b.i.d.: bis in die (twice a day);q.d.: quāque diē (once a day).
Table 17 Anticoagulation therapy for AF complicated with chronic kidney disease.
4.5.3 Patients with AF and Hepatic Disease
The liver is the main organ for synthesizing coagulation factors and metabolizing oral anticoagulants.Patients with abnormal liver function may have coagulation disorders,and oral anticoagulants is contraindicated in patients with severe liver dysfunction.The phase 3 studies of NOACs excluded patients with active liver disease and those with significantly elevated transaminases or bilirubin.For AF patients with liver dysfunction,it is recommended to use the Child-Pugh classification (Table 18)to guide oral anticoagulants treatment.There is no evidence for the use of oral anticoagulants in AF patients with Child-Pugh grade C (10-15 points).AF patients with Child-Pugh grade B (7-9 points) should avoid the use of rivaroxaban because of significant increase in drug plasma concentration.[123]Apixaban,dabigatran,and edoxaban can be used with caution.[124,125]Patients with Child-Pugh grade A (≤ 6 points) can be treated with standard-dose oral anticoagulants.In patients with concomitant hepatic dysfunction,changes in liver function and bleeding complications should be closely monitored.
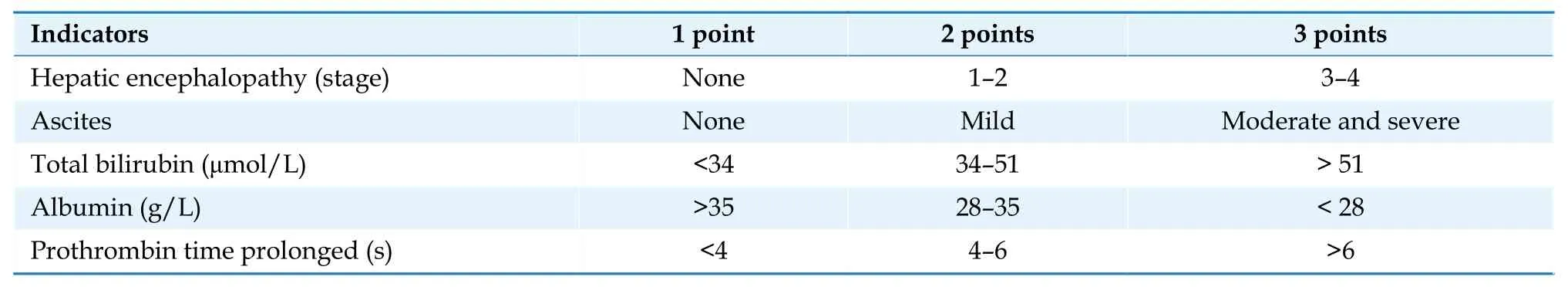
Table 18 Child-Pugh classification.
4.5.4 The advanced-age patients with AF
Age is both a risk factor for thromboembolism and a risk factor for bleeding.Post-hocanalysis of phase 3 clinical trials of NOACs showed that the benefits of anticoagulation therapy in AF patients aged ≥ 75 years were consistent with those in patients aged < 75 years,and NOACs have a better overall risk-benefit profile than warfarin.[126-129]Even in the very elderly population (≥ 90 years),oral anticoagulants can still be beneficial.[130,131]Elderly AF patients often have multiple comorbidities(such as impaired liver and kidney function,and multiple concomitant medications),which increase the risk of adverse reactions.Underdosing of oral anticoagulants is also more common.To ensure the effectiveness of antithrombotic therapy,the anticoagulation treatment in elderly patients should be adjusted according to the dose requirements of NOACs(such as age and renal function) and standard doses should be used to avoid underdosing (Table 11).
For elderly and very elderly patients who are not suitable for standard-dose anticoagulation,the Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF) study provided evidence for the use of very low-dose edoxaban (15 mg once a day) in this population.This study included patients aged ≥ 80 years (mean age: 86.6 years) with at least one risk factor (CrCl: 15-30 mL/min),a history of bleeding from a critical area or organ or gastrointestinal bleeding,low body weight (≤ 45 kg),continuous use of NSAIDs,or current use of an antiplatelet drug.Compared to placebo,the use of very low dose edoxaban (15 mg) can still reduce the incidence of stroke,and although there was an increased risk of major bleeding,the difference was not statistically significant.[132]
4.5.5 Patients with AF and Hypertrophic Cardiomyopathy
Multiple large-sample observational studies have shown that the prevalence and incidence of AF in patients with hypertrophic cardiomyopathy were 23% and 3.1% per year,respectively,which was 4 to 6 times higher than in patients without hypertrophic cardiomyopathy.[133]The prevalence and incidence of thromboembolism in hypertrophic cardiomyopathy patients with AF were 27% and 3.8% per year,respectively,and the risk of stroke was 8-times higher than in hypertrophic cardiomyopathy patients without AF.[133]The annual stroke incidence rate in hypertrophic cardiomyopathy patients with AF who had a CHA2DS2-VASc score of 0 for males and 1 for females was 3.38%.[134]Given the significantly increased risk of stroke in hypertrophic cardiomyopathy patients with AF,anticoagulation therapy should be used in this population regardless of the CHA2DS2-VASc-60 score.[133,135][Table 19] Observational studies have shown that NOACs may be more effective and safer than warfarin in this population.[133]

Table 19 Anticoagulation therapy for AF complicated with hypertrophic cardiomyopathy.
4.5.6 Patients with AF and valvular heart disease
Patients with mechanical heart valve replacement or moderate-to-severe mitral stenosis and AF have a high risk of stroke and should receive anticoagulation therapy with warfarin regardless of their CHA2DS2-VASc-60 score [Table 20].[136,137]The Randomized,Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Re-placement (RE-ALIGN)study evaluated the efficacy and safety of dabigatran in patients with AF and mechanical valve replacement.Owing to a significant increase in thromboembolic and bleeding events in the dabigatran group,the study was terminated early.[137]The Investigation of Rheumatic AF Treatment Using Vitamin K Antagonists,Rivaroxaban or Aspirin Studies (INVICTUS) study showed that in patients with rheumatic heart disease (82% of whom had moderate-to-severe mitral stenosis) and AF,the incidence of major cardiovascular events with warfarin was significantly lower than with rivaroxaban.[136]

Table 20 Anticoagulation therapy for AF complicated with valvular heart disease.
In patients with AF and other valvular disease,including valve regurgitation,bioprosthetic valves,and post-valvular repair,NOACs are safe and effective.The Rivaroxaban for Valvular Heart Disease and Atrial Fibrillation (RIVER) study suggested that rivaroxaban was non-inferior to warfarin in AF patients with bioprosthetic mitral valves.[138]A meta-analysis based on RCTs also demonstrated that the use of NOACs,compared to warfarin,significantly reduced the risk of stroke,systemic embolism,and major bleeding in patients with AF and bioprosthetic valves or prior valve repair.[139]
The Edoxabanvs.Standard of Care and Their Effects on Clinical Outcomes in Patients Having Undergone Transcatheter Aortic Valve Implantation-AF (ENVISAGE-TAVI AF) study demonstrated that when used in AF patients undergoing successful transcatheter aortic valve replacement (TAVR),edoxaban was non-inferior to warfarin in reducing the risk of a postoperative major adverse cardiovascular events.However,the incidence of major bleeding was higher with edoxaban than with vitamin K antagonist.[140]The Society of Thoracic Surgeons-American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT Registry) study showed that in AF patients undergoing TAVR,the use of NOACs compared to warfarin did not result in a significant difference in the occurrence of stroke events at the 1-year follow-up,but had lower rates of bleeding,intracranial bleeding,and all-cause mortality.The differences in the type of NOACs used (with apixaban being the most commonly used in the registry study),differences in patient characteristics (with fewer bleeding risk factors in the NOACs group in the registry study),and differences in antiplatelet treatment strategies may be the reasons for the discrepant conclusions regarding bleeding between the STS/ACC TVT registry study and the ENVISAGE-TAVI AF study.
4.5.7 Peri-cardioversion stroke risk management
4.5.7.1 Anticoagulation strategy for cardioversion in patients with AF lasting ≥ 48 h
Patients with AF lasting for > 48 h and who have not received effective anticoagulation therapy have a significantly higher risk of stroke/transient ischemic attack and systemic embolism within 30 day after cardioversion compared to patients with AF lasting for < 48 h.[141,142]Moreover,98% of thromboembolic events occur within 10 day after cardioversion.[143]The pathophysiological mechanisms include detachment of pre-existing thrombus due to restoration of atrial mechanical function after cardioversion,left atrial stunning following conversion of AF to sinus rhythm,and transient prothrombotic state promoting thrombus formation.Effective anticoagulation for at least 3 weeks before cardioversion significantly reduced the risk of thromboembolism (from 0.71%-2.39% to 0.13%-0.45%).[141]Therefore,in patients with AF lasting ≥ 48 h who have not undergone transesophageal echocardiography,cardioversion should be performed after at least 3 weeks of effective anticoagulation therapy.Anticoagulation should be continued for at least 4 weeks after cardioversion,[144]and the decision to continue anticoagulation thereafter should be based on the risk of stroke.For AF patients with rapid ventricular rate and hemodynamic instability,regardless of the duration of AF,urgent cardioversion should be performed along with the initiation of anticoagulation therapy.
Transesophageal echocardiography-guided cardioversion showed no significant difference in the incidence of embolic events and all-cause mortality compared with effective anticoagulation for 3 weeks before cardioversion,but the incidence of bleeding was lower and the time required for cardioversion was shorter.[145]Patients without a thrombus in the left atrium or left atrial appendage confirmed by transesophageal echocardiography can be cardioverted as soon as possible under effective anticoagulation to replace the 3-week anticoagulant regimen before cardioversion.
4.5.7.2 Anticoagulation strategy for AF lasting< 48 h
In patients with AF duration of < 48 h who have not received anticoagulation therapy,the incidence of thromboembolic events within 30 day after cardioversion was 0.7%.The thromboembolic risks for AF duration of < 12 h and AF duration 12-48 h were 0.33% and 1.1%,respectively.[144]Studies have showed that patients with AF duration of < 12 h but recent history of stroke/transient ischemic attack,patients with AF duration of 12-48 h and mediumto-high thromboembolic risk (CHA2DS2-VASc score≥ 1 for male or ≥ 2 for female patients),or patients with unclear AF duration have a higher risk of thromboembolism.Therefore,it is recommended to treat these patients with an anticoagulation strategy similar to that for AF ≥ 48 h.[144,146,147]Based on the above evidence,the Guidelines recommend that for patients with AF duration of < 12 h and no recent history of stroke/transient ischemic attack,or for patients with AF duration 12-48 h and low thromboembolic risk (CHA2DS2-VASc score=0 for male or 1 for female patients),cardioversion without transesophageal echocardiography examination can be considered,while initiating oral anticoagulants therapy.
4.5.7.3 Oral anticoagulants selection for pericardioversion anticoagulation
A meta-analysis has shown that compared to standard-dose warfarin,NOACs can significantly reduce the risk of stroke/systemic embolism and composite endpoints (stroke,systemic embolism,myocardial infarction,or cardiovascular death) during the peri-cardioversion period,with no significant difference in the risk of major bleeding and allcause mortality.[148,149]Therefore,NOACs should be preferred for anticoagulation during the peri-cardioversion period.Warfarin is recommended in cases of severe mitral stenosis or mechanical valve replacement in the presence of rheumatic heart disease and in situations where NOACs may not be suitable (such as in patients on dialysis or those with decompensated liver disease).A summary of recommendations for anticoagulation therapy for peri-cardioversion is provided in Table 21.
Table 21 Anticoagulation therapy for peri-cardioversion.
4.5.8 Catheter ablation for AF
4.5.8.1 Perioperative anticoagulation for catheter ablation of AF
A meta-analysis showed that even in patients receiving anticoagulation therapy for more than 3 weeks,2.73% of patients still had atrial thrombus detected by transesophageal echocardiography.The thrombus detection rate was 4.81% in non-paroxysmal AF/atrial flutter patients,1.03% in paroxysmal AF/atrial flutter patients,1.65% in patients undergoing catheter ablation,and 5.51% in patients with cardioversion.The thrombus detection rate was 6.31% in patients with a CHA2DS2-VASc score ≥ 3 and 1.06% in patients with a CHA2DS2-VASc score ≤2.[150]Therefore,transesophageal echocardiography examination is recommended before catheter ablation.Delayed-enhancement CT of the left atrium and intraprocedural intracardiac echocardiography can also be considered alternatives to transesophageal echocardiography to exclude atrial thrombus.[30,151-153]For male patients with a CHA2DS2-VASc-60 score ≤2 or female patients with a score of ≤ 3,no history of stroke/transient ischemic attack or systemic embolism,and adequate anticoagulation therapy for > 3 weeks,transesophageal echocardiography examination before catheter ablation may be omitted.
Anticoagulant therapy is an important measure to prevent perioperative stroke/transient ischemic attack and systemic embolism during catheter ablation for AF.Compared to heparin-bridging therapy,uninterrupted oral anticoagulants therapy can significantly reduce the risk of bleeding and thromboembolism.[154]The effectiveness of uninterrupted NOACs and uninterrupted vitamin K antagonist therapy in reducing perioperative thromboembolic events and bleeding risk during catheter ablation is similar.Additionally,uninterrupted dabigatran anticoagulation during the perioperative period can reduce bleeding events compared to uninterrupted vitamin K antagonist anticoagulation.[155-158]During catheter ablation,activated clotting time (ACT)should be monitored regularly (every 15-30 min) to guide heparin usage,with a target ACT value of> 300 s,which can significantly reduce the risk of thromboembolism without increasing the bleeding risk.[159]
Factors such as endocardial injury,inflammatory response,and delayed recovery of left atrial function after catheter ablation may increase the risk of early thrombus formation following AF ablation.[160-162]Therefore,it is recommended to administer oral anticoagulants for at least 3 months after AF ablation,regardless of the patient’s thrombotic risk.[160]
4.5.8.2 Long-term anticoagulation after catheter ablation for AF
Although most current guidelines recommend long-term anticoagulation based on thromboembolic risk scores rather than the outcome of the ablation procedure,there is significant variation in the clinical practice of anticoagulation management after AF ablation.[160,162,163]Evidence shows that discontinuation of oral anticoagulants in patients at high thromboembolic risk (CHA2DS2-VASc score ≥2) is associated with an increased risk of thromboembolism,while discontinuation of oral anticoagulants in patients at low thromboembolic risk(CHA2DS2-VASc score ≥ 0 or 1) does not significantly increase the risk of thromboembolism.[160]Some observational studies have shown that the thromboembolic risk is similar between patients with and without ablation,but patients who continue anticoagulation have a significantly higher risk of bleeding than those who discontinue oral anticoagulants.[162,164]For patients without a history of stroke/transient ischemic attack,systemic embolism,or diabetes,and in the absence of evidence of AF recurrence with reliable monitoring,discontinuation of anticoagulation after 3 months of ablation may be safe.[165]Observational studies suggest that a history of stroke is an important risk factor for increased thromboembolic risk when discontinuing oral anticoagulants after catheter ablation.[165,166]Further research is urgently needed to guide long-term anticoagulation therapy after AF ablation,especially in patients who can safely discontinue anticoagulation under intensive cardiac monitoring.Currently,there is still a lack of reliable evidence for alternative CHA2DS2-VASc score-based guidance for oral anticoagulants use.A summary of perioperative and long-term anticoagulation management after catheter ablation for AF is provided in Table 22.
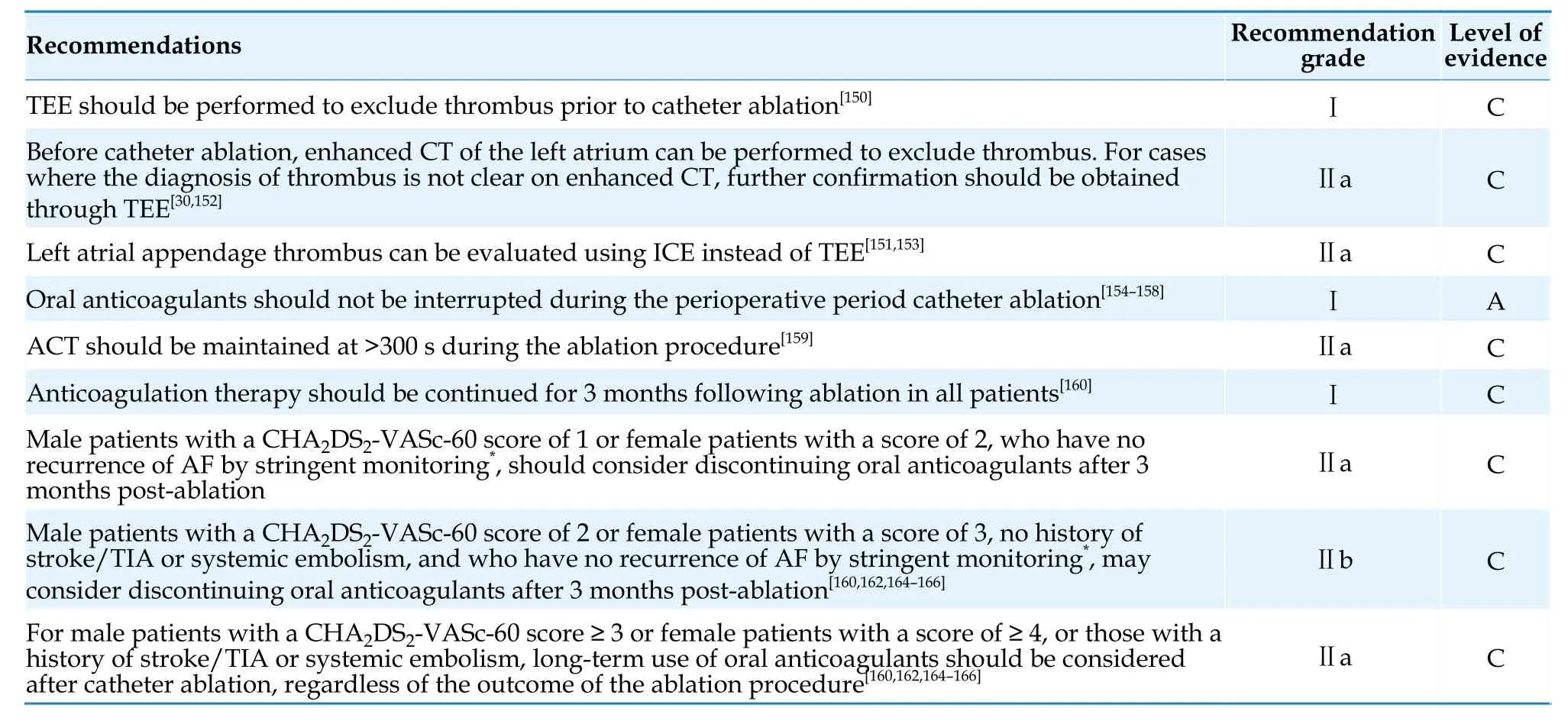
Table 22 Perioperative and long-term anticoagulation therapy for catheter ablation of AF.
4.5.9 Perioperative anticoagulation management for invasive procedures or surgeries
It is estimated that approximately 1 out of every 4 patients receiving oral anticoagulants therapy will undergo invasive procedures or surgeries within 2 years.[167]
Perioperative anticoagulation strategies for longterm oral anticoagulants patients should adhere to the following principles: pay attention to preventing the existing thromboembolic and bleeding risks associated with the patient’s condition or the procedure itself;focus on preventing adverse clinical outcomes related to thromboembolism or bleeding;determine the discontinuation and re-initiation of anticoagulation strategies based on the pharmacokinetic characteristics of oral anticoagulants medications.
4.5.9.1 Patients receiving NOACs treatment
NOACs have the characteristics of rapid onset and short half-life.The preoperative discontinuation time should be determined based on the patient’s renal function and the risk of surgical bleeding (Table 23).If the patient’s renal function is normal and the risk of periprocedural bleeding is minimal,uninterrupted anticoagulation or discontinuation for 1 dose may be considered.For patients with low risk of periprocedural bleeding,it is recommended to discontinue NOACs 1 day before surgery.For patients with high risk of periprocedural bleeding,it is recommended to discontinue NOACs 2 days before surgery.[168,169]Table 24 presents the preoperative discontinuation time determined based on renal function.[87]
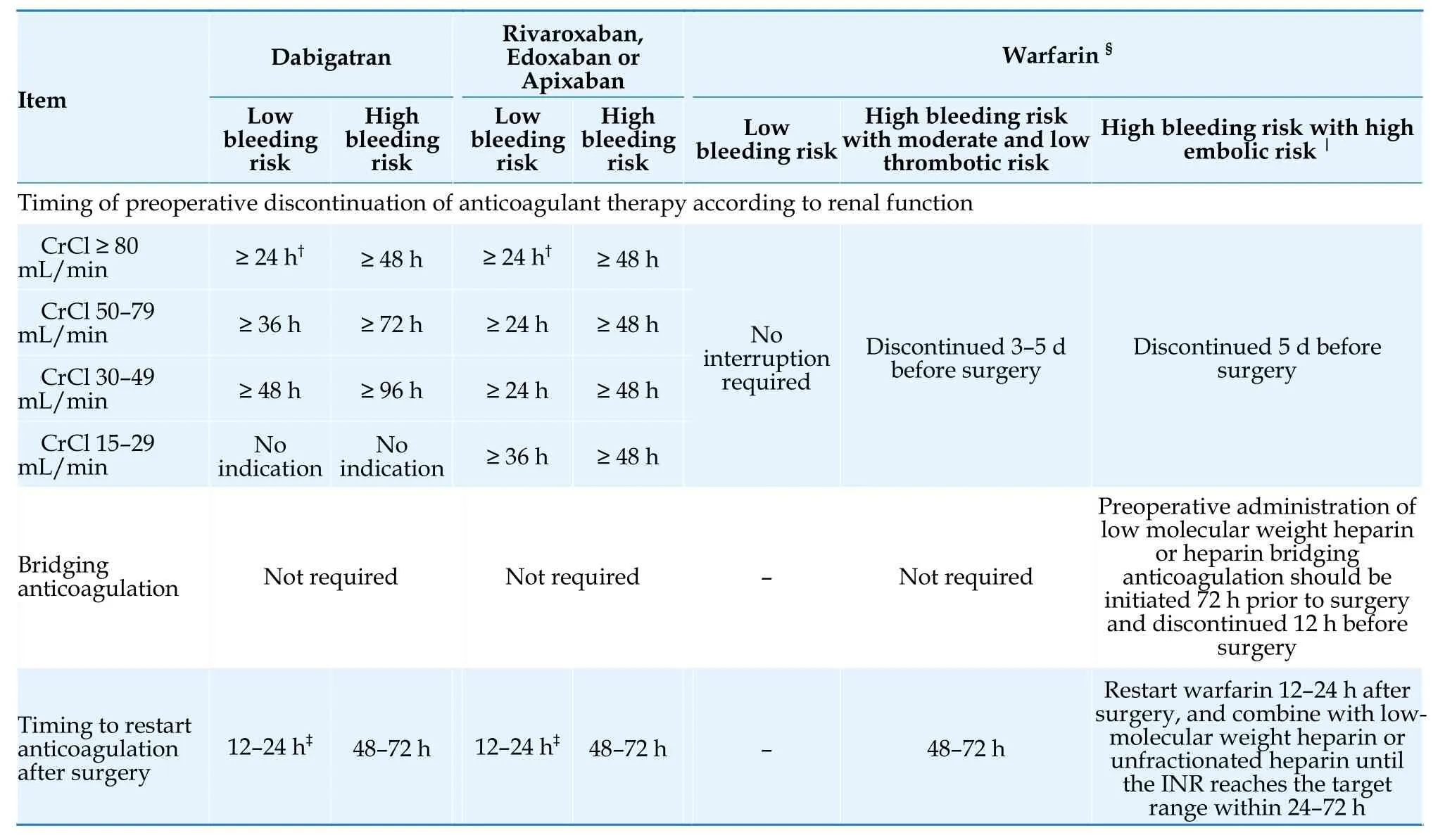
Table 24 Perioperative anticoagulation strategies for patients with AF undergoing invasive procedures or surgeries*.[87,171]
Patients taking NOACs are generally not recommended to undergo bridging anticoagulation during invasive procedures or surgeries.[168]Anticoagulation should be restarted as soon as hemostasis is achieved,or be restarted 6 h post-surgery for patients with mild bleeding risk,12-24 h post-surgery for patients with low bleeding risk,and 48-72 h postsurgery for patients with high bleeding risk.[87,168]
4.5.9.2 Patients on warfarin therapy
If the risk of surgery-related bleeding is low in patients with AF who are taking warfarin,it is not recommended to interrupt anticoagulation.If the risk of surgery-related bleeding is high,it is recommended to discontinue warfarin 3-5 day before surgery.[170]
There was no significant difference in the rate of thromboembolic events between the interruption of vitamin K antagonist with and without low-molecular weight heparin or unfractionated heparin bridging therapy,but the incidence of major bleeding was significantly reduced without bridging therapy (1.3% in the non-bridging groupvs.3.2% in the bridging group).[170]Therefore,for patients taking warfarin,bridging anticoagulation is generally not recommended.Bridging anticoagulation should only be considered for patients with high risk of thromboembolism (including those who have undergone mechanical valve replacement,have a CHA2DS2-VASc-60 score of ≥ 6,and have experienced a stroke or transient ischemic attack within the past 3 months,among non-valvular AF patients).[171]
Patients who discontinue warfarin due to a high risk of periprocedural bleeding can resume warfarin 48-72 h after hemostasis has been achieved.[171]
A summary of risk classification for bleeding associated with invasive procedures or surgeries in patients with AF is presented in Table 23,[87,172]and a summary of perioperative anticoagulation strategies is provided in Table 24.[171]
4.5.10 Diagnosis and management of left atrial appendage thrombus
Transthoracic echocardiography has low sensitivity in diagnosing left atrial appendage thrombus.Transesophageal echocardiography has a high sensitivity (92% to 100%) and specificity (98% to 99%) in diagnosing left atrial appendage thrombus,[173]and is considered the gold standard for diagnosing AFrelated atrial thrombus.[31]Left atrial enhanced CT has a sensitivity of 99% and specificity of 94% in detecting left atrial appendage thrombus,while cardiac MRI has a sensitivity of 80% and specificity of 98%,both of which can be used as alternative screening methods for atrial thrombus.[152]In addition,intracardiac echocardiography has a similar sensitivity and specificity to transesophageal echocardiography in diagnosing left atrial appendage thrombus and can be used as an alternative diagnostic tool.[151,153]
Once a thrombus in the left atrium/left atrial appendage is detected,standardized anticoagulation therapy should be initiated immediately.After confirming the disappearance of the thrombus through repeated transesophageal echocardiography,cardioversion or catheter ablation therapy can be performed.Previous studies have suggested that the thrombus resolution rate is between 50% and 90% by repeated transesophageal echocardiography at 4 weeks after the initiation of anticoagulation therapy.[174]The Exploring the Efficacy of Once Daily Oral Rivaroxaban for Treatment of Thrombus in Left Atrial/Left Atrial Appendage in Subjects With Nonvalvular Atrial Fibrillation or Atrial Flutter (XTRA) study demonstrated that in AF patients with newly diagnosed left atrial appendage thrombus,the thrombus resolution rate was 41.5% after 6 weeks of treatment with standard-dose rivaroxaban,as confirmed by repeated transesophageal echocardiography.[174]
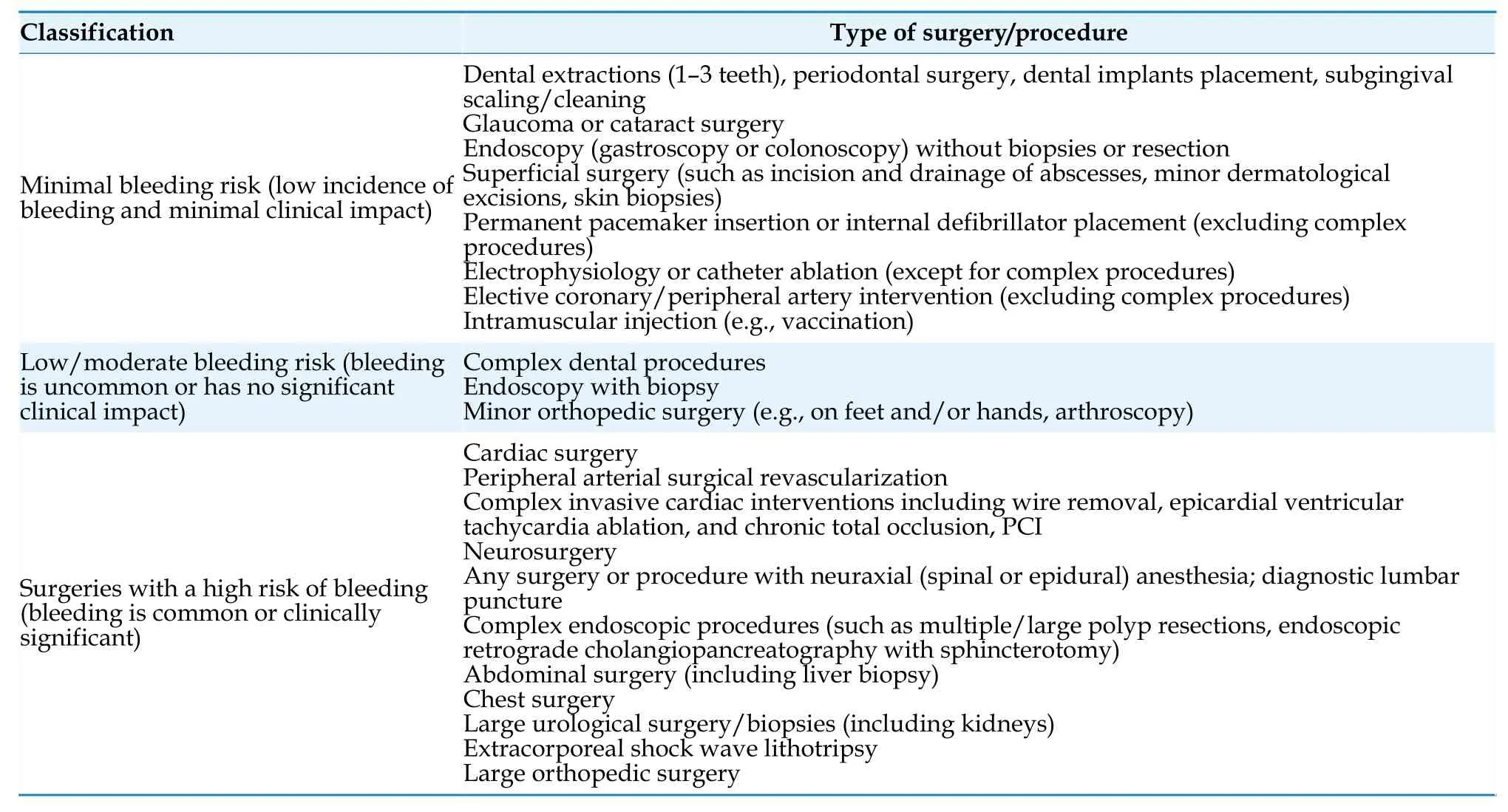
Table 23 Risk classification for bleeding associated with invasive procedures or surgeries in patients with atrial fibrillation.[87,172]
Although there is currently no evidence from RCTs comparing warfarin with NOACs,some smallscale observational studies have shown that NOACs have no significant difference in the efficacy and safety of left atrial appendage thrombus resolution compared with warfarin,and may shorten the time to thrombus elimination.[174,175]For left atrial appendage thrombus that persists despite standardized anticoagulation therapy,treatment strategies include increasing the target INR to 3.0-4.0,switching to or adding low molecular weight heparin,and prolonging the duration of anticoagulation.[176]
4.6 Secondary Stroke Prevention
4.6.1 Ischemic stroke
For patients with AF who experience an ischemic stroke,initiation of oral anticoagulants during the acute phase should carefully balance the risk of stroke recurrence and hemorrhagic transformation.For patients with stroke due to large vessel occlusion within 24 h of onset,mechanical thrombectomy is recommended after ruling out intracranial hemorrhage.[177,178]For patients within the time window(< 4.5 h) and who meet the indications for thrombolysis,thrombolytic therapy can be performed if the patient is taking warfarin and has an INR < 1.7.[179]For patients taking NOACs with normal renal function,the drug would be completely metabolized after more than 48 h since the last dose;thrombolytic therapy is relatively safe at this time,[180]while there is insufficient evidence for thrombolysis within 48 h.Small-scale studies have shown that thrombolytic therapy after specific reversal of anticoagulant effect by reversal agents is safe and feasible in patients taking dabigatran.[181,182]However,for patients taking factor Ⅹa inhibitors and whose anticoagulant intensity cannot be determined,thrombolysis after giving factor Ⅹa inhibitor reversal agents is not recommended.[179]A meta-analysis suggested that compared with patients who had not taken oral anticoagulants or had taken vitamin K antagonist with an INR < 1.7,thrombolysis in acute ischemic stroke patients who had taken NOACs within 48 h did not increase the risk of bleeding or death.[180]An observational study showed that the incidence of symptomatic intracranial hemorrhage after thrombolytic therapy in ischemic stroke patients who had taken NOACs within 48 h was lower than that in patients who had not received anticoagulant treatment,regardless of whether specific reversal agents or NOACs levels were used.[183]As an alternative to thrombolysis,endovascular treatment (such as mechanical thrombectomy) is safe for patients with stroke caused by intracranial anterior circulation occlusion who have used oral anticoagulants within 48 h.[184]
A previously conducted meta-analysis showed that the use of heparin and low-molecular weight heparin for anticoagulation within 48 h after acute cardioembolic ischemic stroke did not reduce the risk of recurrent ischemic stroke but increased the risk of intracranial hemorrhage.[185]Regarding the use of NOACs,the Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation (TIMING) study showed that the use of NOACs within 4 day after mild-to-moderate ischemic stroke with AF (mean National Institute of Health Stroke Scale score: 6) was non-inferior to the strategy of restarting NOACs between 5 and 10 d after stroke in reducing the composite endpoint of recurrent ischemic stroke,symptomatic intracranial hemorrhage,and all-cause death,with a trend towards a reduction in the primary endpoint events.[186]Observational studies have shown that early re-initiation of NOACs based on stroke risk stratification (within 1 day after transient ischemic attack,2 days after mild stroke,3 days after moderate stroke,and 4 days after severe stroke) is associated with a reduction in stroke/embolism risk,without a significant increase in the occurrence of intracranial hemorrhage.[187]Another observational study also showed that early restart of NOACs (within ≤ 5 days) did not significantly increase the risk of intracranial hemorrhage.[188]However,there is a lack of definitive evidence on the timing of restarting anticoagulation in patients with severe stroke.Ongoing studies such as OPtimal TIMing of Anticoagulation After Acute Ischaemic Stroke(OPTIMAS,NCT03759938),Early Versus Late Initiation of Direct Oral Anticoagulants in Post-ischaemic Stroke Patients With Atrial fibrillatioN (ELAN,NCT 03148457),and Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation (START,NCT03021928) will provide more evidence on the timing of restarting anticoagulation after stroke.NOACs are significantly superior to vitamin K antagonist in terms of efficacy in secondary prevention of stroke and reduction of intracranial hemorrhage.[189]The recommendations for secondary prevention of ischemic stroke in patients with AF are summarized in Table 25.
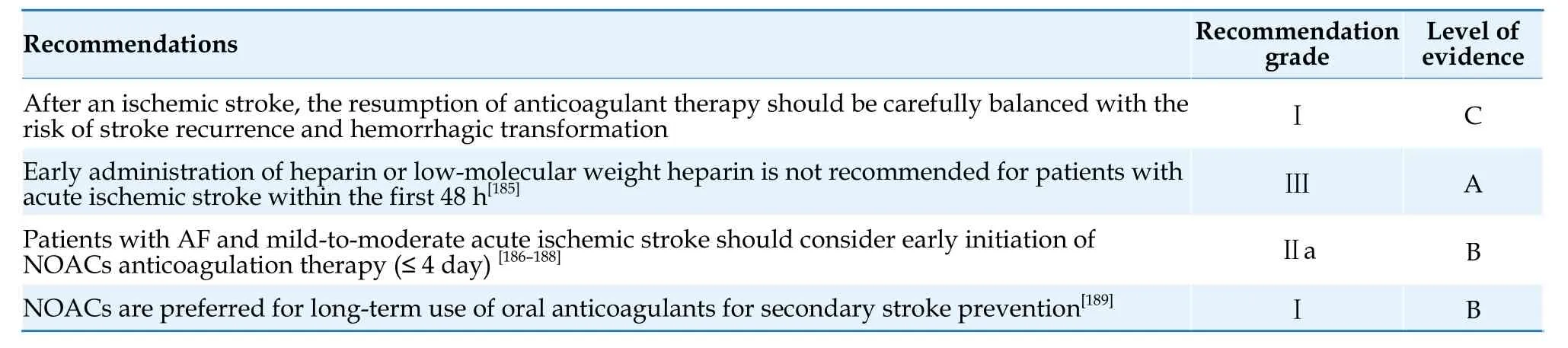
Table 25 Secondary prevention of ischemic stroke in patients with AF.
4.6.2 Hemorrhagic stroke
During the acute phase of intracranial hemorrhage (including primary and traumatic),anticoagulant therapy is contraindicated until reliable bleeding control is achieved.The decision to initiate anticoagulant therapy should be based on the cause and severity of the bleeding.A meta-analysis has shown that continuing oral anticoagulants therapy in AF patients with non-traumatic intracranial hemorrhage can reduce the risk of thromboembolism and all-cause mortality,with no significant increase in the risk of recurrent intracranial hemorrhage.Compared to warfarin,NOACs are more effective in reducing the risk of thromboembolic events and recurrent intracranial hemorrhage.[190]Therefore,NOACs,especially those with specific reversal agents,should be prioritized when restarting anticoagulant therapy in patients with non-traumatic intracranial hemorrhage with AF.The optimal timing for restarting anticoagulant therapy after intracranial hemorrhage is still unclear.Some studies have shown that restarting anticoagulation therapy 7-8 weeks after intracranial hemorrhage provided the greatest benefit.[191,192]For patients at high risk of recurrent intracranial hemorrhage and no correctable cause,left atrial appendage closure may be considered.
4.7 Left atrial Appendage Closure
4.7.1 Transcatheter left atrial appendage closure
Oral anticoagulants can effectively prevent thromboembolism in patients with AF.However,factors such as the risk of bleeding associated with anticoagulation drugs and poor medication adherence will have a certain impact on their practical application.Imaging examinations such as transesophageal echocardiography and autopsy results have confirmed that AF thrombi mainly form in left atrial appendage,with 90% of non-valvular AF left atrial thrombi located in the left atrial appendage.[193]Removal or closure of left atrial appendage may theoretically replace oral anticoagulants and achieve the goal of preventing AF-related stroke without increasing the risk of bleeding.
The ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology (ASAP)study showed that the risk of ischemic stroke in AF patients with contraindications of long-term anticoagulation who underwent transcatheter left atrial appendage closure was lower than the expected risk.[194]The ongoing Assessment of the WATCHMAN device in patients unsuitable for oral anticoagulation(ASAP-TOO) study (NCT0292849) will further provide evidence for left atrial appendage closure in patients with anticoagulation contraindications.Currently,there is no universally accepted definition for absolute contraindications to oral anticoagulants.However,in AF patients with absolute contraindications to long-term oral anticoagulants use,such as a platelet count of < 50×109/L,unexplained severe anemia,[42]irreversible fatal/disabling bleeding (e.g.,amyloid angiopathy,uncorrectable vascular malformation leading to recurrent intracranial hemorrhage,intraspinal hemorrhage,severe bleeding in the digestive/urinary/respiratory system caused by vascular dysplasia),[195]and hereditary hemorrhagic telangiectasia,left atrial appendage closure should be considered to reduce the risk of stroke.left atrial appendage closure may also be considered for patients with relative contraindications to oral anticoagulants,such as malignancies with increased bleeding tendency,end-stage chronic kidney disease,chronic bacterial endocarditis,and specific high-risk occupations (e.g.,pilots,firefighters).[195]
For patients eligible for oral anticoagulants,the WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation (PROTECT AF) study demonstrated that the effectiveness of transcatheter left atrial appendage closure in preventing stroke/systemic embolism/cardiovascular death was non-inferior to warfarin.[196]In the Evaluation of the WATCHMAN Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) study,although left atrial appendage closure did not meet the non-inferiority criteria compared to warfarin for the primary endpoint (stroke,systemic embolism,cardiovascular death,or unexplained death),it was non-inferior to warfarin in preventing long-term stroke/systemic embolism.[197]The Left Atrial Appendage Closurevs.Novel Anticoagulation Agents in Atrial Fibrillation (PRAGUE-17) results showed that left atrial appendage closure,compared to NOACs,was non-inferior in preventing stroke/transient ischemic attack,systemic embolism,major bleeding,clinically relevant nonmajor bleeding,and procedure-related complications.The 20-month and 3.5-year follow-up results of this study demonstrated that left atrial appendage closure was non-inferior to NOACs in reducing the risks of stroke/transient ischemic attack,cardiovascular death,and clinically relevant bleeding,with a significant reduction in non-procedure-related clinically relevant bleeding.However,the incidence of left atrial appendage closure-related severe complications was high at 4.5%.It should be noted that patients included in the PRAGUE-17 study had a CHA2DS2-VASc score of 4.7 ± 1.5 and a HAS-BLED score of 3.1 ± 0.9,representing a population with high risk of stroke and bleeding,and may not represent the majority of AF patients.[198,199]
For patients who experience stroke despite adequate anticoagulation,after excluding stroke confirmed to be caused by cerebral vascular stenosis,left atrial appendage closure may be considered.
The WATCHMAN FLX Versus NOAC for Embolic ProtectION in in the Management of Patients With Non-Valvular Atrial Fibrillation (CHAMPIONAF) study (NCT 04394546) plans to enroll 3,000 AF patients with a CHA2DS2-VASc score of ≥ 2 (male)or ≥ 3 (female),while the Clinical Trial of Atrial Fibrillation Patients Comparing Left Atrial Appendage Occlusion Therapy to Non-vitamin K Antagonist Oral Anticoagulants (CATALYST) study (NCT 04226547) plans to enroll 2,650 AF patients with a CHA2DS2-VASc score of ≥ 3.These studies will compare the effectiveness and safety of the Watchman FLX and Amplatza Amulet occluders with NOACs.With the advancements of procedure techniques and the upgrades in device products,the safety of left atrial appendage closure has significantly improved.[12]Recommendations for left atrial appendage closure in AF patients are summarized in Table 26.

Table 26 Transcatheter LAAC therapy in patients with AF.
4.7.2 Surgical left atrial appendage excision/closure
Observational studies have demonstrated the feasibility and safety of left atrial appendage excision during cardiac surgery or surgical ablation for AF,and have provided preliminary evidence of the potential benefits of left atrial appendage closure.[200]For AF patients at high risk of stroke undergoing cardiac surgery,most patients continue oral anticoagulants therapy,and left atrial appendage closure can reduce the risk of ischemic stroke and systemic embolism,with no significant difference in the risk of all-cause mortality and heart failure readmission.[201]Therefore,for high-risk AF patients undergoing cardiac surgery,concurrent surgical left atrial appendage ligation/excision may be considered [Table 27].[200,201]

Table 27 Surgical left atrial appendage excision/closure in patients with AF.
5.RHYTHMIC CONTROL OF AF
5.1 Rhythm Control Strategy
5.1.1 Choice of rhythm control versus rate control strategy
Rhythm control for AF refers to the restoration and long-term maintenance of sinus rhythm through the use of antiarrhythmic drugs,direct current cardioversion,catheter ablation,or surgical ablation.Studies such as Atrial Fibrillation Follow-up Investigation of Rhythm Management Atrial Fibrillation Follow-up Investigation of Rhythm Management(AFFIRM),Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study (RACE),and Atrial Fibrillation and Congestive Heart Failure(CHF-AF) conducted in the early 21stCentury did not demonstrate improved outcomes with rhythm control strategies in AF patients.[202-204]However,a posthoc analysis of the AFFIRM study showed a significant reduction in mortality in patients who received anticoagulation therapy and successfully maintained sinus rhythm.[205]Observational studies have also shown an association between rhythm control and lower risk of stroke/transient ischemic attack.[206]The Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) study,published in 2020,included patients with early AF (diagnosed within one year before enrollment) and concomitant cardiovascular conditions,such as patients who were diagnosed as AF for the first time,asymptomatic patients (30.5%),and those with persistent AF(26.7%).All enrolled patients were randomly assigned to an early rhythm control group (including antiarrhythmic drugs and ablation) or a usual care group (mainly rate control,with rhythm control used only to mitigate uncontrolled AF-related symptoms).The primary endpoint was a composite of death from cardiovascular causes,stroke (either ischemic or hemorrhagic),or hospitalization with worsening of heart failure or ACS.The study showed a 21% reduction in the primary endpoint events in the early rhythm control group.[11]Large-scale observational studies have shown consistent results with the EASTAFNET 4 study,indicating an association between rhythm control and lower rates of the composite primary endpoint (cardiovascular death,ischemic stroke,heart failure,or hospitalization for ACS) compared to rate control in newly diagnosed AF patients.[207]
Why do the results of rhythm control show different outcomes in studies conducted in different eras? The main reason is the difference in rhythm control approach,and the improvement in anticoagulation rates has also contributed to an improved prognosis.In early studies,the only rhythm control approach was antiarrhythmic drugs with amiodarone and sotalol,which could increase mortality,and accounted for more than two-thirds of all antiarrhythmic drugs.The adverse reactions of antiarrhythmic drugs may offset the benefits of maintaining sinus rhythm.In the EAST-AFNET 4 study,more advanced rhythm control methods were used,and the use of antiarrhythmic drugs was more reasonable.The main antiarrhythmic drugs used were flecainide,amiodarone,and dronedarone.Amiodarone was used in 19.6% of the patients at the beginning of the study,which decreased to 11.8% after two years.Additionally,approximately 20% of patients received catheter ablation as a rhythm control strategy.The differences in other treatments are shown in Table 28.
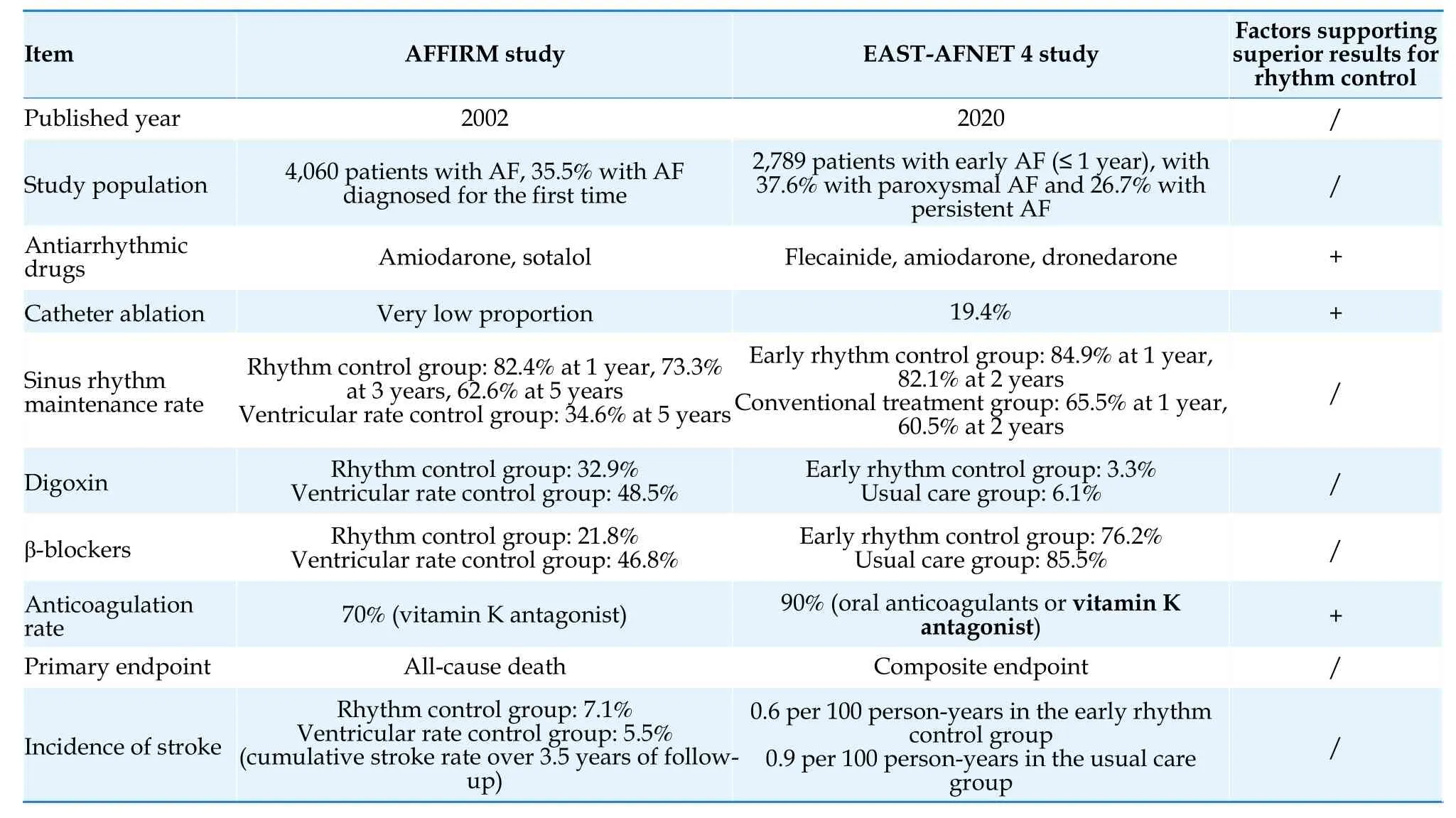
Table 28 Comparison between the EAST-AFNET 4 study and AFFIRM study.
Safe and effective rhythm control is the ideal strategy for the treatment of AF.Increasing evidence from numerous studies supports the early rhythm control strategies for patients with early AF or AF complicated with heart failure.However,current guidelines and clinical practices in China and abroad have not fully embraced this concept.Early rhythm control strategies can effectively reduce atrial remodeling,prevent AF-related death,heart failure,and stroke in high-risk populations,and play an important role in delaying AF progression and reducing AF-related symptoms.Therefore,early rhythm control should be actively applied in larger AF populations [Table 29].[11,207,208]With the improvement of integrated management of AF,an increasing number of patients with AF will be diagnosed at an early stage.In the future,patients who meet the criteria for early rhythm control will become the mainstream population of AF patients.

Table 29 Rhythm control of AF.
5.1.2 Strategy for the selection of antiarrhythmic drugs and catheter ablation
Antiarrhythmic drugs and catheter ablation are the main approaches for rhythm control.Owing to good acceptability and efficacy in restoring sinus rhythm and relieving symptoms,antiarrhythmic drugs has long been recommended as a first-line treatment for symptom improvement by the guidelines.Although previous studies have shown only moderate effectiveness of antiarrhythmic drugs in maintaining sinus rhythm,recent results from the EASTAFNET 4 study have shown that early rhythm control therapy primarily based on safe and rational antiarrhythmic drugs was both safe and effective,and improved prognosis.In the early rhythm control group,84% patients received antiarrhythmic drugs treatment,while 19.4% underwent catheter ablation,resulting in a sinus rhythm maintenance rate of 80%after 2 years.[209]
There is robust evidence supporting the use of catheter ablation for rhythm control.Compared to antiarrhythmic drugs,catheter ablation can significantly reduce the risk of AF recurrence and cardiovascular hospitalizations.[210-213]Catheter ablation as a first-line treatment for paroxysmal AF is superior to antiarrhythmic drugs in reducing symptomatic AF recurrence and improving patients’ quality of life.[211]However,reliable studies on whether catheter ablation can improve the prognosis of AF patients are still lacking.The Catheter Ablation vs.Antiarrhythmic Drug Therapy for Atrial Fibrillation(CABANA) study implied that although the catheter ablation group showed a reduction in the primary composite endpoint (death,disabling stroke,serious bleeding,and cardiac arrest) compared to the drug therapy group,the difference was not statistically significant.The main reason for the negative results of the primary endpoint in the CABANA study was the high crossover rate between the groups,which impacted the power of the intention-to-treat analysis.Additionally,there were limitations such as lowerthan-expected event rates and inadequate sample size,which prevented answering the question of whether catheter ablation is superior to drug therapy in improving prognosis.[214]An observational study included 183,760 AF patients who underwent catheter ablation or drug therapy during the same period as the CABANA study.Among the patients who met the CABANA inclusion criteria (73.8%),catheter ablation was significantly associated with a reduction in the composite endpoint of all-cause death,stroke,major bleeding,and cardiac arrest.[215]Studies have confirmed that catheter ablation is more effective than antiarrhythmic drugs in delaying the progression from paroxysmal AF to persistent AF.[7,8]Restoring the sinus rhythm can reverse cardiac chamber enlargement and alleviate functional valve regurgitation.[216]
AF and heart failure share common risk factors and often coexist.[20]The risk of developing heart failure in patients with AF is 1-2-times higher than in those without AF,while the risk of developing AF in patients with heart failure is 2-times higher than in those without heart failure.[217]The prevalence of AF in patients with heart failure with reduced ejection fraction (HFrEF) ranges from 36.7% to 44.9%,while in patients with heart failure with preserved ejection fraction(HFpEF),the prevalence of AF ranges from 40% to 50%.[218]Additionally,heart failure is a major cause of mortality in patients with AF.The Randomized Evaluation of Long-Term Anticoagulation Therapy(RE-LY) AF registry study showed that heart failure was the leading cause of death within 1 year in patients who had previously sought emergency care for AF.[219]
For patients with AF and HFrEF,multiple RCTs have shown that catheter ablation can improve the prognosis.The Catheter Ablationvs.Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) study demonstrated that catheter ablation significantly reduced the composite endpoint of all-cause mortality and hospitalization due to worsening heart failure in AF patients with LVEF < 35% who have an implantable cardioverter-defibrillator or cardiac resynchronization therapy-defibrillator (CRT-D)device.[9]The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AATAC) study,which had the primary endpoint of sinus rhythm maintenance rate,also showed that catheter ablation reduced mortality and rehospitalization rates in heart failure patients with LVEF < 40%.[220]Subsequently,several meta-analyses have confirmed the benefits of catheter ablation in patients with heart failure and AF.One meta-analysis,which included 18 RCTs,showed that catheter ablation significantly reduced all-cause mortality in AF patients,with the greatest benefit observed in those with AF and HFrEF.[210]Another meta-analysis,which included 7 RCTs,demonstrated that catheter ablation,compared to rate control strategies,reduced all-cause mortality and rehospitalization rates,increased sinus rhythm maintenance rate,and improved cardiac function and quality of life in patients with AF and heart failure,while rhythm control strategies primarily using amiodarone showed no significant difference in all-cause mortality,stroke,and thromboembolic events compared to rate control strategies.[10]Catheter ablation in AF patients with HFrEF led to significant improvement in left ventricular function and fibrosis.[221,222]For patients with heart failure caused by AF,catheter ablation may result in greater improvement in left ventricular function and prognosis.
When HFrEF is combined with AF and complete left bundle branch block,the treatment strategy should take into account factors such as the etiology of heart failure,the degree of heart failure control,and the extent of myocardial fibrosis.First,catheter ablation should be actively performed.Patients with left bundle branch block secondary to dilated cardiomyopathy and a high degree of myocardial fibrosis shown on cardiac MRI may warrant multidisciplinary discussion regarding the indication for heart transplantation.For patients with unsatisfactory control of heart failure who are intolerant to catheter ablation and have sufficient viable myocardium,priority should be given to CRT-D therapy.Subsequently,when significant improvement in cardiac function and relative stability of pacing electrodes(3-6 months) have been achieved,AF catheter ablation can be performed.If there is no possibility of restoring sinus rhythm from AF,combined atrioventricular node ablation and CRT-D therapy may also be considered.[223,224]
For patients with concomitant AF and HFpEF,catheter ablation can improve hemodynamic parameters,exercise tolerance,and quality of life.[225,226]Posthoc analysis of the CABANA study showed that catheter ablation is significantly associated with reduced mortality,decreased AF recurrence,and improved symptoms in patients with concomitant stable heart failure,predominantly HFpEF.[227]
For patients with end-stage heart failure who have concomitant AF and are awaiting cardiac transplantation,studies are also being conducted to evaluate the role of AF catheter ablation in reducing the risk of the composite endpoint including all-cause mortality and the need for urgent transplantation or implantation of a left ventricular assist device due to worsening heart failure.[228]
In clinical practice,it is important to respect the patient’s preferences and comprehensively evaluate the patient’s comorbidities,risk factors,symptoms,cardiac function,medication adherence,tolerance,and treatment outcomes,and select appropriate strategies for ventricular rate control and rhythm control [Figure 3].
Figure 3 Flowchart of ventricular rate control and rhythm control strategies in AF patients.AAD: Antiarrhythmic drugs;AF: Atrial fibrillation;HF: Heart failure.*In patients undergoing catheter ablation,if the rhythm control effect is not satisfactory,AAD treatment can be performed.In patients undergoing AAD treatment,if the rhythm control effect is not satisfactory,catheter ablation can be performed.If neither of these rhythm control treatment strategies can achieve the desired therapeutic effect despite reasonable and adequate application (such as unsatisfactory improvement of AF-related symptoms,high risk of AF recurrence),a ventricular rate control strategy can be performed.
5.2 Long-term Antiarrhythmic Drug Therapy
antiarrhythmic drugs has moderate efficacy in reducing recurrent episodes of AF and maintaining sinus rhythm in the long term,but they are associated with relatively common adverse reactions.[229-232]When using antiarrhythmic drugs for sinus rhythm maintenance,it is important to prioritize safety and consider the associated adverse reactions.The principle of “safety first and efficacy second”should be emphasized in the selection and application of antiarrhythmic drugs.Individualized treatment strategies should be chosen based on the patient’s condition,and careful evaluation of the timing,duration,and dosage of antiarrhythmic drugs should be conducted to avoid excessive use.Continuous monitoring,assessment,and adjustment of medication should be carried out,with attention to the proarrhythmic effects and cardiac toxicity of antiarrhythmic drugs.For patients with poor response to antiarrhythmic drugs treatment or those with adverse reactions,alternative treatment methods,such as changing the type of medication or opting for catheter ablation,should be promptly considered,while respecting the patient’s preferences.
Amiodarone is a class Ⅲ antiarrhythmic drugs with multi-channel blocking effects.It is the most effective among all antiarrhythmic drugs,[233,234]but also has the most side effects (Table 30).[229]Amiodarone should ideally be used as a second-line or last-resort option for the treatment of AF with antiarrhythmic drugs.However,in current clinical practice in China,overuse of amiodarone is common.There should be a stronger emphasis on the limitations of amiodarone use.If other alternative antiarrhythmic drugs or catheter ablation are available,usage of amiodarone should be avoided as far as possible or only last for a short period.
Table 30 Incidence,manifestations,and treatment of adverse reactions of amiodarone.[235]
The main cardiovascular side effect of amiodarone is sinus bradycardia (with ventricular rate decreased by 10-12 beats/min).Prolongation of the QT interval is also common but rarely associated with torsade de pointes (incidence < 0.5%).The incidence,manifestations,and treatment of adverse reactions in extracardiac organs in patients treated with amiodarone are shown in Table 30.[235]Baseline evaluation should be performed before initiating amiodarone therapy,and regular monitoring should be conducted during its use to promptly detect adverse reactions.Owing to potential drug interactions,patients receiving warfarin should have their INR monitored at least once a week during the first 6 weeks of amiodarone treatment.[235]
Pulmonary toxicity of amiodarone: The incidence of pulmonary toxicity induced by amiodarone is 2%to 17%,which is the most serious extracardiac adverse reaction of amiodarone and is closely related to the cumulative dose of amiodarone.It often occurs months to years after the administration of amiodarone.[235]Patients with a daily dose of amiodarone ≥400 mg,treatment duration exceeding 6 to 12 months,and age > 60 years have a higher risk of developing pulmonary toxicity than others.[236]The most common manifestation is interstitial pneumonia,with dry cough and progressively worsening dyspnea as the main clinical symptoms.Imaging findings show patchy interstitial infiltrates,and pulmonary function tests reveal decreased diffusion capacity.Other manifestations include eosinophilic pneumonia,organizing pneumonia,acute respiratory distress syndrome (annual incidence < 1%),diffuse alveolar hemorrhage,pulmonary nodules or solitary masses,and pleural effusion.Patients taking amiodarone for a long time undergo chest radiographic examination at the beginning of treatment,and annually thereafter.[237]When symptoms suggestive of pulmonary toxicity occur,chest radiography or chest CT and pulmonary function tests should be performed immediately.Amiodarone should be discontinued once pulmonary toxicity is suspected.Even after discontinuation,pulmonary toxicity may continue to progress.In severe cases,glucocorticoids may be used concurrently with the discontinuation of amiodarone.It is not recommended to re-administer amiodarone after the pulmonary condition has recovered.
Amiodarone-induced thyroid toxicity: Amiodarone can affect thyroid function via 2 pathways: amiodarone's high iodine content and its direct toxic effect on the thyroid.This can manifest as either hypothyroidism or hyperthyroidism,with incidences of 6% and 2% respectively.[235]Hyperthyroidism typically occurs several months after starting amiodarone treatment,[238]while hypothyroidism usually occurs within 2 weeks to several months of initiating treatment.Thyroid function (including T3,T4,and thyroid-stimulating hormone) should be monitored 3 months after starting amiodarone therapy.If the thyroid-stimulating hormone levels are normal,longterm users should have their thyroid function checked at least every 6 months.[239]In patients who must continue using amiodarone and develop hypothyroidism,supplementation with T4 should be initiated while continuing the medication.[239]
The antiarrhythmic mechanism of dronedarone is similar to that of amiodarone,but it is not as effective as amiodarone in reducing AF recurrence.The A Placebo-Controlled,Double-Blind,Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from any Cause in Patients with Atrial Fibrillation/Atrial Flutter (ATHENA) study showed that dronedarone can reduce the risk of cardiovascular hospitalization or all-cause mortality in patients with paroxysmal or persistent AF.[240]The Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease (ANDROMEDA) study showed that dronedarone increased the mortality rate in AF patients with severe left ventricular systolic dysfunction (defined as wall motion score index ≤ 1.2,approximately equivalent to LVEF< 35%).[241]The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)study showed that dronedarone increased the risk of heart failure,stroke,and cardiovascular death in patients with permanent AF.[242]Sotalol showed betablocking effects but increased the risk of death.[232]The safety and efficacy of flecainide and propafenone are moderate,[229]and they can be used for rhythm control in patients with normal left ventricular systolic function and no structural heart disease.[232,243-245]Dofetilide has similar effectiveness to amiodarone in reducing atrial arrhythmia recurrence when used under reasonable monitoring,[246]but it should not be used in patients with prolonged QT interval,and its effect on QT interval should be monitored at the initiation of administration.Combining dofetilide with moricizine can reduce the impact of dofetilide on QT interval and potentially improve treatment effectiveness and safety.[247]The long-term use and precautions of various antiarrhythmic drugs are summarized in Table 31.Recommendations for antiarrhythmic drugs treatment of AF are summarized in Table 32.[229,230,232,248]
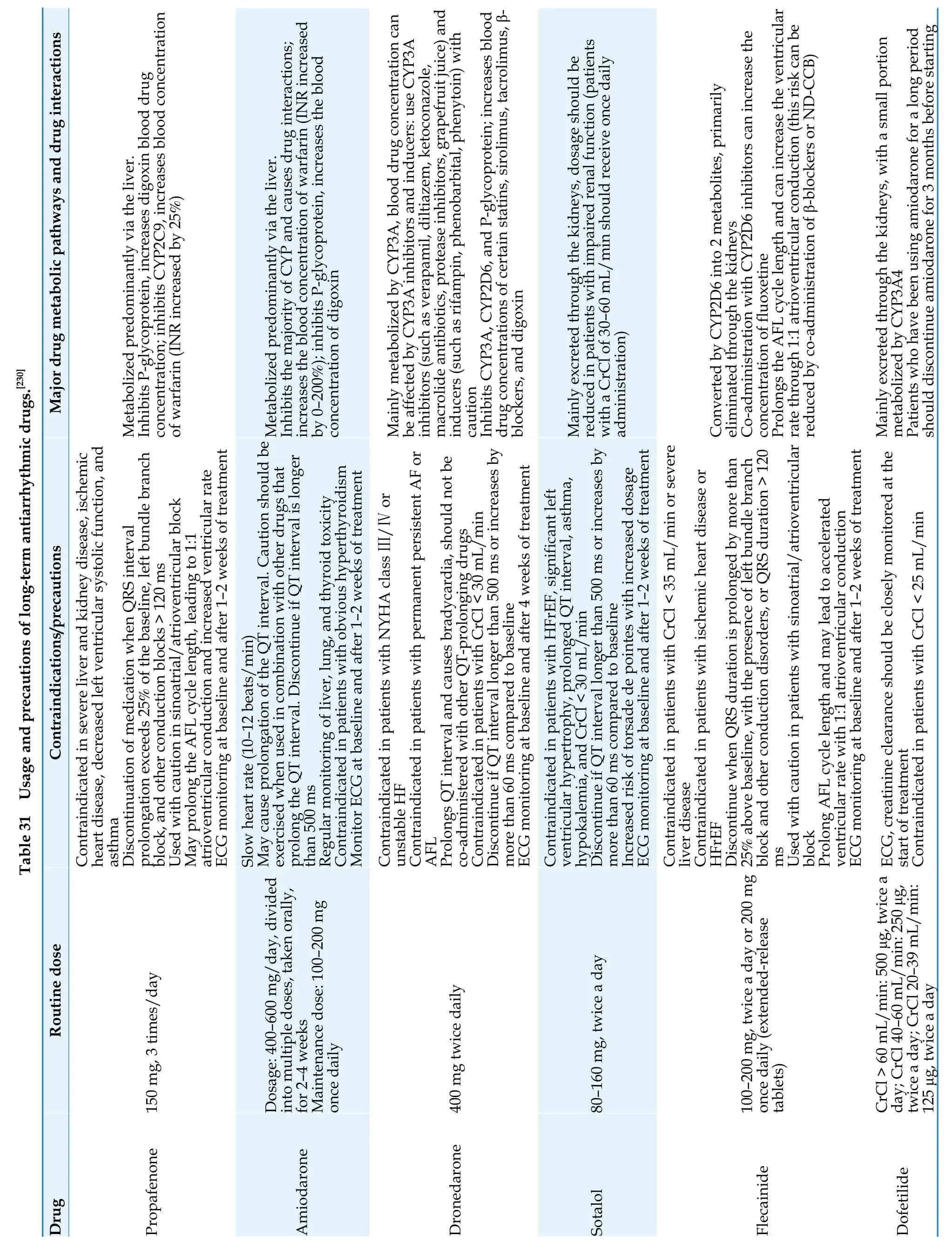
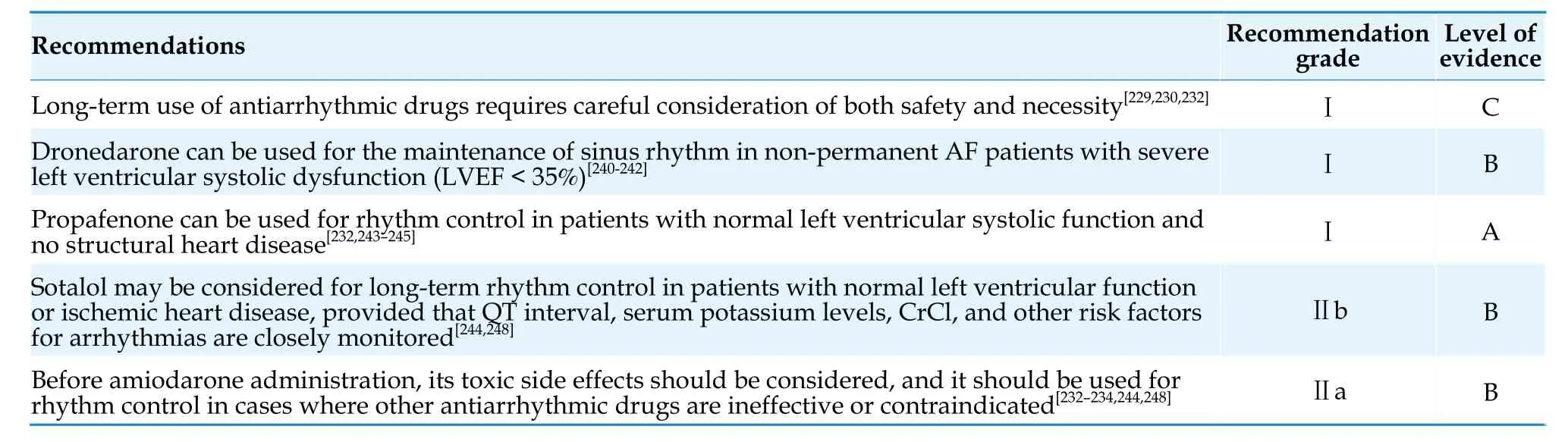
Table 32 Antiarrhythmic drug therapy for AF.
5.3 Catheter Ablation
5.3.1 Indications
For patients undergoing catheter ablation for AF,a comprehensive clinical evaluation should be performed,including identification and correction of reversible factors contributing to AF onset,assessment of procedural risks and the major risk factors for AF recurrence,evaluation of prognosis,and careful consideration of patient preferences.
Compared to antiarrhythmic drugs,catheter ablation can significantly reduce the risk of AF recurrence and cardiovascular hospitalizations.[210-213]Therefore,symptomatic AF patients who are either unresponsive to or intolerant of antiarrhythmic drugs treatment should undergo catheter ablation to improve symptoms.For paroxysmal AF,catheter ablation is clearly superior to antiarrhythmic drugs,as it can significantly lower the recurrence rate of AF,improve AF-related symptoms,and reduce the risk of rehospitalization and visitation rate without increasing the risk of serious adverse events.It is also considered as a first-line rhythm control strategy before antiarrhythmic drugs treatment with robust evidence.[211]For persistent and long-standing persistent AF,catheter ablation is associated with a reduction in AF recurrence rate and improvement in quality of life.[249-251]
Indications for catheter ablation in patients with AF and heart failure can be found in the “Rhythm Control Strategy”section of the Guidelines.
5.3.2 Strategy for the selection of antiarrhythmic drugs and catheter ablation
Asymptomatic AF patients also face an increased risk of stroke,systemic embolism,all-cause mortality,and cardiovascular mortality.[25,252]Subgroup analysis of the EAST-AFNET 4 study showed that early rhythm control in asymptomatic AF patients with cardiovascular risk factors provided similar benefits as in symptomatic AF patients.[253]Observational studies have shown that catheter ablation can improve the quality of life,exercise tolerance,and cardiac functional parameters in asymptomatic AF patients.[250,254]Catheter ablation may be considered in some asymptomatic patients after a thorough discussion with them regarding its benefits and risks.[255]However,if there are multiple risk factors for recurrence,catheter ablation is not recommended.
For patients with symptomatic cardiac arrest/long pauses (ie,tachy-brady syndrome) during the conversion of AF to sinus rhythm,successful treatment of AF by catheter ablation can eliminate such long pauses,thereby avoiding the need for permanent pacemaker implantation.[256-258]
Catheter ablation is equally safe and effective in advanced-age patients with AF,but current research has inconsistent conclusions regarding its impact on prognosis.[259,260]
Observational studies have shown that in patients with AF and concomitant severe functional mitral and/or tricuspid regurgitation,restoration of the sinus rhythm by catheter ablation can significantly reduce the degree of mitral and/or tricuspid regurgitation.[216,261]
A summary of the indications for catheter ablation of AF is shown in Table 33.[249,262-264]
5.3.3 Ablation technique
Pulmonary veins are the most common source of ectopic electrical activity that triggers AF.[265]Achieving pulmonary vein isolation (PVI) should be the cornerstone of all AF catheter ablation procedures.[266-268]For persistent and long-standing persistent AF,the success rate of the ablation procedure with PVI alone is low,with a clinical success rate of 57% after a single ablation and an improvement to 71% after multiple ablations.[267]A study showed that the sinus rhythm maintenance rates in paroxysmal AF patients were 67.8%,56.3%,and 47.6% at 5,10,and 15 years,respectively,while for persistent AF,the rates were 46.6%,35.6%,and 26.5%,and for long-standing persistent AF,the rates were 30.4%,18.0%,and 3.4%.[269]
Currently,different centers are practicing different additional ablation strategies with PVI,including linear ablation,substrate modification (complex fractionated electrogram ablation),rotor (driver) ablation,posterior wall isolation,extra-pulmonary vein trigger ablation,and left atrial appendage isolation.However,the effectiveness of these ablation strategies remains controversial.[267,270,271]Ethanol ablation of the vein of Marshall can improve the rate of mitral isthmus blockage and significantly increase the success rate of persistent AF ablation.[272-275]
In terms of the energy source for ablation,radiofrequency ablation and cryoballoon ablation have shown similar safety and efficacy in studies focusing on PVI as the endpoint.[276-279]
Pulse field ablation theoretically has selective effects on myocardial cells,with minimal impact on adjacent tissues such as blood vessels,nerves,and esophagus.Multiple clinical studies have shown its good safety and efficacy.[280-282]Pulse field ablation also significantly improves procedural efficiency and reduces operation time,which is beneficial for shortening the learning curve.However,potential adverse reactions of pulse field ablation,such as coronary artery spasm,asymptomatic brain injury,and tracheal injury require further evaluation and understanding.
Intracardiac echocardiography has significant value in catheter ablation procedures,as it can assist in locating relevant anatomical structures,guide transseptal puncture,monitor the ablation process,identify thrombus formation,and detect pericardial effusion in an early phase.[283]A meta-analysis has shown that the use of intracardiac echocardiography in AF ablation can shorten fluoroscopy time,reduce radiation exposure,and even achieve zero-fluoroscopy AF ablation,[284,285]without affecting efficacy and safety.[283]The recommended AF ablation techniques are listed in Table 34.

Table 34 Ablation techniques for AF.
5.3.4 Complications
The incidence of complications in catheter ablation for AF is approximately 2.9% to 10%,[286-292]with an incidence of 0.7% to 0.9% for potentially fatal complications.[290,292,293]The perioperative mortality rate is 0.05% to 0.55%,[292,294,295]with the most common cause being cardiac tamponade.[292]Most catheter ablation-related complications occur during the procedure and within 24 h post-procedure,but they can also occur 1 to 2 months after the procedure,primarily related to catheter manipulation and ablation injury.With advancements in catheter ablation techniques for AF such as the use of pressure-sensing catheters,ultrasound-guided venous puncture,[296]esophageal protection measures,[297]ablation/injury index-guided ablation,pulsed field ablation,[280-282]the accumulation and dissemination of experience in operation and process management and improvements in training quality,the safety of catheter ablation for AF has steadily improved,and the incidence of AF ablation-related complications has been decreasing year by year.[298-300]
Pulmonary vein stenosis can be asymptomatic or present with dyspnea,cough,chest pain,and hemoptysis.Owing to the non-specific nature of these symptoms,it often leads to missed diagnosis or misdiagnosis.[301]When these symptoms occur after AF ablation,the possibility of pulmonary vein stenosis should be considered.Two studies evaluated the incidence of pulmonary vein stenosis by comparing pre-and post-ablation pulmonary vein CT angiography or MRI.One study showed that in 41 out of 197 patients (20.8%),47 out of 573 pulmonary veins (8.2%)had pulmonary vein stenosis,with mild stenosis(stenosis rate< 50%) accounting for 7.3%,moderate stenosis (stenosis rate: 50%-70%) accounting for 0.9%,and no severe pulmonary vein stenosis.[302]In another study,out of 976 patients,306 (31.4%) had mild stenosis,42 (4.3%) had moderate stenosis,and 7 patients (0.7%) had at least one pulmonary vein with severe stenosis.[303]Standardized procedures can prevent the most occurrences of pulmonary vein stenosis.Pulmonary vein stenosis mainly occurs immediately during the procedure,and a certain proportion of patients may progress or improve after the procedure.Currently,there is no clear treatment strategy for pulmonary vein stenosis detected immediately during the procedure.Asymptomatic pulmonary vein stenosis generally does not require special treatment,while treatment options for symptomatic pulmonary vein stenosis include balloon dilation and stent placement.[301]However,there is still a high rate of long-term restenosis (> 30%),which can be significantly reduced by stent placement.[304]Severe pulmonary vein stenosis can lead to adverse clinical outcomes,and preventive measures should be taken to ensure zero occurrence.
Atrioesophageal fistula (AEF) is one of the most serious complications of catheter ablation for AF.The latest large-scale study,PrOgnosis following oesophageal fisTula formaTion in patients undergoing cathetER ablation for AF (POTTER-AF),reported an incidence rate of 0.025% for AEF (0.038% for radiofrequency ablation,0.0015% for cryoballoon ablation).[305]AEF mainly occurs within a few days to 2 months after the procedure.[305-306]The most common symptoms are infection-related symptoms(eg,chills,high fever,intracardiac neoplasm) and embolic symptoms (e.g.,myocardial infarction,stroke),as well as odynophagia,chest pain,hemoptysis,and other manifestations.[305-306]Once these symptoms occur,AEF should be considered as the first possibility,and an immediate enhanced CT scan of the left atrium should be performed.It is also important to involve experts with experience in the diagnosis and management of AEF in the medical de-cision-making process.Once diagnosed,surgical treatment should be performed as soon as possible,or endoscopic treatment,such as esophageal stenting,can be considered.Otherwise,the prognosis is extremely poor.Studies have shown that the mortality rate after surgical treatment of AEF is 51.9%,the mortality rate after endoscopic treatment is 56.5%,and the mortality rate with medical treatment alone is as high as 89.5%.[305]When there is high suspicion of AEF,esophagoscopy examination should be avoided.If there are symptoms such as odynophagia before the occurrence of AEF,and no fever or embolic symptoms are present,esophagoscopy can be cautiously performed.If severe damage or ulceration and exudative changes are found,semi-recumbent position,fasting,water deprivation,parenteral nutrition,intravenous administration of proton pump inhibitors and antibiotics should be immediately initiated.If the symptoms significantly improve after 1 week,esophagoscopy can be repeated.The flowchart of management for suspected esophageal injury after catheter ablation is shown in Figure 4.

Figure 4 Flowchart of management for esophageal injury/esophageal fistula after AF ablation.AF: atrial fibrillation;CRP: C-reactive protein;IE: infective endocarditis;PPI: proton pump inhibitor;TEE: transesophageal echocardiography.
The incidence of complications in AF ablation is significantly correlated with the procedure volume of the center and the operator.Centers with an annual volume of < 50 cases of AF ablation procedures,as well as operators with an annual volume of < 25 cases of AF ablation procedures will lead to a significantly increased risk of complications.[294,307]
Complications related to catheter ablation for AF are summarized in Table 35,[286,287,289-293,302,303,305,306,308]and the management and treatment recommendations for major complications are summarized in Table 36.[295,304-306]
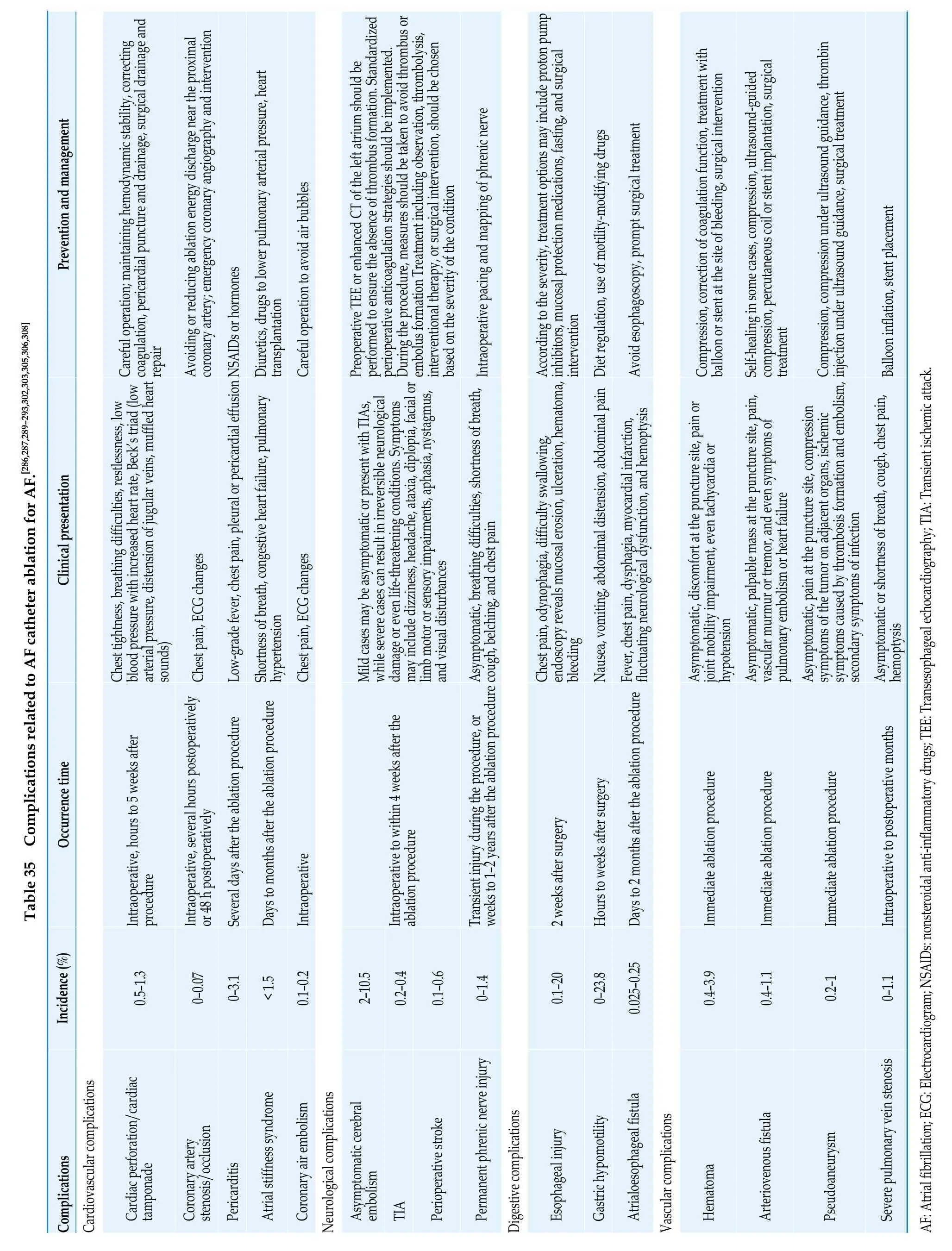

Table 36 Management of complications of AF catheter ablation.
5.3.5 Post-ablation management
Patients should be thoroughly educated on how to recognize delayed complications,including delayed cardiac tamponade,AEF,and pulmonary vein stenosis,to reduce misdiagnosis and delayed medical treatment.It is important for patients to seek prompt medical care if they experience any relevant symptoms.
Symptom recurrence after catheter ablation for AF may be caused by AF recurrence or other factors(including but not limited to anxiety,premature beats,or other non-sustained arrhythmias),which can be further evaluated through ECG or ambulatory ECG.
After ablation,the use of antiarrhythmic drugs for 6 weeks to 3 months can reduce early recurrence but does not decrease long-term recurrence rates.For patients with paroxysmal AF,the value of using antiarrhythmic drugs after ablation is limited.Whether to use antiarrhythmic drugs beyond 3 months after the procedure should be evaluated based on the patient’s clinical condition.[309]Regular followup after the procedure can detect asymptomatic AF.Monitoring should be strengthened especially for patients who discontinue anticoagulant therapy after the procedure.Wearable monitoring devices such as smartwatches/wristbands with ECG monitoring function and ECG patches have great potential for application.
For anticoagulation therapy after catheter ablation,please refer to the section “4.5.Anticoagulation therapy in special populations and special situations:Catheter ablation for AF”in the chapter “Stroke Prevention”in the Guidelines.
5.3.6 Risk assessment for recurrence after ablation
Risk factors for AF recurrence after ablation include age,duration of AF,left atrial size,structural heart disease,and degree of atrial fibrosis.Currently,although there are prediction models such as CHADS2,ALARMEc,BASE-AF2,CAAP-AF,APPLE,MB-LATER,ATLAS,and LAGO,the accuracy of predicting recurrence is not sufficiently high.[310]In clinical practice,before deciding to perform catheter ablation,a comprehensive evaluation of the patient’s risk factors for AF recurrence is necessary.For patients with a high risk of recurrence,the decision to perform catheter ablation should be made based on the patient’s condition,and thorough communication with the patients and their family should be ensured to make informed decisions while actively controlling modifiable risk factors.
The current recognized definition of successful AF ablation procedure is the absence of AF/atrial flutter/atrial tachycardia events lasting longer than 30 s without the use of antiarrhythmic drugs after the blanking period (defined as the first 3 months after ablation).The Guidelines still recommend a blanking period of 3 months after ablation,during which the occurrence of atrial tachyarrhythmias (usually defined as early recurrence) is not considered as AF recurrence after the ablation procedure.However,increasing evidence shows that early recurrence is very common and significantly associated with late recurrence.[311-313]In the Cryoballoon vs.Irrigated Radiofrequency Catheter Ablation (CIRCA-DOSE)study,follow-up of AF patients with implantable cardiac monitors showed that 61% patients had early recurrence,of which nearly 75% were asymptomatic.The timing and burden of early recurrence have predictive value for late recurrence.[312]There are also studies suggesting that shortening the blanking period to 1 month may be more reasonable.[314-316]In addition,the definition of recurrence as “≥ 30 s”is also debatable.Studies using implantable cardiac monitors monitoring have shown that patients with recurrence lasting < 1 h and AF burden < 0.1% have no significant difference in healthcare resource utilization than patients without recurrence,while recurrence events lasting > 1 h and AF burden > 0.1%are associated with increased healthcare resource utilization.[317]Therefore,in the future,AF burden may be considered as a determining factor for successful AF ablation,which needs further research validation.
For patients who experience symptomatic arrhythmia after ablation,if effective anticoagulation is not interrupted,direct electrical cardioversion may be considered.Whether to perform repeated catheter ablation in patients with recurrence during the blanking period should be determined based on the severity of symptoms,long-term recurrence risk,and risk of procedure complications.Generally,repeated ablation during the blanking period is not recommended because some patients with early recurrence do not have further episodes in the long term,therefore,ablation during the blanking period would increase exposure to unnecessary procedures.[318]However,if the patient has severe symptoms,or even hemodynamic instability,and the mechanism of recurrence is clear,repeated ablation during the blanking period may be considered.[318]
5.4 Surgical Treatment of AF
Surgical treatment for AF was first proposed by James Cox in 1987.[319]It involves extensive cutting and resewing of the atrial wall to create regional electrical isolation,eliminating the anatomical basis for re-entry circuits,which is known as the Maze Ⅲprocedure.The Maze Ⅳ procedure,which utilizes energy sources such as radiofrequency or cryotherapy instead of sutures,has become the most commonly used ablation technique in surgical treatment for AF.
Surgical treatment for AF can be classified into the following 2 categories: (1) concomitant surgical treatment for AF during cardiac surgery;(2) surgical treatment for AF alone or as hybrid surgery combining catheter ablation and surgical treatment.
Concomitant surgical treatment of AF during cardiac surgery can be divided into 2 categories: cardiac surgery with atrial incision and cardiac surgery without atrial incision.In cardiac surgeries that involve atrial incision (such as mitral valve replacement or repair (with or without tricuspid valve surgery) and atrial septal defect repair),it is relatively easy to perform surgical ablation for AF.In patients undergoing mitral valve surgery with concomitant surgical treatment for AF (including Maze Ⅲ and Maze Ⅳ procedures),the sinus rhythm maintenance rate after 5 years is significantly higher in the ablation group than the no ablation groups,and the risk of death and thromboembolic events is significantly lower in the ablation group than the no ablation groups.[320]A meta-analysis has shown that surgical ablation for AF during cardiac surgery can significantly improve the 1-year maintenance rate of sinus rhythm without increasing the risk of perioperative death.[321]In patients with concomitant AF who require cardiac surgery for other heart diseases,concomitant surgical treatment for AF should be considered.[322]
In terms of surgical treatment specifically for the purpose of treating AF,the 5-year AF recurrence rate for Maze Ⅲ surgery in the treatment of persistent AF is >90%,[323]while the 5-year sinus rhythm maintenance rate for Maze Ⅳ surgery in the treatment of persistent AF ranges from 83% to 92%.[324,325]With the advancement of minimally invasive surgical ablation techniques for AF,epicardial ablation under thoracoscopy is increasingly being used.The Ablation or Surgical Treatment: A Randomized Study Comparing Non-pharmacologic Therapy in Patients With Drug-refractory Atrial Fibrillation (FAST) study showed that while epicardial ablation under thoracoscopy is superior to endocardial catheter ablation in maintaining sinus rhythm,it carries a higher risk of complications than the latter.In the subgroup analysis,the sinus rhythm maintenance rate is better with epicardial ablation under thoracoscopy than catheter ablation in patients with paroxysmal AF;however,there was no difference between the 2 approaches for patients with persistent AF.[326,327]In the recent Catheter Ablationvs.Thoracoscopic Surgical Ablation in Long-Standing Persistent AF (CASAAF) study,the sinus rhythm maintenance rate at 12 months after epicardial ablation under thoracoscopy and percutaneous catheter ablation showed no significant difference in patients with persistent AF,as evaluated by an implanted loop recorder.[328]For patients with recurrent AF after previous catheter ablation,an RCT showed that regardless of whether it is paroxysmal or persistent AF,epicardial ablation under thoracoscopy is superior to catheter ablation in terms of sinus rhythm maintenance rate,but it has a higher incidence of serious adverse events.[329]
To further improve the efficacy of surgical ablation for AF,the combination of thoracoscopic epicardial ablation with interventional mapping and catheter ablation techniques has been developed,forming a hybrid ablation procedure.In addition to surgical PVI and atrial linear ablation,concomitant or staged endocardial ablation of the mitral and/or tricuspid isthmus and/or fractionated electrogram ablation or surgical ablation lines can be performed by an electrophysiologist,which helps to improve the success rate of AF treatment.The Convergence Of Epicardial And Endocardial Radiofrequency Ablation For The Treatment Of Symptomatic Persistent AF (CONVERGE) study showed that combined epicardial and endocardial ablation is superior to endocardial catheter ablation in terms of sinus rhythm maintenance rate in patients with persistent/long-standing persistent AF,[330]but a systematic review and meta-analysis showed a slight increase in the risk of complications with the hybrid ablation for AF.[331]Recommendations for surgical treatment of AF are summarized in Table 37.
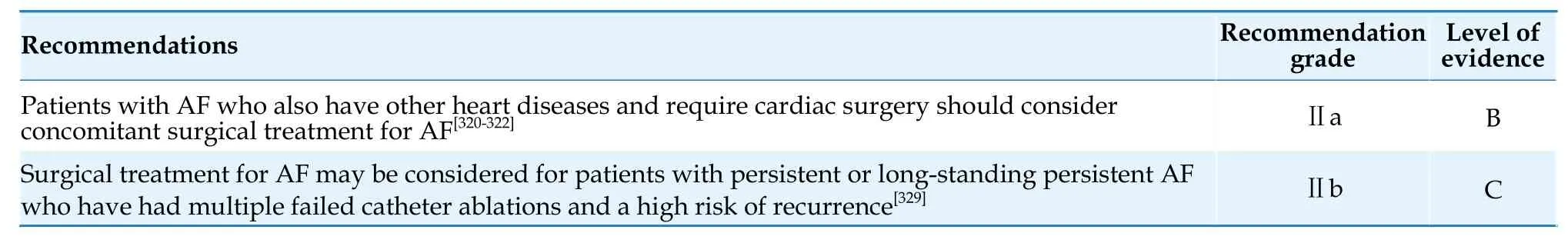
Table 37 Surgical treatment of AF.
6.Ventricular rate control in AF
6.1 Objectives of Ventricular Rate Control
Ventricular rate control strategies for patients with AF include strict ventricular rate control (resting heart rate ≤ 80 beats/min,heart rate < 110 beats/min during moderate-intensity exercise) and lenient ventricular rate control (resting heart rate < 110 beats/min).The Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient versus Strict Rate Control II (RACE Ⅱ) study enrolled 614 patients with permanent AF,of which 34.8% had concomitant heart failure (New York Heart Association class Ⅱ-Ⅲ),and found that the primary composite endpoint (death,hospitalization,stroke,bleeding,and malignant arrhythmias)of lenient ventricular rate control was non-inferior to strict ventricular rate control.[332]Therefore,the initial objective of ventricular rate control for patients with AF can be set as a resting heart rate< 110 beats/min,and if the patient's symptoms persist,stricter ventricular rate control should be considered [Table 38].AF combined with heart failure is a common clinical problem,and international guidelines have not provided consistent recommendations for the objectives of ventricular rate control in these patients.Thus,the objectives of ventricular rate should be determined on the basis of satisfactory control of heart failure symptoms.

Table 38 Long-term ventricular rate control objectives in patients with AF.
6.2 Drugs for ventricular rate control
Commonly used drugs for long-term ventricular rate control include β-blockers,non-dihydropyridine calcium channel blockers (ND-CCBs),digoxin,and some antiarrhythmic drugs.The selection of the type and dosage of ventricular rate control drugs should be based on the individual’s condition and response to medication (Figure 5) (Table 39).If a single drug fails to achieve the target heart rate,combination therapy with different types of ventricular rate control drugs should be considered.[333,334]
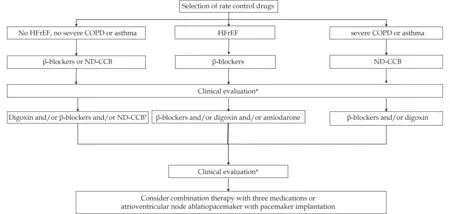
Figure 5 Flowchart of drug selection for ventricular rate control.*Clinical evaluation includes evaluating resting heart rate,symptoms related to atrial fibrillation/atrial flutter,and quality of life.?The combination of β-blockers and ND-CCBs has a synergistic effect.Caution should be taken to avoid bradycardia.Digoxin is preferable for use in combination with β-blockers or ND-CCBs from the firstline drugs.COPD: chronic obstructive pulmonary disease;HFrEF: heart failure with reduced ejection fraction;ND-CCB: non-dihydropyridine calcium channel blockers.
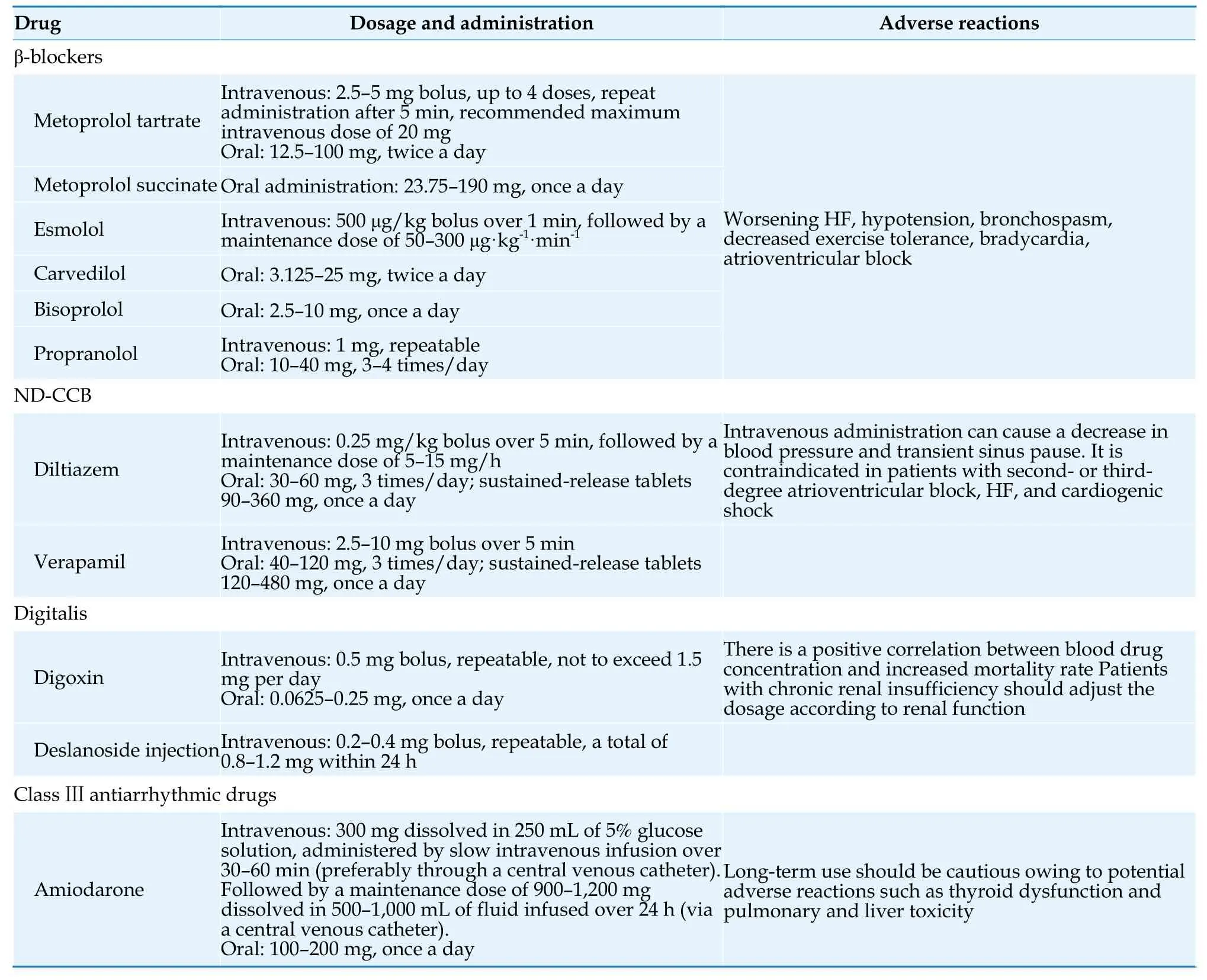
Table 39 Drugs and usage for ventricular rate control.
Owing to the effectiveness in ventricular rate control and good tolerability across all age groups,βblockers are still the first-line treatment for ventricular rate control in patients with AF.However,a meta-analysis suggested that β-blockers did not improve the prognosis of patients with AF and HFrEF.[335]
ND-CCB can effectively control the resting and exercise heart rate in patients with persistent AF,and improve exercise tolerance.[336,337]Unlike β-blockers,ND-CCB can preserve the exercise capacity of patients with persistent AF and reduce NT-proBNP levels.[338]ND-CCB has negative inotropic effects and is therefore only used in non-HFrEF patients.
The Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) study showed that there was no significant difference in the improvement of quality of life in AF patients with heart failure who were treated with low-dose digoxin (62.5-250 μg/day,mean: 161 μg/day) compared to lowdose bisoprolol (1.25-15 mg/day,mean: 3.2 mg/day).[339]Although observational studies have suggested an increased mortality rate in AF patients treated with digoxin,subsequent analysis of the Digitalis Investigation Group (DIG) study showed that the results from observational studies were likely influenced by confounding factors.[340]Therefore,in patients with HFrEF and AF who have unsatisfied ventricular rate control with β-blockers or are unable to use β-blockers,digoxin can still be used to control the heart rate.
When the above-mentioned combination of drugs fails to effectively control the ventricular rate in patients with AF,amiodarone can be considered as the last option for pharmacological control of the ventricular rate,[341]but the risk of adverse reactions should be taken into account.The recommended guidelines for pharmacological treatment for ventricular rate control in AF patients is presented in Table 40.
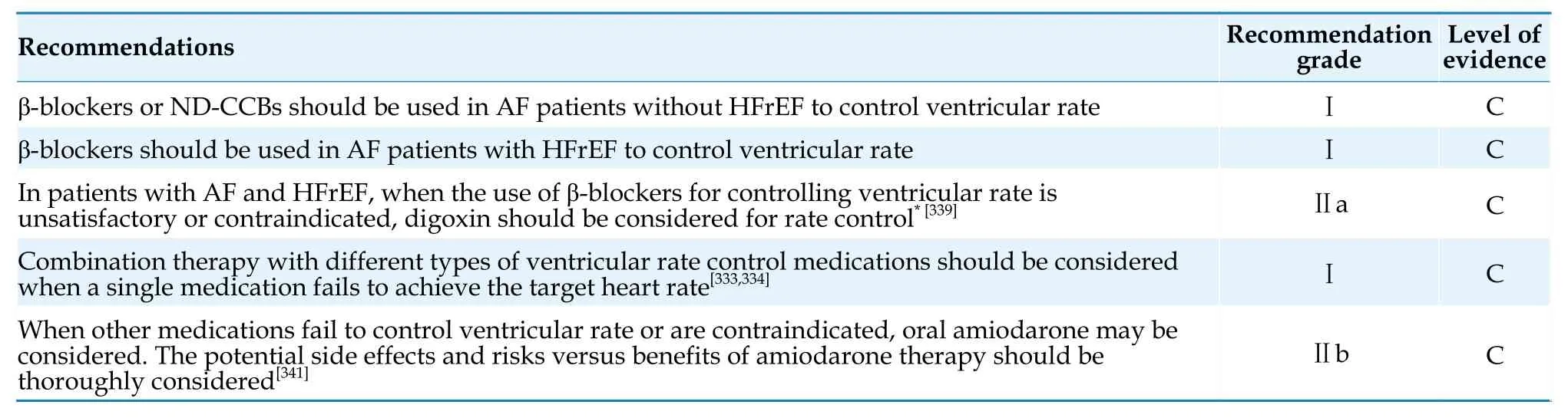
Table 40 Drugs for long-term ventricular rate control in AF patients.
6.3 Atrioventricular Node Ablation Combined with Pacemaker Implantation
The Ablation for Paroxysmal Atrial Fibrillation(APAF) study showed that for patients with severe symptoms of AF,which affects their quality of life,or for patients with poor response to medication treatment,reduced left ventricular function,and wide QRS complex,CRT is superior to right ventricular pacing in reducing heart failure exacerbation and hospitalization events.[224]The APAF-CRT study showed that for patients with permanent AF and heart failure with narrow QRS complex (≤ 110 ms),catheter ablation of the atrioventricular node combined with CRT implantation effectively reduced all-cause mortality compared to pharmacological therapy.[223]The safety and efficacy of atrioventricular node ablation combined with His bundle pacing have also been validated in AF patients with narrow QRS complex.[342]However,the results of the Pulmonary vein antrum isolation vs AV node ablation with Biventricular pacing for treatment of Atrial fibrillation in patients with Congestive Heart Failure (PABACHF) study showed that for patients with AF and heart failure,catheter ablation was superior to atrioventricular node ablation combined with biventricular pacing therapy.[343]Therefore,atrioventricular node ablation combined with synchronized pacing therapy is only suitable for patients who cannot maintain sinus rhythm through catheter ablation and have severe symptoms and poor response to medication treatment.The recommendations for the use of atrioventricular node ablation combined with pacemaker implantation for ventricular rate control in AF patients can be found in Table 41.[223,224,342-344]
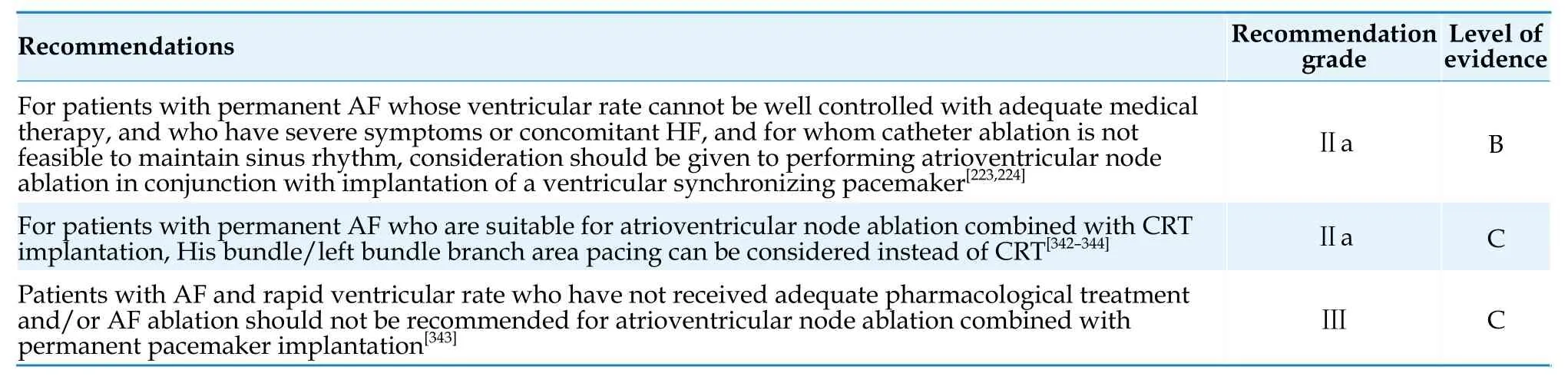
Table 41 Atrioventricular node ablation combined with pacemaker implantation.
7.EMERGENCY MANAGEMENT OF AF
New-onset AF,paroxysmal AF,and sudden onset of rapid ventricular rate in persistent AF often require emergency management.The principles of emergency management for AF include stabilizing hemodynamics,relieving symptoms,and reducing short-term and long-term risk of thromboembolism.It is important to note the following aspects: (1) Identify the hemodynamic status: when symptoms of hemodynamic instability such as severe hypotension,syncope,acute pulmonary edema,or cardiogenic shock occur during an episode of AF,immediate synchronized direct current cardioversion should be performed.(2) Identify and manage precipitating factors for acute AF: AF may be secondary to certain emergency conditions or systemic diseases,and attention should be paid to identifying and managing associated conditions,such as ACS,pulmonary embolism,or hyperthyroidism.In addition,acute episodes of AF may be accompanied by reversible triggers such as infection,alcohol abuse,diarrhea,electrolyte disturbances,medications.If these triggers are not promptly corrected,the effectiveness of rhythm control and rate control treatment will be compromised.Therefore,when managing acute AF,a comprehensive evaluation and active management of potential triggers should be simultaneously performed.[345]
7.1 Emergency Ventricular Rate Control
Before initiating rate control in AF,potential causes of increased ventricular rate should be evaluated,such as myocardial ischemia,infection,anemia,and pulmonary embolism,and it should be determined whether the increased ventricular rate is a compensatory mechanism.In patients with acute onset of AF,symptoms usually improve rapidly after urgent rate control.If accompanied by acute decompensated heart failure,rate control can lower capillary wedge pressure and increase stroke volume,thereby improving cardiac function.[346]
Drugs commonly used to control rapid ventricular rate in AF include β-blockers,ND-CCBs,and digitalis.In specific situations,amiodarone can also be used for ventricular rate control.[341]In emergency situations,intravenous formulations are generally preferred,and once the heart rate is controlled,it can be switched to oral formulations.
7.2 Emergency Rhythm Control
Approximately two-thirds of paroxysmal AF cases can convert to sinus rhythm spontaneously within 48 h of onset.For patients with newly diagnosed AF and stable hemodynamics,a delayed rhythm control strategy can be considered,which involves initial treatment with rate-control medication only and delayed cardioversion if the AF does not resolve within 48 h.[347]Delayed rhythm control strategy was no significant difference to early cardioversion in achieving a return to sinus rhythm at 4 weeks.[347]Patients with AF and pre-excitation syndrome with antegrade conduction via the bypass should not be treated with β-blockers,ND-CCBs,digoxin,or intravenous amiodarone for heart rate control.[348]In cases with unstable hemodynamics,immediate cardioversion should be performed.If the hemodynamics is stable,intravenous ibutilide or procainamide can be considered,as well as propafenone,flecainide,or dofetilide.After conversion to sinus rhythm,long-term rhythm control and anticoagulation strategies should be determined,as described in other relevant content.
7.2.1 Pharmacological cardioversion
In cases of acute AF with stable hemodynamics,pharmacological cardioversion may be considered.The choice of antiarrhythmic drugs should be based on the patient's underlying conditions and the characteristics of various antiarrhythmic drugs (Table 42).
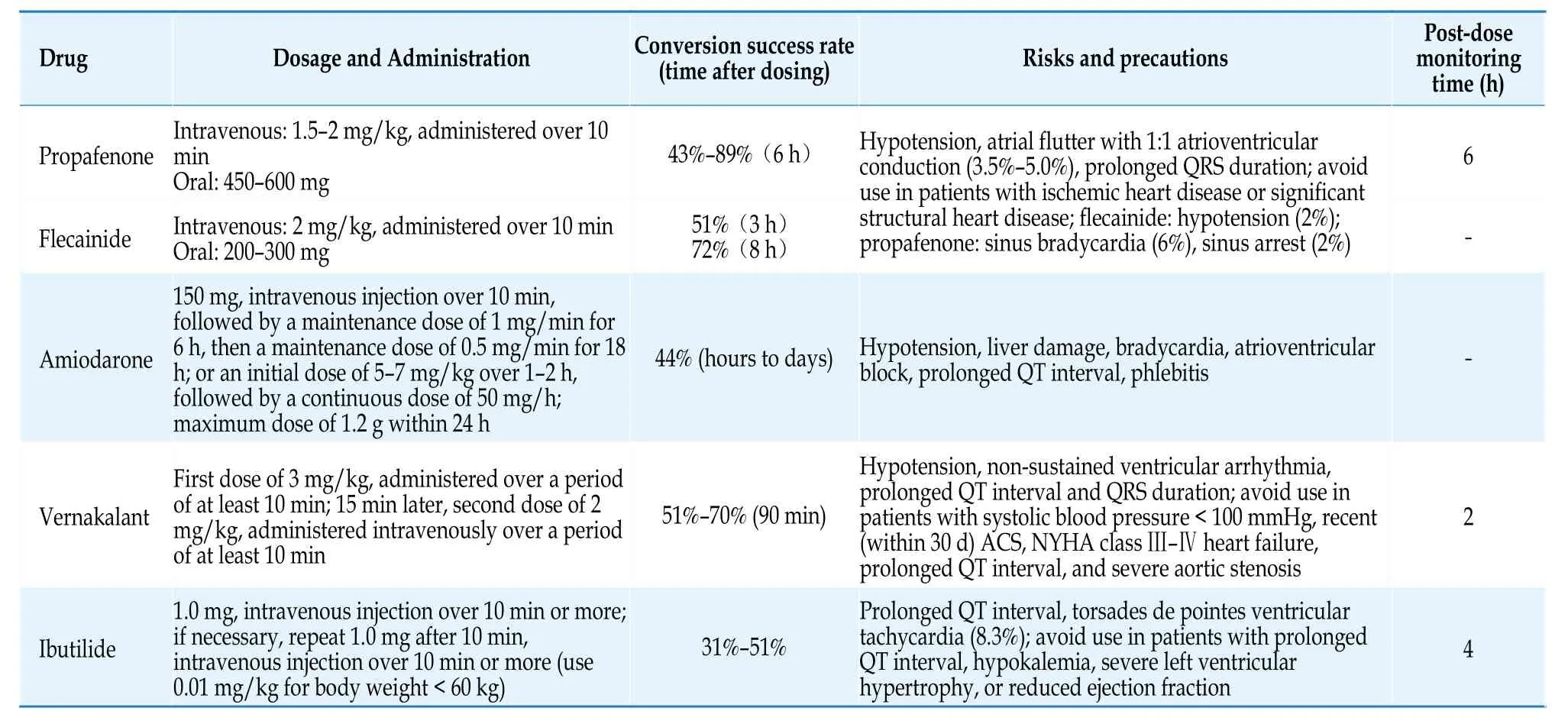
Table 42 Rhythm control medications and usage in acute onset of AF.[42]
Emergency antiarrhythmic drugs are typically administered intravenously.Propafenone and flecainide are suitable for patients with AF who have no or mild structural heart disease,but they are contraindicated in patients with heart failure,previous myocardial infarction,coronary artery disease,myocardial ischemia,and left ventricular hypertrophy.Both drugs have good conversion effects for acute AF (within 48-72 h of onset),with a high rate of sinus rhythm restoration within 1-2 h after intravenous infusion (80%-90%).Oral formulations can also be used for emergency cardioversion of AF,known as the“pill-in-the-pocket”approach.For patients weighing≥ 70 kg,flecainide 300 mg or propafenone 600 mg is recommended;for patients weighing < 70 kg,flecainide 200 mg or propafenone 450 mg is recommended.The onset time is 2-4 h,and the efficacy rate is relatively high (flecainide 56%-83%,propafenone 57%-91%).Owing to the risk of reversible QRS widening,transient hypotension,and left ventricular dysfunction,the first-time use of “pill-in-the-pocket”approach should be conducted under medical supervision.[349]
Vernakalant is a fastest-acting antiarrhythmic drugs,with the median to conversion ranging between 8 and 14 min,51%-70% of patients can restore sinus rhythm.It can be used for pharmacological cardioversion of recent-onset AF (≤ 7 day) and early (≤ 3 day) post-operative AF.[350]
The efficacy of ibutilide in converting AF is moderate,and its effect lasts for a relatively short duration (approximately 4 h),but it has a better effect on converting atrial flutter.Ibutilide can be used in patients with moderate structural heart disease other than heart failure.The major limitation of this medication is the high incidence of torsades de pointes (up to 4%),which usually occurs within 0.5 h after administration.Monitoring for at least 4 h after administration is recommended.[351]
Amiodarone is the only antiarrhythmic drugs recommended for patients with severe structural heart disease,especially those with concomitant left ventricular systolic dysfunction and heart failure.Additionally,amiodarone can slow atrioventricular conduction and control the ventricular rate.
For patients with sick sinus syndrome,second-degree type Ⅱ or higher atrioventricular block,or prolonged QTc interval (> 500 ms),pharmacological cardioversion is not recommended.
7.2.2 Electrical cardioversion
Synchronized direct current cardioversion is more effective than pharmacological cardioversion and is the preferred method for restoring sinus rhythm in hemodynamically unstable AF.Pre-treatment with antiarrhythmic drugs (such as amiodarone,ibutilide,or vernakalant) can improve the success rate of cardioversion.[352-354]Bradycardia may occasionally occur after electrical cardioversion,so preoperative preparation with medications such as atropine or isoproterenol,or temporary pacing,is necessary.
7.2.3 Improving the efficiency of AF management in the emergency department
To manage AF more efficiently and reduce the hospitalization rate of AF patients in the emergency department,it is recommended to establish a multidisciplinary collaboration and transitional AF clinic.After the acute episode of AF is stabilized,patients are advised to seek follow-up care at the AF clinic,where assessment of AF-related risk factors and indications for rhythm control can be conducted.Recommendations for managing AF in the emergency department are summarized in Table 43.
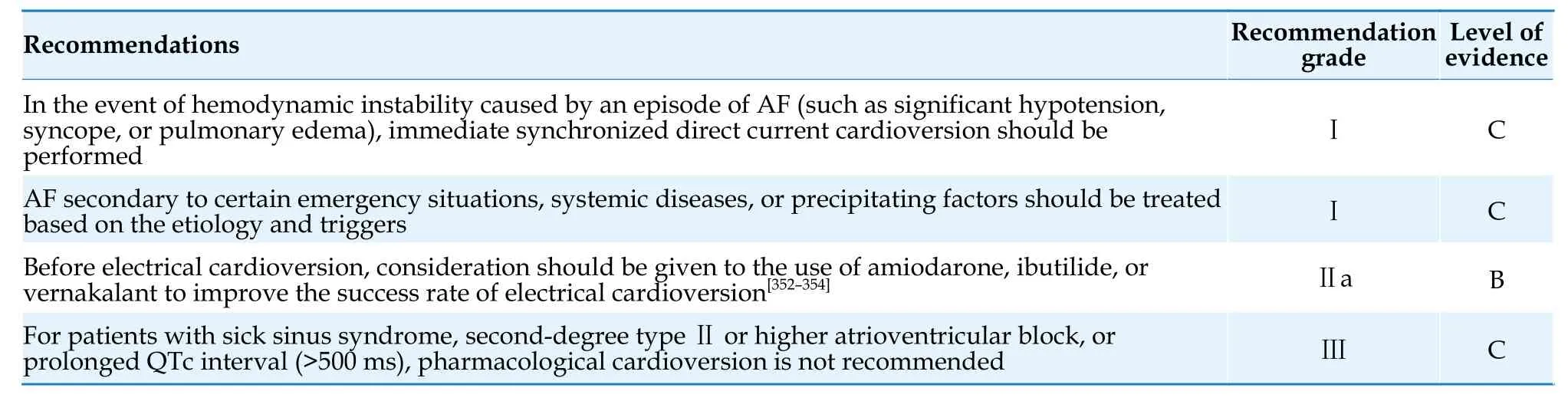
Table 43 Emergency management of patients with AF.
8.Integrated management of AF
Integrated management of AF emphasizes the holistic management of patients,including the management of cardiovascular risk factors and comorbidities.
8.1 Integrated Management Pathway of AF
The objective of integrated management of AF is to provide individualized diagnosis and management plans for patients via a multidisciplinary collaboration led by cardiovascular internal medicine,including stroke prevention,relieving symptoms through rhythm and/or rate control,controlling cardiovascular risk factors,treating comorbidities,and providing support for patient self-management;lifestyle modifications;and psychosocial support.[355]It is important to fully utilize internet technology,new media,and specialized disease management software tools to carry out diverse forms of highquality patient education that involve collaboration between specialists and general practitioners,as well as patient and family numbers.The development and widespread use of remote health management and wearable devices have greatly improved the efficiency of disease management.[356]In the future,AF management will integrate medical services provided both in healthcare facilities and through remote health management.Recommendations for integrated management of AF are summarized in Table 44.

Table 44 Integrated management of AF.
8.2 Management of Cardiovascular Risk Factors
Cardiovascular risk factors,comorbidities,and unhealthy lifestyles are closely associated with the occurrence and progression of AF.Strict management of these risk factors and comorbidities is an important component of comprehensive AF management.
8.2.1 Obesity
Obesity significantly increases the risk of stroke and mortality in patients with AF,and it is also an independent risk factor for recurrence after AF ablation.[357]Weight loss can alleviate AF-related symptoms,improve quality of life,and reduce the rate of recurrence after catheter ablation.[358,359]
8.2.2 Exercise
The relationship between exercise and the risk of AF is complex.Moderate exercise can help prevent the occurrence of AF,[360,361]while athletes engaged in long-term,high-intensity exercise have a higher risk of developing AF.[362]Engaging in appropriate exercise (≥ 150 min of moderate-intensity exercise or ≥ 75 min of high-intensity exercise per week) is associated with a reduced risk of long-term cardiovascular mortality and all-cause mortality in patients with AF.[363]
8.2.3 Alcohol consumption
Alcohol consumption is a risk factor for AF incidence,evident even in individuals with low alcohol consumption.[364]Quitting alcohol can reduce AF episodes and lower the burden of AF.[365]Alcohol consumption also increases the risk of bleeding and stroke in patients with AF.[68,366]Observational studies have shown that quitting alcohol is associated with a decrease in AF recurrence after catheter ablation.[367]
8.2.4 Smoking
The risk of AF is higher in smokers,and there is a dose-dependent relationship.The risk of AF in former smokers is significantly lower than in current smokers.[368]
8.2.5 Diabetes
The risk of AF in patients with diabetes is increased by 34%.Additionally,diabetes also increases the risk of stroke and death in patients with AF.[369]A meta-analysis has shown that sodium-glucose cotransporter-2 inhibitors (SGLT-2i) can reduce the risk of new-onset AF in patients with diabetes.[370]In patients with both AF and diabetes,SGLT-2i can reduce the risk of major cardiovascular events.[371,372]A small-scale RCT suggested that SGLT-2i can reduce AF recurrence after catheter ablation.[373]
8.2.6 Hypertension
Hypertension is an important risk factor for the development of AF.Subsequent analysis of the Systolic Blood Pressure Intervention Trial (SPRINT)study has shown that intensive blood pressure lowering (targeting a systolic blood pressure < 120 mm-Hg) is associated with a lower risk of incident AF.[374]The prevalence of hypertension in patients with AF is as high as 60% to 80%,and hypertension significantly increases the risk of adverse cardiovascular events in this population.[375]A meta-analysis of RCTs has shown that the benefits of blood pressurelowering therapy in patients with AF are similar to those in patients without AF.[376]A subgroup analysis of the SPRINT study has shown that intensive blood pressure lowering therapy provides greater absolute cardiovascular benefits in patients with AF than in those without.[377]Further research is needed to determine the optimal blood pressure target in patients with AF.
8.2.7 Obstructive sleep apnea syndrome
Approximately 20% to 70% of AF patients have obstructive sleep apnea.[378]Obstructive sleep apnea affects the success rate of electrical cardioversion,catheter ablation,and the efficacy of antiarrhythmic drugs in AF patients.[379,380]However,evidence from RCTs suggests that continuous positive airway pressure therapy does not reduce AF burden or increase the success rate of catheter ablation.[381]
The management of risk factors for AF is summarized in Table 45.[357-382]
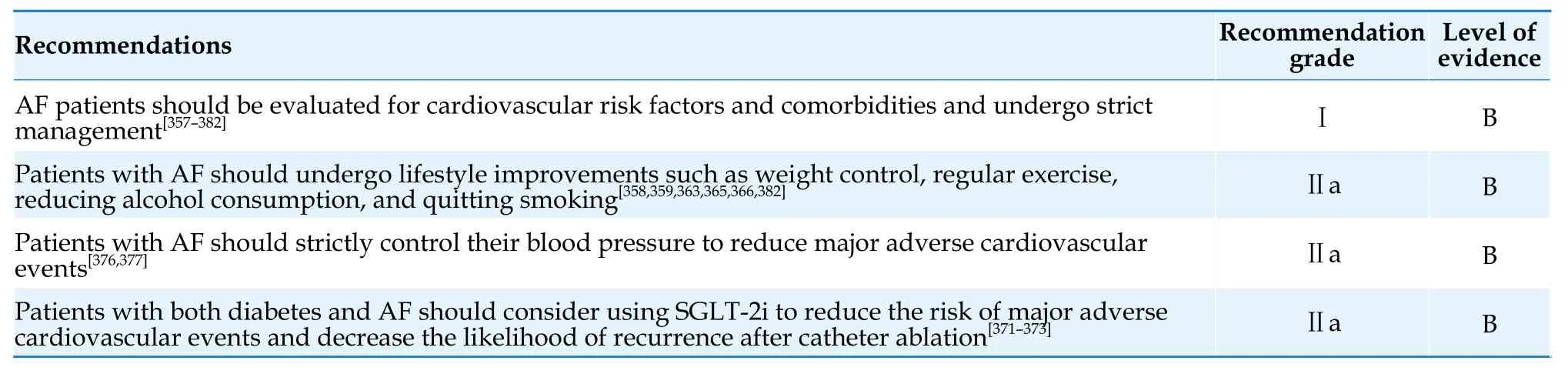
Table 45 Risk factor management for AF.
8.3 AF after Cardiac or Noncardiac Surgery
Although postoperative AF (POAF) mainly occurs in patients receiving cardiac surgery,with the highest incidence after valve-related surgeries (40%-50%),it also occurs after non-cardiac surgeries.POAF can prolong the length of hospitalization,increase medical costs,and increase the risk of heart failure,stroke,myocardial infarction,death,and long-term recurrence of AF.[383]
Amiodarone can effectively reduce the incidence of POAF and shortens the length of hospitalization,but it does not differ from placebo in reducing stroke or death rates.[384]β-blockers also reduce the occurrence of POAF after cardiac surgery,but have no effect on hospital stay,stroke,or mortality.[384]In noncardiac surgery,prophylactic use of β-blockers can reduce the risk of new-onset AF,non-fatal myocardial infarction,and cardiac arrest,but may increase postoperative all-cause mortality and stroke.[385]RCTs have failed to prove that statins,colchicine,and corticosteroids reduce the incidence of POAF,[386-388]while perioperative oral berberine can effectively reduce the incidence of POAF,possibly because of its anti-inflammatory effects.[389]Targeted injection of high-concentration calcium chloride into major atrial ganglionated plexi during coronary artery bypass grafting surgery significantly inhibits autonomic activity,resulting in a decrease in the incidence of new-onset POAF from 36% to 15%.[390]
For patients with POAF causing hemodynamic instability,cardioversion should be performed promptly.For POAF with hemodynamic stability,an RCT showed that strategies for rate control and rhythm control to treat POAF were associated with equal numbers of days of hospitalization,similar death and complication rates.[391]
Observational studies suggest that the use of oral anticoagulants in patients with POAF undergoing non-cardiac surgery is associated with reduced risk of stroke and death.[392]However,there is still controversy regarding the benefits of early oral anticoagulants use in patients with newly diagnosed AF after coronary artery bypass grafting surgery.[393-395]
8.4 Management of Patients with AF Combined with Tumors
The risk of AF is increased in patients with malignant tumors/cancer,but the risk of AF varies among different types and stages of malignant tumors.Patients with hematological malignancies have a risk of AF more than twice that of individuals without tumors,while patients with solid tumors such as thoracic malignancies (lung cancer,esophageal cancer) and central nervous system tumors also have a significantly increased risk of AF.[396]Factors related to the cancer disease itself (such as tumor progression,inflammation,hypoxia,autonomic dysfunction,and paraneoplastic syndrome),treatment factors (surgery,specific types of chemotherapy,radiation therapy),as well as common conditions in cancer patients such as advanced age and underlying heart disease,can all contribute to an increased risk of AF.[396]
Various anti-tumor drugs are associated with the occurrence of AF,such as tyrosine kinase inhibitors,alkylating agents,and anti-metabolites,which can participate in atrial remodeling through multiple pathways,increasing the risk of AF.[397]AF can occur immediately after treatment or several weeks to months after treatment.[397]After using the aforementioned drugs,it is important to strengthen ECG monitoring to promptly detect AF and initiate appropriate anticoagulation therapy.
The predictive value of the CHA2DS2-VASc score for thromboembolic risk in AF patients with concomitant cancer has not yet been fully validated.The risk of thromboembolism is increased in male patients with a CHA2DS2-VASc score of 0 and female patients with a score of 1,who have had a recent history of cancer.[398]Certain tumors themselves,tumor metastasis,and anti-tumor drugs all increase the risk of thromboembolism.Anticoagulant therapy should be given due consideration in AF patients with concomitant cancer.When using NOACs in these patients,drug interactions,renal function,bleeding risk (including bleeding risk associated with the underlying disease and bone marrow suppression caused by anti-cancer treatment),as well as the impact of tumors in the digestive and urinary systems on drug absorption and metabolism should be taken into account.[399]For AF patients with concomitant cancer,a meta-analysis suggested that NOACs were significantly more effective and safer than warfarin.[400]The anticoagulation regimen for these patients should be developed through collaboration among multidisciplinary teams,taking into consideration various factors.
Currently,there is limited research evidence on catheter ablation for AF in cancer patients.For patients with generally good health,who can tolerate surgery and have a longer expected survival,catheter ablation may be considered.
DISCLOSURE
Core Expert Group Members
Changsheng Ma (Beijing Anzhen Hospital,Capital Medical University),Huishan Wang (General Hospital of Northern Theater Command),Shaowen Liu(Shanghai First People’s Hospital),Ribo Tang (Beijing Anzhen Hospital,Capital Medical University),Yihong Sun (China-Japan Friendship Hospital),Xin Du (Beijing Anzhen Hospital,Capital Medical University),Jingjie Li (The First Affiliated Hospital of Harbin Medical University),Shulin Wu (Guandong Provincial People’s Hospital),Yan Yao (Fuwai Hospital,Chinese Academy of Medical Sciences),Caihua Sang(Beijing Anzhen Hospital,Capital Medical University),Dong Chang (Xiamen Cardiovascular Hospital,Xiamen University),Yan Liang (Fuwai Hospital,Chinese Academy of Medical Sciences),Yaling Han(General Hospital of Northern Theater Command),Jianzeng Dong (Beijing Anzhen Hospital,Capital Medical University),Chenyang Jiang (Sir Run Run Shaw Hospital,Zhejiang University School of Medicine)
Expert Group Members (No Specific Order of Names)
Yansheng Ding (Peking University First Hospital),Bo Yu (The First Hospital of China Medical University),Yongjun Wang (Beijing Tiantan Hospital),Yan Wang (Tongji Hospital (Wuhan)),Zulu Wang (General Hospital of Northern Theater Command),Ji-Guang Wang (Ruijin Hospital (Shanghai)),Quan Fang (Peking Union Medical College Hospital),Xiaomeng Yin (The First Affiliated Hospital of Dalian Medical University),Deyong Long (Beijing Anzhen Hospital,Capital Medical University),Hua Fu (West China Hospital,Sichuan University),Xiufen Qu (The First Affiliated Hospital of Harbin Medical University),Shuwang Liu (Peking University Third Hospital),Qiming Liu (The Second Xiangya Hospital of Central South University),Bin Liu (The Second Hospital of Jilin University),Jing Liu (Beijing Anzhen Hospital,Capital Medical University),Gang Liu (The First Hospital of Hebei Medical University),Lisheng Jiang (Shanghai Chest Hospital),Hong Jiang (Renmin Hospital of Wuhan University),Baopeng Tang (The First Affiliated Hospital of Xinjiang Medical University),Yingxian Sun (The First Hospital of China Medical University),Guangping Li(The Second Hospital of Tianjin Medical University),Shufeng Li (The Second Affiliated Hospital of Harbin Medical University),Xuebin Li (Peking University People's Hospital),Shaolong Li (Yan’an Hospital of Kunming City),Shuyan Li (The First Hospital of Jilin University),Yigang Li (Xinhua Hospital Affiliated To Shanghai Jiao Tong University School of Medicine),Yanzong Yang (The First Hospital of Dalian Medical University),Bing Yang (Eastern Hospital,Tongji University School of Medicine),Jiefu Yang (Beijing Hospital),Yanmin Yang (Fuwai Hospital,Chinese Academy of Medical Sciences),Xinchun Yang (Beijing Chaoyang Hospital,Capital Medical University),Ben He (Shanghai Chest Hospital),Shuyang Zhang (Peking Union Medical College Hospital),Jian Zhang (Fuwai Hospital,Chinese Academy of Medical Sciences),Ping Zhang (Beijing Tsinghua Changgung Hospital),Yihan Chen (Shanghai East Hospital),Songwen Chen (Shanghai General Hospital),Minglong Chen (Jiangsu Province Hospital),Shenghua Zhou (The Second Xiangya Hospitalof Central South University),Qiangsun Zheng (The Second Affiliated Hospital of Xi’an Jiaotong University),Jingquan Zhong (Qilu Hospital of Shandong University),Kui Hong (The Second Affiliated Hospital of Nanchang University),Yuquan He (China-Japan Union Hospital of Jilin University),Yiqiang Yuan (The Seventh People’s Hospital of Zhengzhou),Zuyi Yuan(The First Affiliated Hospital of Xi’an Jiaotong University),Shaoping Nie (Beijing Anzhen Hospital,Capital Medical University),Wei Xu (Nanjing Drum Tower Hospital),Lianjun Gao (The First Affiliated Hospital of Dalian Medical University),Yutao Guo(Chinses People′s Liberation Army General Hospital),Min Tang (Fuwai Hospital,Chinese Academy of Medical Sciences),Jianhong Tao (Sichuan Provincial People’s Hospital),Weibin Huang (Zhongshan Hospital,Xiamen University),Weijian Huang (The First Affiliated Hospital of Wenzhou Medical University),He Huang (Renmin Hospital of Wuhan University),Zhaoguang Liang (The First Affiliated Hospital of Harbin Medical University),Bin Liang(The Second Hospital of Shanxi Medical University),Bing Han (Xuzhou Central Hospital),Maoqin Shu (The First Affiliated Hospital of Army Medical University),Zhibing Lu (Zhongnan Hospital of Wuhan University),Ruiqin Xie (The Second Hospital of Hebei Medical University),Jun Cai (Fuwai Hospital,Chinese Academy of Medical Sciences),Yumei Xue (Guangdong Provincial People's Hospital)
Authors
Wei Wang (Beijing Anzhen Hospital,Capital Medical University),Ying Bai (Beijing Tongren Hospital,Capital Medical University),Nian Liu (Beijing Anzhen Hospital,Capital Medical University),Xiaoxia Liu (Beijing Anzhen Hospital,Capital Medical University),Ribo Tang (Beijing Anzhen Hospital,Capital Medical University),Songnan Li (Beijing Anzhen Hospital,Capital Medical University),Changyi Li (Beijing Anzhen Hospital,Capital Medical University),Xin Zhao (Beijing Anzhen Hospital,Capital Medical University),Caihua Sang (Beijing Anzhen Hospital,Capital Medical University),Shijun Xia (Beijing Anzhen Hospital,Capital Medical University),Xueyuan Guo (Beijing Anzhen Hospital,Capital Medical University),Qi Guo (Beijing Anzhen Hospital,Capital Medical University),Chao Jiang (Beijing Anzhen Hospital,Capital Medical University),Chenxi Jiang (Beijing Anzhen Hospital,Capital Medical University)
Guideline Secretariats
Chao Jiang (Beijing Anzhen Hospital,Capital Medical University),Changyi Li (Beijing Anzhen Hospital,Capital Medical University),Mingyang Gao (Beijing Anzhen Hospital,Capital Medical University)
Conflicts of Interest
None.
 Journal of Geriatric Cardiology2024年3期
Journal of Geriatric Cardiology2024年3期
- Journal of Geriatric Cardiology的其它文章
- Community-based prevention and treatment of cardiovascular diseases
- Significance of balloon aortic valvuloplasty as palliative procedure for symptom benefit in patients with severe aortic stenosis
- Sleep quality and characteristics of older adults with acute cardiovascular disease
- The diagnostic value of tenascin-C in acute aortic syndrome
- Association of acute glycemic parameters at admission with cardiovascular mortality in the oldest old with acute myocardial infarction
- Effects of loneliness and isolation on cardiovascular diseases:a two sample Mendelian Randomization Study
